Fertility preservation in patients diagnosed with cancer during pregnanc
Aim. To investigate the feasibility of in vitro maturation (IVM) of human oocytes as a fertility-sparing technology in patients diagnosed with cancer during pregnancy.Kirillova A.O., Bunyaeva E.S., Koval’skaya E.V., Kamaletdinov N.S., Khabas G.N., Farmakovskaya M.D, Sirotkina E.A., Mishieva N.G., Nazarenko T.A., Abubakirov A.N.
Materials and methods. Ovariectomy was performed during a cesarean section (CS) or elective surgery. Immature oocyte-cumulus complexes were retrieved from the ovarian medulla and underwent 48–72 h in vitro maturation. All mature oocytes were vitrified. The percentage of matured and degenerative oocytes and the number of cryopreserved oocytes were estimated.
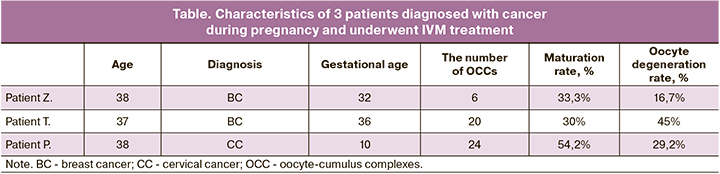
Results. In all three cases of patients diagnosed with cancer during pregnancy, oocyte-cumulus complexes were obtained. Six, 20, and 24 oocyte-cumulus complexes were isolated from the first, the second, and the third patient, respectively. The oocyte maturation rate was 33.3%, 30.0%, and 54.2%, resulting in vitrification of 2, 6, and 12 oocytes, respectively.
Conclusion. Oocyte-cumulus complexes can be successfully isolated from the ovarian medulla after ovary removal during cesarean section or elective surgery. In vitro-matured oocytes can be vitrified or used for in vitro fertilization (IVF) to preserve fertility.
Keywords
The incidence of cancer in pregnant women is estimated to be one case per 1000 pregnancies, which represents from 0.07 to 0.1% of all malignancies [1, 2]. The most common malignant neoplasms occurring during gestation are breast cancer, cervical cancer, Hodgkin's lymphoma, melanoma, and leukemia [2]. These cancers are the most common among young women of reproductive age [3].
The management of these women should always be based on the following optimal gold standards: 1. to maintain and improve the life of the mother; 2. treat the curable malignant disease of pregnant women; 3. protect of the fetus and newborn from harmful effects of cancer treatment; 4. retain the mother's reproductive system intact for future gestations [2].
Even though fertility preservation is the last treatment priority for this group of patients, this aspect should not be neglected. Pregnant women with cancer should be consulted on possible methods for preserving reproductive function.
Currently, fertility-sparing treatments include embryo and oocyte cryopreservation after controlled ovarian hyperstimulation, cryopreservation of oocytes and/or embryos after in vitro maturation (IVM), ovarian tissue cryopreservation, or a combination of these methods [4].
In international practice, there have been only a few clinical cases of fertility preservation in patients during pregnancy or immediately after childbirth. In 1997, a case was reported of a pregnancy in a woman with primary infertility after a donation of immature oocyte- cumulus complexes (OCC), aspirated during cesarean section (CS). [5]. Seven immature oocytes were obtained during the operation, of which two successfully matured after 48 hours of IVM. Both oocytes were fertilized using ICSI with sperm from the recipient’s husband resulting in healthy pregnancy [5].
A case also was reported of direct aspiration of small ovarian follicles from the right ovary during CS, isolation of immature oocyte-cumulus complexes (OCC) from the left ovary after ovariectomy, and ovarian tissue cryopreservation in a patient with rhabdomyosarcoma at the 37 weeks’ gestation [6]. Such integrated fertility-sparing management led to cryopreservation of 14 fragments of ovarian tissue and 12 mature oocytes [6].
Besides, a case of ovarian tissue freezing after removal of half of the left ovary during CS was reported, combined with controlled ovarian hyperstimulation a week after delivery in a patient with Hodgkin’s lymphoma at the 40th gestational week [7]. During transvaginal follicular puncture, four oocytes were retrieved, of which only two were mature. One oocyte was fertilized and cryopreserved [7].
Our study includes three clinical cases and provides additional insight into the possibility of maturation of oocytes retrieved from ovarian tissue after its removal in patients diagnosed with cancer during pregnancy. In two cases, patients with breast cancer underwent ovariectomy during CS at 32–36 weeks of gestation and in one case during a total hysterectomy with the termination of pregnancy at 10 weeks’ gestation. In all three cases, immature oocytes were isolated, matured in vitro, and were cryopreserved. In the future, these oocytes can be used in in-vitro fertilization (IVF) programs.
Material and methods
Ovariectomy and cryopreservation of ovarian tissue
Bilateral ovariectomy was performed during a laparotomy or CS. Both ovariectomy specimens were transported to the Laboratory for Assisted Reproductive Technologies (ART) in a 0.9% NaCl solution preheated to 37°C for 15 minutes. After aspiration of the visible antral follicles with a 21G needle, the ovarian cortical layer (about 1 mm thick) was separated from the ovarian medulla on a heated surface (37°C) in a laminar box. Cortical tissue was cut into 0.5 cm x 0.5 cm fragments and equilibrated for 30 min on ice in a cryopreservation solution containing ethylene glycol and sucrose, and then placed in cryovials (Nunc, Thermo Fischer Scientific, Copenhagen, Denmark) 4 fragments in each tube. Samples were frozen using the slow cryopreservation protocol [8] in a programmable controlled rate freezer (Kryo 10; series 10/20, Planer Biomed, Sunbury on Thames, UK).
Oocyte collection and maturation
The collection and maturation of oocytes were carried out according to the previously described technique [9].
Denudation and cryopreservation of oocytes
All oocytes were denudated with a hyaluronidase solution (SynVitro Hyadase, Origio, CooperSurgical) by gentle pipetting to remove cumulus cells. After denudation, the morphological features of mature oocytes were determined using an inverted microscope (Ti-U eclipse, Nikon).
All mature oocytes were cryopreserved by vitrification according to the manufacturer's guidelines (Kitazato vitrification kit, Kitazato).
Results
Case 1
A 38-year-old patient Z. presented at 18 weeks' gestation and reported finding redness of her left breast over the scar area after surgical excision of fibroadenoma at the age of 36 years. She underwent a core needle biopsy at the N.N. Blokhin Russian Oncology Center.
The results of a histological examination of biopsy specimens were diagnostic of T3N3M0 left breast cancer. The patient underwent one course of palliative chemotherapy. At the beginning of the second trimester of pregnancy, an increase in liver enzymes to 220 IU/l was noted, and subsequent chemotherapy was canceled.
Based on the results of the oncologist's examination and taking into account the hepatotoxicity of cytostatic therapy and the spread of the tumor, early delivery was indicated at a gestational age of 32–33 weeks.
The present gestation was her second pregnancy. The patient had a history of vacuum aspiration of the gestational sac after a missed miscarriage at 7 weeks' gestation. Her family history was unremarkable, but she had a history of viral hepatitis A and B. Menarche occurred at the age of 15 and continued with regular menstruation.
In February 2019, at 32 weeks and 2 days of gestation, the patient underwent C.S. and a bilateral adnexectomy resulting in the delivery of a live male preterm infant weighing 1857 g with a height of 42 cm and Apgar score of 7/8.
The ovarian cortical layer was cryopreserved. From both ovaries, 6 OCCs were obtained. After 48 h of maturation, 2 and 1oocytes reached stage MII and MI, respectively, 2 oocytes ceased developing at stage G.V. and 1 oocyte degenerated (table). All mature oocytes had normal morphology. After an additional 24 hours of oocyte culture, no oocyte reached stage MII. Oocyte maturation rate in this patient was 2/6 (33.3%). Mature oocytes were vitrified.
Case 2
A 37-year-old pregnant woman at 28 weeks’ gestation discovered two lumps in her left breast and sought medical attention at a private medical center. No pathology was detected by ultrasound examination. She was diagnosed as having lactostasis, and breast milk expression was recommended. After following the recommendation for two days, the patient developed a hematoma in the area of the lumps of her left breast, with a bright hyperemic corolla. At the same medical center, she was diagnosed with an abscess and was offered surgical treatment. The patient was self-referred to the N.N. Blokhin Russian Oncology Center with her ultrasound findings, where she underwent a core needle biopsy. The results of histological and immunohistochemical (IHC) examination were consistent with T3N2M0 retro- positive subtype B breast cancer. She was recommended early delivery at 35–35 weeks and 5 days’ gestation, disabling ovarian function, suppressing lactation, and neoadjuvant palliative chemotherapy.
The present gestation was her sixth pregnancy. She had a history of one surgical delivery, one missed miscarriage at 8 weeks, and 3 spontaneous miscarriages at 8, 21, and 12 weeks’ gestation, of which two were accompanied by multiple fetal malformations. The index pregnancy was complicated by a congenital fetal brain malformation in the form of ventriculomegaly of the left lateral ventricle. Menarche occurred at the age of 13 and continued with regular menstruation.
In February 2019, at 36 weeks and 2 days' gestation, the patient underwent CS resulting in the delivery of a live female preterm infant weighing 2610 g with a height of 47 cm and Apgar score of 8/9. From both ovaries, 20 OCCs were obtained. After 48 h of culture in maturation medium, 6 and 3oocytes reached stage MII and MI, respectively, 3 oocytes ceased developing at stage GV, 9 oocytes degenerated, and one oocyte was fragmented (table). All mature oocytes had an expanded perivitelline space, and one oocyte had a fragmented polar body. After an additional 24 hours of oocyte culture, no oocyte reached stage MII. Oocyte maturation rate in this patient was 6/20 (30%). All mature oocytes were vitrified. The ovarian cortical layer was cryopreserved.
Case 3
At 9 weeks of gestation, a 38-year-old woman presented to an antenatal clinic complaining of vaginal spotting. A vaginal examination revealed a cervical tumor up to 5 cm in diameter, extending to the left vaginal fornix. Histological and IHC findings showed a small cell neuroendocrine cervical cancer, after which the patient was self-referred to the Center.
The patient had a history of chronic gastritis in remission, and her family history was unremarkable. At the age of 32 years, she underwent laparoscopic cholecystectomy. She was a nicotine-dependent smoker for 15 years and reported a smoking habit of one pack of cigarettes per day.
Menarche occurred at the age of 16 and continued with regular and painless menstruation. The patient had a history of 4 pregnancies, including two that ended in spontaneous full-term birth, one missed miscarriage at early gestation, and the index pregnancy.
Considering the presence of histologically verified small cell cervical cancer diagnosed during the first trimester of pregnancy, contraindications to neoadjuvant chemotherapy, and the high risk of cancer progression, the Center’s medical Concilium advised radical surgery with the termination of pregnancy at 10 weeks’ gestation. In June 2019, a laparotomy through a midline incision was performed, followed by a Piver class III radical hysterectomy with the removal of fallopian tubes (type C-2, Wertheim operation) and bilateral ovariectomy. Histological examination revealed a low- grade neuroendocrine carcinoma of the cervix uteri (NEC G3, small cell neuroendocrine cancer, Ki-67 = 100%) with an invasion of the entire thickness of the cervical wall, spreading to the upper third of the vagina (3 cm), but without parametrial invasion. Also, there was a lymphovascular invasion and the left ovary macrometastases of neuroendocrine cancer. Ovarian tissue of the right ovary was cryopreserved.
From two ovaries, 24 OCCs were obtained. After 48 hours of culture in a maturing medium, 10 oocytes reached stage II, 7 oocytes ceased developing at the GV stage, and 7 oocytes degenerated. All mature oocytes had normal morphology. After an additional 24 hours of oocyte culture, another 3 oocytes reached stage MII (table). One of them had abnormally large size, one had an abnormal shape and contained a cytoplasmic vacuole, and one had a fragmented polar body. Oocyte maturation rate in this patient was13/24 (54.2%). Twelve stages II oocytes (except the giant one) were vitrified.
Discussion
In our study, we demonstrated the potential of in vitro maturation of immature oocytes obtained from surgically removed ovarian tissue in pregnant women with cancer. Currently, only a few studies are known that describe the isolation and maturation of immature OCCs during pregnancy in an animal model or as sporadic clinical cases [5, 6].
Of great interest is the fact that oocytes retain the ability to mature even in the late stages of gestation. It has been previously shown that retrieving oocytes at any phase of the menstrual cycle does not affect the quantity and quality of the immature oocytes in IVM programs, the percentage of matured and fertilized oocytes, and the number of frozen embryos [10]. Typically, two or three antral follicular waves are recruited during the human menstrual cycle [11]. During pregnancy, there is a unique hormonal background with a low gonadotropin concentration and the absence of cyclic endocrine changes, which is unfavorable for folliculogenesis [12]. Besides, during pregnancy, ovulation is suppressed, resulting in the degeneration of antral follicles that reach 4–5 mm in size [13, 14]. During pregnancy, folliculogenesis is irregular, and there are several spikes in the growth of follicles, which are replaced by their atresia. During the first 7 weeks of pregnancy, there is little evidence of follicular activity. It resumes at week 10 when antral follicles reappear. Since the 20th week of gestation, folliculogenesis almost does not occur, and most ovarian antral follicles remain atretic. From 33 weeks new Graafian follicles, rarely exceeding 4 mm in diameter, appear in progressively increasing numbers and reach a maximum by the 40th week [13]. By the end of pregnancy, 15–20 antral follicles up to 6 mm in diameter can be observed in the ovary [13, 15].
In our study, 30–54% of oocytes retained the competency to complete meiosis. Interestingly, the most significant ability to mature in vitro was observed in a patient in the first trimester of pregnancy (54.2%). In contrast, in patients in the third trimester, this ability was lower (30–33%).
Previous studies have reported that most antral ovarian follicles during pregnancy were degenerative [13, 15], and only 7% of the follicles were viable [15]. In our study, the percentage of degenerative oocytes ranged from 16.7 to 45%, and most of the oocytes were viable.
Immature OCCs can be retrieved by aspiration during CS or from the ovarian medulla after ovary removal. In our study, OCCs were isolated by ex vivo extraction of antral ovarian follicles, which we believe is the most effective method of retrieving OCCs. It allows the isolation of even small 0.5–2 mm follicles located deep in the ovary, which are impossible to aspirate. So it has been shown that the ex vivo retrieval of OCCs from the ovarian medullar layer results in twice as many OCCs than using aspiration technology [6].
A limitation of our study is that it consists of individual clinical cases. More extensive studies are needed to investigate the effectiveness of ex vivo IVM technology in patients diagnosed with cancer during pregnancy.
However, our study provides encouraging results. Ex vivo IVM can be used to preserve fertility in patients diagnosed with cancer during pregnancy. The ovary can be removed during an elective cesarean section. After an oophorectomy, the ovarian cortical layer can be cryopreserved, and immature oocytes capable of maturation in vitro can be isolated from the medullar layer.
Several authors have proposed this approach to obtain donor [16] or the patients' own oocytes [6] for IVF. This approach may help avoid additional surgical intervention, reduce the risks associated with controlled ovarian hyperstimulation, and reduce treatment costs.
Conclusion
Immature OCCs can be successfully isolated from the ovarian medulla after ovary removal during CS. Ex vivo IVM is a promising fertility-sparing procedure in patients diagnosed with cancer during pregnancy. In vitro-matured oocytes can be vitrified or used for IVF.
References
- Donegan W.L. Cancer and pregnancy. CA: Cancer J. Clin. 1983; 33(4): 194-214. https://dx.doi.org/10.3322/canjclin.33.4.194.
- Pavlidis N.A. Coexistence of pregnancy and malignancy. Oncologist. 2002; 7(4): 279-87.
- Hepner A., Negrini D., Hase E.A., Exman P., Testa L., Trinconi A.F. et al. Cancer during pregnancy: The oncologist overview. World J. Oncol. 2019; 10(1): 28-34. https://dx.doi.org/10.14740/wjon1177.
- Medrano J.V., Andrés M. del M., García S., Herraiz S., Vilanova-Pérez T., Goossens E, Pellicer A. Basic and clinical approaches for fertility preservation and restoration in cancer patients. Trends Biotechnol. 2018; 36(2): 199-215. https://dx.doi.org/10.1016/j.tibtech.2017.10.010.
- Hwang J.L., Lin Y.H., Tsai Y.L. Pregnancy after immature oocyte donation and intracytoplasmic sperm injection. Fertil. Steril. 1997; 68(6): 1139-40.https://dx.doi.org/10.1016/s0015-0282(97)00398-1.
- Ben-Haroush A., Sapir O., Fisch B. Aspiration of immature oocytes during cesarean section for fertility preservation and future surrogacy. Am. J. Obstet. Gynecol. 2010; 203(1): e12-4. https://dx.doi.org/10.1016/j.ajog.2010.04.011.
- Gundelach T., Stuck D., Widschwendter P., Weiss J.M., Janni W., Hancke K. Fertility preservation after caesarean delivery in a woman diagnosed with Morbus Hodgkin disease during pregnancy. J. Assist. Reprod. Genet. 2014; 31(1): 51-3. https://dx.doi.org/10.1007/s10815-013-0126-7.
- Schmidt K.L.T., Byskov A.G., Andersen A.N., Muller J., Andersen C.Y. Density and distribution of primordial follicles in single pieces of cortex from 21 patients and in individual pieces of cortex from three entire human ovaries. Hum. Reprod. 2003; 18(6): 1158-64. https://dx.doi.org/10.1093/humrep/deg246.
- Ковальская Е.В., Кириллова А.О., Буняева Е.С., Хабас Г.Н., Камалетдинов Н.С., Назаренко Т.А., Абубакиров А.Н. Эффективность дозревания ооцитов, полученных в ходе овариэктомии у онкологических пациенток. Акушерство и гинекология. 2019; 9: 87-91. [Kovalskaya E.V., Kirillova A.O., Bunyaeva E.S., Khabas G.N., Kamaletdinov N.S., Nazarenko T.A., Abubakirov A.N. Efficiency of maturation of oocytes obtained from cancer patients during ovariectomy. Obstetrics and Gynecology/Akusherstvo i ginekologiya. 2019; (9): 87-91. (in Russian).]https://dx.doi.org/10.18565/aig.2019.9.87-91.
- Creux H., Monnier P., Son W.Y., Tulandi T., Buckett W. Immature oocyte retrieval and in vitro oocyte maturation at different phases of the menstrual cycle in women with cancer who require urgent gonadotoxic treatment. Fertil. Steril. 2017; 107(1): 198-204. https://dx.doi.org/10.1016/j.fertnstert.2016.09.041.
- Yang D.Z., Yang W., Li Y., He Z. Progress in understanding human ovarian folliculogenesis and its implications in assisted reproduction. J. Assist. Reprod. Genet. 2013; 30(2): 213-9. https://dx.doi.org/10.1007/s10815-013-9944-x.
- Gougeon A. Dynamics of follicle development in the human ovary. In: Chang R.J., ed. Polycystic ovary syndrome. New York, NY: Springer; 1996: 21-36.
- Govan A.D.T. Ovarian follicular activity in late pregnancy. J. Endocrinol. 1970; 48(2): 235-41. https://dx.doi.org/10.1677/joe.0.0480235.
- Dizerega G., Hodgen G.D. Pregnancy-associated ovarian refractoriness to gonadotropin: A myth. Am. J. Obstet. Gynecol. 1979; 134(7): 819-22.https://dx.doi.org/10.1016/0002-9378(79)90953-0.
- Westergaard L., McNatty K.P., Christensen I.J. Steroid concentrations in fluid from human ovarian antral follicles during pregnancy. J. Endocrinol. 1985; 107(1): 133-6. https://dx.doi.org/10.1677/joe.0.1070133.
- Brzyski R.G., Leland M.M., Eddy C.A. In vitro maturation of baboon oocytes retrieved at the time of cesarean section. Fertil. Steril. 1999; 71(6): 1153-6. https://dx.doi.org/10.1016/s0015-0282(99)00130-2.
Received 29.01.2020
Accepted 07.02.2020
About the Authors
Anastasia O Kirillova, Ph.D. (bio.sci.), Senior Researcher at the 1st Department of Gynecology, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov. Tel.: +7(926)781-55-24. E-mail: stasia.kozyreva@gmail.com.4 Oparina str., Moscow, 117997, Russian Federation.
Ekaterina S Bunyaeva, Ph.D. Student at the 1st Department of Gynecology, National Medical Research Center for Obstetrics, Gynecology and Perinatology named
after Academician V.I. Kulakov. Tel.: +7(495)438-26-22. E-mail: es_bunyaeva@mail.ru. 4 Oparina str., Moscow, 117997, Russian Federation.
Evgeniya V Kovalskaya, Embryologist at the 1st Department of Gynecology, National Medical Research Center for Obstetrics, Gynecology and Perinatology named
after Academician V.I. Kulakov. E-mail: kovalskaya.evgeniya@gmail.com. 4 Oparina str., Moscow, 117997, Russian Federation.
Nail S. Kamaletdinov, Embryologist at the 1st Department of Gynecology, National Medical Research Center for Obstetrics, Gynecology and Perinatology named
after Academician V.I. Kulakov. E-mail: sunsh86@mail.ru. 4 Oparina str., Moscow, 117997, Russian Federation.
Grigory N. Khabas, Ph.D., Head of the Department of Innovative Oncology and Gynecology, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov. E-mail: khabas@list.ru. 4 Oparina str., Moscow, 117997, Russian Federation.
Maria D. Farmakovskaya, Ph.D., Researcher at the 1st Department of Gynecology, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov. E-mail: farmakovskaya@gmail.com. 117997, Russia, Moscow, Ac. Oparina str. 4.
Elena A. Sirotkina, Ph.D., Obstetrician Gynecologist at the 2nd Obstetric Physiological Department, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov. E-mail: lenasirotkina@gmail.com.
4 Oparina str., Moscow, 117997, Russian Federation.
Nona G. Mishieva, MD, Leading Researcher at the 1st Department of Gynecology, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov. E-mail: nondoc555@mail.ru. 4 Oparina str., Moscow, 117997, Russian Federation.
Tatyana A. Nazarenko, MD, Professor, Director of the Institute of Reproductive Medicine, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov. E-mail: t.nazarenko@mail.ru. 4 Oparina str., Moscow, 117997, Russian Federation.
Aydar N. Abubakirov, Ph.D., Head of the 1st Department of Gynecology, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov. Тel.: +7(495)438-26-22. Е-mail: nondoc555@yahoo.com.
4 Oparina str., Moscow, 117997, Russian Federation.
For citation: Kirillova A.O., Bunyaeva E.S., Koval’skaya E.V., Kamaletdinov N.S., Khabas G.N., Farmakovskaya M.D, Sirotkina E.A., Mishieva N.G., Nazarenko T.A., Abubakirov A.N. Fertility preservation in patients diagnosed with cancer during pregnancy.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2020; 4: 59-64. (In Russian).
https://dx.doi.org/10.18565/aig.2020.4.59-64



