The role of complex neurophysiological studies in diagnosing pudendal neuropathy in patients with pelvic organ prolapse and pain syndrome
Objective. To assess the role of complex neurophysiological studies in detecting pudendal neuropathy in patients with painful syndrome associated with pelvic organ prolapse.Fomenko О.Yu., Аchkasov S.I., Krasnopolsky V.I., Мartynov М.Yu., Poryadin G.V., Popov А.А., Salmasi Zh.М., Belousova S.V., Аleshin D.V., Kozlov V.А., Fedorov А.А., Nekrasov М.А., Еfremova Е.S.
Materials and methods. The study included 44 patients with pelvic organ prolapse and pelvic pain syndrome. All patients underwent a complex examination including physical examination, colonoscopy, defecography, sphincterometry and complex neurophysiological examination using stimulation electroneuromyography for the investigation of latency of motor nerve conduction (M-response latency), deep pudendal reflex (DPR) and bulbocavernous reflex (BCR).
Results. According to the findings of the neurophysiological study, neuropathy in patients with pain syndrome associated with the pelvic organ prolapse occurred unilaterally or bilaterally in all cases. Neuropathy was diagnosed in 65.9% of patients on the basis of the increased M-response latency (in combination with changed parameters of BCR/DPR), and in 34.1% of cases this condition was identified only due to changed parameters of DPR and/or BCR.
Conclusion. The neurophysiological studies have shown that neuropathy in patients with pain syndrome associated with the pelvic organ prolapse occurred unilaterally or bilaterally in all cases. Neuropathy was diagnosed in 65.9% of patients on the basis of the increased M-response latency (in combination with changed parameters of BCR/DPR), and in 34.1% of cases this condition was identified only due to changed parameters of deep pudendal reflex and/or bulbocavernous reflex.
Keywords
Impairment of pelvic floor muscle innervation can be caused mainly by pudendal neuropathy, as pudendal nerve is the main nerve of the perineum [1]. In describing the problems of pelvic innervation, authors of publications have used a great number of terms: pudendal neuropathy, pudendal neuralgia, pudendal canal syndrome, Alcock’s canal syndrome, pudendal nerve compression syndrome, pudendopathy, interligamental pudendal neuropathy, subpyriform pudendoneuropathy. Pudendal neuropathy can manifest as a pain syndrome in the dermatomes innervated by the pudendal nerve, as well as the loss of sensitivity, impairment of such functions as continence and evacuation of feces and urine, sexual dysfunction.
However, due to the polymorphism of etiopathogenetic factors that cause chronic pelvic pain, the term ‘chronic neurogenic pelvic pain syndrome’ would be more precise for determining neurogenic origin of pain [2, 3]. The neurogenic nature of chronic pelvic pain was first described by J. Boisson et al. [4] and M. E. Neil and H. M. Swash [5]. R. A. Schmidt [6] identifies two main causes of chronic neurogenic pelvic pain, namely myofascial pain syndrome and pudendal nerve compression. Neurogenic pelvic pain syndrome is a diagnosis of exclusion, when all other inflammatory and organic causes of pelvic pain are excluded.
In recent studies on coloproctology, chronic pelvic pain has been considered as a result of functional disorders and topographic anatomic relationship of musculoligamentous structures of the pelvic floor and pelvic organs. Descending perineum syndrome, pelvic floor weakness, spastic pelvic floor syndrome are not just concomitant disorders of defecation, urination, pelvic organ prolapse, but in most cases they are accompanied by chronic pain in the perineal area during straining efforts or after prolonged standing [7].
Each of the possible causes of chronic neurogenic pelvic pain should be considered separately. According to R. E. Baldry [8], in myofascial pain syndrome, various pelvic floor muscles are involved in the pathological process with the formation of trigger points in them [9–15]. Trigger points represent neurofibromatosis areas or knots in the muscle tissue. They form around the ending of the nerve fiber and tense nodules can be palpated. Patients usually determine their location very clearly. A characteristic feature is that the pain is localized not only in the trigger point itself, but also radiates to various areas, namely the perineum, genitals, perianal area, and coccyx [11].
The second cause of chronic neurogenic pelvic pain syndrome is a compression/tension of the pudendal nerve [3, 7, 15–19]. Despite numerous publications on the study of neurogenic causes of pelvic pain, the diagnostic algorithm for identifying neurogenic causes of pelvic pain is not fully defined. A number of authors highlight the importance of studying only M-response latency using stimulation electroneuromyography (ENMG) [20], some researchers suggest studying deep pudendal reflex (DPR) and bulbocavernous reflex (BCR) [21, 22], other scientists consider the use of ENMG studies optional [23].
Therefore, the purpose of our work was to evaluate the role of complex neurophysiological studies in detecting pudendal neuropathy in patients with painful syndrome associated with pelvic organ prolapse.
Materials and Methods
A total of 44 women with pelvic organ prolapse and pain syndrome were examined in Ryzhikh National Medical Research Centre for Coloproctology (Moscow) in 2019. Some of the patients were referred from Moscow Regional Research Institute of Obstetrics and Gynecology. Rectocele as a form of pelvic organ prolapse was identified in 29/44 (65.9%) patients, its distribution was the following: 1st degree – 18/29 (62.1%) patients, 2nd degree – 9/29 (31.0%) patients, 3rd degree – 2/29 (6.9%) patients. The combination of rectocele and internal rectal invagination was detected in 15/44 (34.1%) patients. The average age of patients was 48.8 (12.2) years. All 44 patients complained of the pain in the rectum and anus, the pain was radiating to the coccyx, genitals and perineal skin and was not associated with defecation. Tenderness, numbness, paresthesia in the genital area, inner thigh and perineum were observed in 14/44 (31.8%) women; decreased sensitivity during sexual intercourse and lack of orgasm were revealed in 15/44 (34.1%) patients; dyspareunia was noted in 8/44 (18.2%) cases. In addition to the pain syndrome, 35/44 (79.5%) patients complained of incontinence of gases and liquid stool.
All patients underwent a complex examination including physical examination, colonoscopy, defecography, sphincterometry and complex neurophysiological examination using stimulation ENMG for the investigation of latency of motor nerve conduction (M-response latency), as well as DPR and BCR. All patients consulted a urologist and gynecologist, they were also performed magnetic resonance imaging of the lumbosacral part of the vertebral column in order to exclude gynecological, urological and neurosurgical pathologies.
The intensity of pain syndrome in all patients was assessed using Visual Analog Scale (VAS) [23, 24]. The presence and nature of complaints of intestinal incontinence were evaluated in accordance with the Wexner Incontinence Score [25].
In order to exclude myofascial pain syndromes, appropriate manual tests were performed to determine the levator syndrome, internal obturator muscle syndrome, piriformis syndrome, bulbocavernous syndrome. HowshipRomberg phenomenon and iliopsoas muscle syndrome were excluded. For determining neuropathy, manual testing of the genitofemoral, iliohypogastric, ilioinguinal and sciatic nerves was performed.
Anorectal manometry in the form of complex sphincterometry was performed according to the original method of National Medical Research Centre for Coloproctology (“Method for Functional Assessment of Rectum Closing Apparatus”, patent No. 2576445, dated 05.12.16). The average pressure in the anal canal at rest and the maximum pressure during volitional contraction were recorded. The parameters of the normal indicators for this method are 41–63 and 110–178 mm Hg, respectively [26].
A complex neurophysiological study was performed using Neuro-EMG-Micro System (Neurosoft, Russia) as stimulating ENMG for the pelvic floor muscles using St. Mark’s electrode (“Method for Determining the Neurophysiological State of the Pelvic Floor Muscles”, patent No. 2708052). At first, standard stimulating ENMG was performed for the investigation of latency of motor nerve conduction (M-response latency) with normal indicators of the electrode for intravaginal use 1.54–2.55 ms [21]. Further, electrode being located at the same place, DPR was registered by studying the mixed backreflex response in the single stimulation mode under submaximal, maximal and supramaximal stimuli with the assessment of DPR latency. The DPR normal indicator is 36.18 (4.29) ms (or within 21–41 ms) [22]. Further, using the same St. Mark’s electrode, which was applied in this case as a recording electrode, the BCR was investigated with the registration of the response from the pelvic floor muscles with a latency norm of 34.88 (4.29) ms (or within 25–42 ms) [22].
The statistical package Stata 14.2 (StataCorp) was used for statistical data analysis. Descriptive statistics formats are presented as follows: the format M (SD) is used for quantitative data with a normal distribution, where M is the arithmetic mean and SD is the standard deviation; for quantitative data with a distribution other than normal, the median (Me) and first quartile (Q1) and third quartile (Q3) are presented in the format Me (Q1; Q3).
The normality of trait distribution including residuals in the regression equations was checked on the basis of the Shapiro-Wilk Test (the null hypothesis of a normal distribution). The nonparametric Mann-Whitney U test was used to determine the statistical significance of differences between the study groups.
Correlation analysis (Spearman’s correlation coefficient) was used in the study; the result is presented as a correlation coefficient (r) and the upper and lower bounds of the confidence interval (CI).
Univariate and multivariate linear regressions were used to identify statistically significant predictors of decreased tone using sphincterometry. In the multivariate model, we did not use a trait selection procedure that includes all the factors simultaneously. To estimate the possible multicollinearity, Variance Inflation Factor (VIF) was used, and for estimating the possible outliers in all models, the interquartile range (IQR) was applied.
In this paper, the results were considered statistically significant at a significance level of p<0.05.
Results
Visual Analog Scale (VAS) median score was 5 (1.0; 9.8), the distribution is not normal (p=0.37). When assessing fecal incontinence on the Wexner Incontinence Score, the mean value is 7.5 (5.2) points, the distribution is normal (p<0.001). According to complex sphincterometry, the average indicators of pressure in the anal canal at rest, showing the state of the anal sphincters tone, were reduced to 38.6 (11.4) mm Hg (which were lower by 5.9% from the lower limit of physiological norms). The pressure parameters for volitional contraction that characterize the contractility of the anal sphincters were 109.7 (39.8) mm Hg (almost at the lower limit of the age-related physiological norm).
M-response latency was increased in 29/44 (65.9%) patients. At the same time, a bilateral increase in latency was registered in 12/29 (41.4%) cases, monolateral increase was noted in 17/29 (58.6%) patients; of these, an increased response was registered on the right in 6/17 (35.3%) patients, and on the left in 11/17 (64.7%) women. The distribution of M-response indicators is not normal. Median indicators of latency in all patients were 2.35 (2.10; 2.78) ms on the right and 2.60 (2.03; 2.70) ms on the left.
DPR dysfunction (increased latency, impairment or complete lack of response) was revealed in 37/44 (84.1%) patients. BCR latency was increased in 39/44 (88.6%) cases, with an average of 53.6 (8.0) ms on the right and 55.5 (6.3) ms on the left.
Thus, there were no patients who would have increased only one M-response without changing DPR and BCR; however, 5/29 (17.2%) patients had increased M-response along with the DPR dysfunction, while other 5/29 (17.2%) patients demonstrated increased M-response latency and BCR latency. In 15/44 (34.1%) cases, an increase in latency was registered only for DPR or BCR with normal M-response.
The statistical analysis was conducted using the MannWhitney U test which did not show statistically significant differences in the assessment of pain intensity on the VAS scale among patients (p=0.60) with normal and increased M-response latency. As it was noted above, there were no patients in the sample who did not show abnormal indicators, at least one of them (M-response latency, DPR dysfunction, BCR latency). At the same time, only 1/44 (2.3%) patient had only one of the mentioned signs, 25/44 (56.8%) patients had two signs, and 18/44 (40.9%) patients had all three signs. However, a statistically significant correlation between the pain indicators in points on the VAS scale and the number of signs could not be detected r = 0.01 (-0.296; 0.298), p=0.99.
Thus, we could not find statistically significant predictors of pain in the examined patients in the form of neurophysiological indicators of increased M-response latency and BCR, as well as DPR dysfunction, separately or in combination.
We also developed regression models (estimates were made using a least squares method), in which the obtained neurophysiological indicators were the independent variables acting as predictors, and VAS scores and sphincterometry indicators at rest and during volitional contraction, as well as the degree of rectocele were taken into account as dependent variables. Significant coefficients with independent variables could not be obtained in any of these equations, neither in univariate nor in multivariate models. Thus, for VAS scale, the indicators p for the coefficients were the following (here and further they are presented in the form of a univariate model/multivariate model): for M-response indicator, p=0.70/0.71; for BCR indicator, p=0.39/0.46; for DPR indicator, p=0.22/0.33.
For the pressure parameters at rest, the statistical significance for M-response was p=0.15/0.38, it was p=0.11/0.16 for BCR, and it was p=0.52/0.48 for DPR. For the pressure parameters with volitional contraction, the statistical significance for M-response was p=0.81/0.45, it was p=0.08/0.07 for BCR latency, and p=0.86/0.79 for DPR. In case of different degrees of rectocele, the statistical significance for M-response was p=0.77/0.81, it was p=0.13/0.12 for BCR, and p = 0.70/0.51 for DPR.
Moreover, none of the models showed a statistically significant positive relationship between VAS score and anal sphincter tone parameters and the fact whether deviations from the norm of independent variables were bilateral or monolateral. There was no relationship between pain indicators (VAS score) and anal sphincter tone (Wexner score, pressure at rest and during voluntary contraction) and the number of simultaneously observed deviations, both in the univariate and multivariate models.
Mann-Whitney U tests demonstrate that VAS indicator does not depend on the combination of increased BCR latency and DPR dysfunction or only one of these signs (p=0.78) in the patient, and the same result is observed in patients with a normal M-response (p=0.24).
When conducting statistical analysis, we tried to identify the relationship between neurophysiological indicators and parameters that characterize fecal incontinence. The following evident factor was noted: pressure at rest and pressure in volitional contractions according to complex sphincterometry was significantly higher in patients without complaints of incontinence of fecal contents components than in patients with complaints (35/44 (79.5%)). These differences were statistically significant: p<0.001 for pressure at rest and p<0.001 for pressure in volitional contractions.
When performing regression analysis, we used linear regression to identify the determinants of the assessment of fecal incontinence. Wexner Incontinence Score was used as a dependent variable in the regression equation.
The obtained neurophysiological indicators were independent variables that acted as predictors of the development of incontinence:
- М-response latency: 0 – normal, 1 – changed;
- DPR: 0 – normal, 1 – dysfunction on at least one side;
- BCR latency: 0 – not enlarged, 1 – enlarged on at least one side.
When performing the regression analysis, we considered both univariate and multivariate models. It should be noted that IQR suggests that there are no outliers that affect the result.
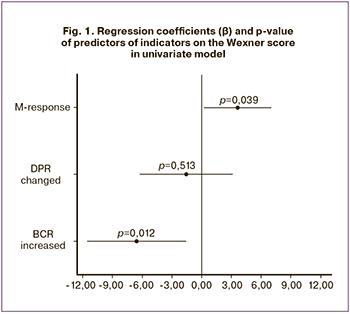 As can be seen from Fig. 1 and Table, the impaired M-response is a positive predictor of an increase in score on the Wexner scale in the univariate model: β-coefficient is 3.64 (p=0.04). The indicator of increased BCR does not have a positive relationship with scores on the Wexner scale (the relationship is negative), and the DPR indicator is not significant. Moreover, as mentioned above, combinations of neurophysiological indicators with each other are common; so, when all of them are included simultaneously in the multivariate model, the significance of the increased M-response latency decreases to p=0.18, and β – to 2.37. In this case, VIF in the model is equal to 1.11 (which indicates the presence of multicollinearity), and the remainder is distributed abnormally (p=0.21); therefore, it is preferable to use the results of a univariate analysis.
As can be seen from Fig. 1 and Table, the impaired M-response is a positive predictor of an increase in score on the Wexner scale in the univariate model: β-coefficient is 3.64 (p=0.04). The indicator of increased BCR does not have a positive relationship with scores on the Wexner scale (the relationship is negative), and the DPR indicator is not significant. Moreover, as mentioned above, combinations of neurophysiological indicators with each other are common; so, when all of them are included simultaneously in the multivariate model, the significance of the increased M-response latency decreases to p=0.18, and β – to 2.37. In this case, VIF in the model is equal to 1.11 (which indicates the presence of multicollinearity), and the remainder is distributed abnormally (p=0.21); therefore, it is preferable to use the results of a univariate analysis.
An interesting pattern has been found. If we consider the indicators only for normal M-responses, the significance of the coefficients for DPR will be p=0.36; if BCR is considered, then in all cases there is a deviation of BCR from the norm for the registered normal M-response.
It should be noted that in the case of the Wexner score indicator, we had statistically significant differences (p=0.02) when using the Mann-Whitney U test for assessment of the patients whose BCR and DPR are impaired at the same time, and patients who have at least one of these neurophysiological indicators changed. And when considering similar differences in patients with normal M-response, statistical significance is observed, but only at a 10% level (p=0.1).
This fact may indicate the need for further obtaining clinical material when using all neurophysiological methods in order to identify possible statistical patterns for correlations between clinical manifestations of fecal incontinence and changes in latency/impairment of BCR and DPR, respectively.
Discussion
When analyzing the obtained results, we drew attention to the presence of 15/44 (34.1%) patients with clinical manifestations of anal sphincter insufficiency and pain syndrome; the patients had normal indicators of M-response latency of the pudendal nerve with changed parameters of DPR and/or BCR latency. In this regard, it is of fundamental importance for us to expand the methods of stimulation ENMG to detect impairments at all links of the reflex arc, including both afferent and efferent links of reflex responses. When registering M-response from the external sphincter muscles or/and pelvic floor muscles, the study of the latent period of excitation by the motor fibers of n. pudendus is informative in our opinion for assessing the state of innervation in the distal part of the nerve.
However, DPR evaluation is not sufficiently comprehensive to assess afferent (from receptors to the posterior horn of the spinal cord) and efferent innervation (from the anterior horn to the muscle effectors), as the stimulation in the case of registration of DPR is not from receptors; antidromic signal activation occurs directly from the sensitive fibers of the pudendal nerve at the point of its irritation at the tubercle of the sciatic spine.
In this regard, the study of BCR, in our opinion, is the most optimal for evaluating the activity of the pelvic floor muscles, since it makes possible to evaluate the neuroreflex reactions that occur when the sensitive areas of the perineum are stimulated.
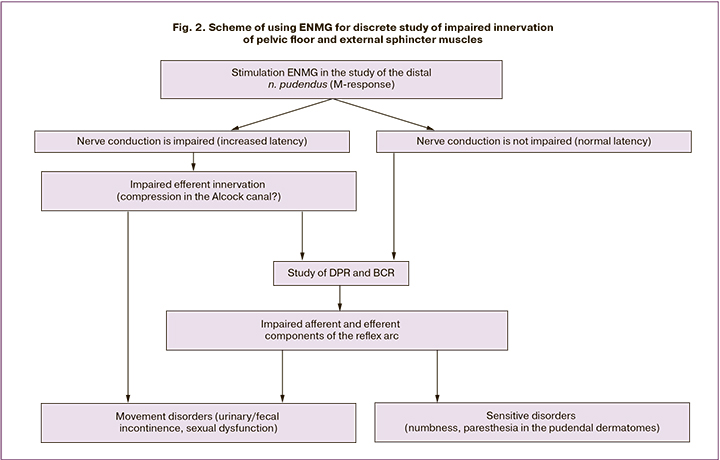
In our opinion, a comprehensive sequential assessment of the stimulation ENMG, namely, M-response, then the mixed reflex response, and then the BCR, allows us to discretely study the impairment of innervation in the distal section of the efferent link in the proximal area of the pudendal nerve, as well as the entire reflex pathway (Fig. 2).
To date, it is difficult to explain the impact of DPR and BCR impairments on the pathogenesis of fecal incontinence in patients with genital prolapse in the presence of pain syndrome. However, this fact requires more extensive use of stimulation neurophysiological techniques and accumulation of material in order to identify certain diagnostic markers of anal sphincter insufficiency and pain syndrome in this category of patients.
Conclusion
According to neurophysiological studies, neuropathy in patients with pain syndrome associated with pelvic organ prolapse occurs monolaterally or bilaterally in all cases.
Neuropathy can be diagnosed in 65.9% of patients on the basis of the increased M-response latency (in combination with changed parameters of BCR/DPR), and in 34.1% of cases this condition can be identified only due to changed parameters of DPR and/or BCR.
3. When evaluating the excitation of motor fibers of the pudendal nerve, increased M-response latency can be considered as a predictor of clinical manifestations of fecal incontinence in patients with pelvic organ prolapse and pain syndrome.
The algorithm of neurophysiological research of patients with pain syndrome associated with pelvic organ prolapse may include the study of M-response latency and the assessment of the mixed back response reflex in the form of DPR, as well as the study of BCR.
References
- Аполихина И.А., Миркин Я.Б., Эйзенах И.А., Малинина О.Ю., Бедретдинова Д.А. Тазовые дисфункции и болевые синдромы в практике уролога. Экспериментальная и клиническая урология. 2012; 2: 84-90. [Apolihina I.A., Mirkin Ya.B., Eyzenah I.A., Malinina O.Yu., Bedretdinova D.A. Perineal Pain and Dysfunction in Urological Practice. Experimental and Clinical Urology. 2012; 2: 84-90. (in Russian)].
- Воробьев Г.И., Древаль О.Н., Шелыгин Ю.А., Благодарный Л.А., Чагава Д.А. Нейрогенные причины хронического тазового болевого синдрома. Колопроктология. 2004; 3: 41-4. [Vorobyov G.I., Dreval O.N., Shelygin Yu.A., Blagodarny L.A., Chagava D.A. Neurogenic causes of chronic pelvic pain syndrome. Coloproctology. 2004; 3(9): 41-4. (in Russian)].
- Antolak S.J., Hough D.M., Pawlina W., Spinner R.J. Anatomical basis of chronic pelvic pain syndrome: the ischial spine and pudendal nerve entrapment. Med. Hypotheses. 2002; 59(3): 349-53. https://dx.doi.org/10.1016/s0306-9877(02)00218-9.
- Boisson J, Debbasch L, Bensaude A. Les algies anorectales essentielles. Arch. Fr. Mai. Appar. Dig. 1966; 55: 3-24.
- Neil M.E., Swash M. Chronic perineal pain: An unresolved problem. J. R. Soc. Med. 1982; 75(2): 96-101.
- Shmidt R.A. Technique of pudendal nerve localization for block or stimulation. J. Urol. 1989; 42(6): 1528-31. https://dx.doi.org/10.1016/s0022-5347(17)39150-4.
- Попова И.С., Перов Ю.В., Михайлов И.А. Хроническая тазовая боль в колопроктологии – стратификация терминологических понятий. Современные проблемы науки и образования. 2017; 3: 30-9. [Popova I.S., Perov Yu.V., Mikhailov I.A. Chronic pelvic pain in Coloproctology. Stratification of terminological concepts. Modern problems of science and education. 2017; 3: 30-9. (in Russian)].
- Baldry P.E. Myofascial pain and fibromyalgia syndromes. A clinical guide to diagnosis and management. Edinburgh: Churchill Livingstone; 2001. 401p.
- Sinaki M., Meritt J.L., Stillwell O.K. Tension myalgia of the pelvic floor. Mayo Clin. Proc. 1977; 52(11): 717-22.
- Smith W.T. Levator spasm syndrome. Minn. Med. 1959; 42(8): 1076-9.
- Travell J.G., Simons D.G. Myofascial pain and disfunction. The trigger point manual. Baltimore: Williams&Wilkins; 1983.
- Иваничев Г.А. Клинические болевые мышечные синдромы. Казанский медицинский журнал. 2011; 92(2): 224-8. [Ivanichev G. A. Clinical muscle pain syndromes. Kazan medical journal. 2011; 2 (92): 224-8. (in Russian)].
- Трэвелл Ж.Г., Симонс Д.Г. Миофасциальные боли и дисфункции. т.1. Пер. с англ. 2-е изд. М.: Медицина; 2005. 1192с. [Travell J. G., Simons D. G. Myofascial Pain and Dysfunction. In 2 Volumes. Trans. from English. 2nd edition, revised and enlarged. M.: Medicine. 2005; V.1: 1192 p. (in Russian)].
- Шостак Н.А. Правдюк Н.Г. Миофасциальный болевой синдром: диагностика и лечение. Клиницист. 2010; 1: 55-9. [Shostak N.A., Pravdyuk N.G. Myofascial pain syndrome: diagnosis and treatment. Klinicist. 2010;(1):55–9. (in Russian)].
- Kukreja A.N. Anorectal surgery made easy. JayPee Brothers; 2013: 243-50.
- Шелыгин Ю.А., Благодарный Л.А., ред. Справочник по колопроктологии. М.: Литтерра; 2012. 596p. [Shelygin Yu.A., Blagodarny L. A. Handbook on Coloproctology. M. Litterra, 2012: 596 p. (in Russian)].
- Amarenco G., Kerdraon J. Pudendal nerve terminal sensitive latency: technique and normal values. J. Urol. 1999; 161(1): 103-6.
- Amarenco G., Savatovsky I., Budet C., Perrigot M. Nevralgies perineales et syndrome du canal d’Alcock. Ann. Urol.(Paris). 1989; 23(6): 488-92.
- Antolak S.J., Hough D.M. Ejaculatory pain associated with noninflammatory urogenital pain. Rochester: Mayo Foundation; 2002.
- Hough D.M., Wittenberg K.H., Pawlina W. Chronic perineal pain caused by pudendal nerve entrapment: anatomy and CT guided perineural injection technique. A.J.R. Am. J. Roentgenol. 2003; 181(2): 561-7. https://dx.doi.org/10.2214/ajr.181.2.1810561.
- Olsen A.L., Ross M., Stansfield R.B., Kreiter C. Pelvic floor nerve conduction studies: establishing clinically relevant normative data. Am. J. Obstet. Gynecol. 2003; 189(4): 1114-9. https://dx.doi.org/10.1067/s0002-9378(03)00551-9.
- Contreras Ortiz O., Bertotti A.C., Rodriguez Nuñez J.D. Pudendal reflexes in women with pelvic floor disorders. Zentralbl. Gynak. 1994; 116(10): 561-5.
- Fall M., Baranowski A.P., Elneil S., Engeler D., Hughes J., Messelink E.J., Oberpenning F., de C.Williams A.C. Синдром хронической тазовой боли. Алымов Ю.В. перевод; Коган М.И. ред. Европейская ассоциация урологов; 2011: 75.
- Tan G., Jensen M.P., Thornby J.I., Shanti B.F. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J. Pain. 2004; 5(2): 133-7. https://dx.doi.org/10.1016/j.jpain.2003.12.005.
- Jorge J.M., Wexner S.D. Etiology and management of fecal incontinence. Dis. Colon Rectum. 1993; 36(1): 77-97. https://dx.doi.org/10.1007/BF02050307.
- Шелыгин Ю.А., Фоменко О.Ю., Веселов В.В., Белоусова С.В., Алешин Д.В., Вязьмин Д.О. Нормативные показатели давления в анальном канале при неперфузионной манометрии. Колопроктология. 2015; 3: 4-9. [Shelygin Yu.A., Fomenko O.Yu., Veselov V.V., Belousova S.V., Aleshin D.V., Vyazmin D.O. Normative indicators of pressure in the anal canal in nonperfusion manometry. Coloproctology. 2015; 3(53): 4-9. (in Russian)].
Received 14.04.2020
Accepted 14.05.2020
About the Authors
Oksana Yu. Fomenko, Head of the Laboratory of clinical pathophysiology, Associate Professor, MD, PhD, Ryzhikh National Medical Research Centre for Coloproctology of the Ministry of Health of Russia. Tel.: +7(499)199-06-55. E-mail: info@gnck.ru. ORCID: 0000-0001-9603-6988. 2 Salyama Adilya st., Moscow, 123423, Russia.Sergey I. Achkasov, Head of the Department of oncology and colon surgery, Professor, MD, PhD, Ryzhikh National Medical Research Centre for Coloproctology of the Ministry of Health of Russia. Tel.: +7(499)199-86-22. E-mail: info@gnck.ru. ORCID: 0000-0001-9294-5447. 2 Salyama Adilya st., Moscow, 123423, Russia.
Vladislav I. Krasnopolsky, Academician of the Russian Academy of Sciences, MD, PhD, Professor, President of Moscow Regional Research Institute of Obstetrics and Gynecology. Tel.: +7(495)625-73-32. E-mail: gyn_endoscopy@mail.ru. ORCID: 0000-0001-7041-9024. 22а Pokrovka st., Moscow, 101000, Russia.
Mikhail Yu. Martynov, Corresponding Member of the Russian Academy of Sciences, MD, PhD, Professor of the Department of neurology, neurosurgery and medical genetics of Pirogov Russian National Research Medical University (RNRMU). Tel.: +7(495)434-14-22. E-mail: rsmu@rsmu.ru. ORCID: 0000-0003-2797-7877.
1 Ostrovityanova st., Moscow, 117997, Russia.
Gennady V. Poryadin, Corresponding Member of the Russian Academy of Sciences, MD, PhD, Honorary Head of the Department of pathophysiology and clinical pathophysiology of Pirogov Russian National Research Medical University (RNRMU). Tel.: +7(495)434-14-22. E-mail: rsmu@rsmu.ru. ORCID: 0000-0003-2010-3296.
1 Ostrovityanova st., Moscow, 117997, Russia.
Aleksandr A. Popov, MD, PhD, Professor, Head of the Department of endoscopic surgery of Moscow Regional Research Institute of Obstetrics and Gynecology.
Tel.: +7(495) 625-73-32. E-mail: gyn_endoscopy@mail.ru. ORCID: 0000-0003-3692-2421. 22а Pokrovka st., Moscow, 101000, Russia.
Jean M. Salmasi, MD, PhD, Head of the Department of pathophysiology and clinical pathophysiology of Pirogov Russian National Research Medical University (RNRMU).
Tel.: +7(495)434-14-22. E-mail: rsmu@rsmu.ru. ORCID: 0000-0001-8524-0019. 1 Ostrovityanova st., Moscow, 117997, Russia.
Svetlana V. Belousova, senior researcher of the Laboratory of clinical pathophysiology, MD, PhD, Ryzhikh National Medical Research Centre for Coloproctology of the Ministry of Health of Russia. Tel.: +7(499)199-06-55. E-mail: info@gnck.ru. ORCID: 0000-0003-1475-2599.
2 Salyama Adilya st., Moscow, 123423, Russia.
Denis V. Aleshin, Нead of the operating unit, MD, PhD, Ryzhikh National Medical Research Centre for Coloproctology of the Ministry of Health of Russia,
Tel.: +7(499)642-54-40. E-mail: info@gnck.ru. ORCID: 0000-0001-8863-2229. 2 Salyama Adilya st., Moscow, 123423, Russia.
Vladimir A. Kozlov, Associate Professor, Researcher, PhD in economics, Ryzhikh National Medical Research Centre for Coloproctology of the Ministry of Health of Russia. Tel.: +7(499)199-06-55. E-mail: info@gnck.ru. ORCID: 0000-0003-1788-1484. 2 Salyama Adilya st., Moscow, 123423, Russia.
Anton A. Fedorov, MD, PhD, leading researcher of the Department of endoscopic surgery of Moscow Regional Research Institute of Obstetrics and Gynecology.
Tel.: +7(495)625-73-32. E-mail: gyn_endoscopy@mail.ru. ORCID: 0000-0003-2590-5087. 22а Pokrovka st., Moscow, 101000, Russia.
Maksim A. Nekrasov, MD, junior researcher of the Laboratory of clinical pathophysiology, Ryzhikh National Medical Research Centre for Coloproctology of the Ministry of Health of Russia. Tel.: +7(499)199-06-55. E-mail: info@gnck.ru. ORCID: 0000-0002-5767-0123. 2 Salyama Adilya st., Moscow, 123423, Russia.
Elena S. Efremova, surgeon of the Department of endoscopic surgery of Moscow Regional Research Institute of Obstetrics and Gynecology.
Tel.: +7(495)625-73-32. E-mail: gyn_endoscopy@mail.ru. ORCID: 0000-0002-1438-5701. 22а Pokrovka st., Moscow, 101000, Russia.
For reference: Fomenko О.Yu., Аchkasov S.I., Krasnopolsky V.I., Мartynov М.Yu., Poryadin G.V., Popov А.А., Salmasi Zh.М., Belousova S.V., Аleshin D.V., Kozlov V.А., Fedorov А.А., Nekrasov М.А., Еfremova Е.S. The Role of Complex Neurophysiological Studies in Diagnosing Pudendal Neuropathy in Patients with Pelvic Organ Prolapse and Pain Syndrome.
Akusherstvo i Ginekologiya / Obstetrics and Gynecology. 2020; 6: 72-79 (in Russian)
https://dx.doi.org/10.18565/aig.2020.6.72-79



