Основными причинами ранних репродуктивных потерь до 6 недель гестации являются как генетические, так и иммунологические факторы, а также их сочетание. При спорадическом прерывании беременности на ранних сроках у 60% абортусов диагностируется аномальный кариотип. Известно, что частота спонтанных геномных и хромосомных мутаций у плода коррелирует со сроком остановки развития беременности. Соответственно, чем раньше остановилось развитие зародыша, тем больше вероятность обнаружения у него хромосомной патологии [1]. В исследовании Чиряевой О.Г. и соавт. [2] при остановке развития плода на сроке менее 7 недель число аномальных кариотипов практически в 2 раза превышало число нормальных. Аномалии кариотипа, выявленные в хорионе при неразвивающейся беременности, четко дифференцировались по их значимости для раннего эмбрионального развития. Было доказано, что причинами остановки развития плодов с аномальным кариотипом могут служить как нарушения собственно формирования эмбриона, так и патология процесса плацентации. Неоспоримым фактором риска невынашивания беременности может также явиться носительство родителями сбалансированных хромосомных мутаций [3].
В последнее время пристальное внимание уделяется семейному анамнезу пар с повторными выкидышами. У родственников I, II и III степени родства этих пациентов привычное невынашивание беременности (ПНБ) было в 2–3 раза чаще, чем в популяции [4]. Полногеномный анализ позволил выявить в таких семьях критические локусы, ассоциированные с ПНБ: 3p14.2,6q16.3, 6q27, 9p22.1, 9q33.1, 11q13.4, Xp22.11 [5, 6]. Таким образом, при оценке риска остановки развития беременности важно учитывать наследственную предрасположенность к ПНБ. При этом при повторных потерях плода частота генетически неполноценных эмбрионов значительно ниже [7]. В случае исключения генетических аномалий на первое место среди причин повторных репродуктивных неудач ранних сроков выходят иммунологические нарушения [8]. Иммунологические факторы в 50–80% служат основными причинами ранних репродуктивных потерь при нормальном кариотипе плода, так как доказано, что в процессах оплодотворения, имплантации и плацентации одна из главных ролей отведена иммунной системе [9].
В 2000 г. исследователи в области репродуктивной иммунологии Grene E. и соавт. впервые выделили 5 категорий, ассоциированных с невынашиванием беременности, неудачами экстракорпорального оплодотворения (ЭКО) и бесплодием неясного генеза: совместимость супругов по антигенам системы главного комплекса гистосовместимости (Human Leukocyte Antygen — HLA); антифосфолипидный синдром, наличие антинуклеарных, антигистоновых антител, наличие антиспермальных антител, тяжелая иммунологическая патология с нарушением процесса имплантации и высокое содержание клеток CD19+CD5+ [10].
Спустя 20 лет данная классификация постоянно обновляется. Так, в некоторых лабораториях предлагается для пациентов с ПНБ большая иммунологическая панель, в которую включены более 100 параметров исследования, таких как гены KIR-рецепторов, определение гаплогруппы HLA (как пациента и партнера, так и донора спермы), исследование внутриклеточного содержания цитокинов (полноценное исследование процентного соотношения каждого типа в цитокиновой панели – CD4+ T-клетки, CD8+ T-клетки, NKT-клетки, NK-клетки, фактор некроза опухоли (TNF-α), интерферон (IFN)-γ, интерлейкин (IL)-17, IL-4, IL-10), исследование соотношения цитотоксичных NK-клеток, анти-HLA антител, TNF-α, IL-6, IL-8, IL-17, исследование аутоантител (антинуклеарные антитела, антифосфолипидные антитела, антитела к тканям щитовидной железы, антитела к тиреопероксидазе, антитела к тиреоглобулину, антитела к рецепторам тиреотропного гормона (ТТГ), ревматоидный фактор, антицитруллиновые антитела) и другие (активность C3- и C4-компонентов системы комплемента, общее содержание IgM, IgA, IgG и IgE, уровень витамина D, уровень гомоцистеина, полиморфизм метилентетрагидрофолатредуктазы (MTHFR) (C677T и A1298C), ТТГ). Одной из основных иммунологических панелей является панель, исследующая неклассические молекулы HLA I класса (G, E, C), а также антитела к HLA и их гаплотипы.
Основополагающей всего иммунитета является система HLA — высокополиморфный локус, содержащий гены, продукты которых осуществляют контроль взаимодействия всех иммунокомпетентных клеток организма, распознавание своих и чужеродных (в том числе измененных собственных) клеток, запуск и реализацию иммунного ответа [11, 12]. Гены, кодирующие эти белковые продукты экспрессии, расположены на коротком плече хромосомы 6 и могут быть разделены на HLA класса I (a и b) и класса II. Антигены HLA класса I необходимы для распознавания трансформированных клеток цитотоксическими Т-лимфоцитами. Важнейшая функция антигенов HLA класса II – обеспечение взаимодействия между Т-лимфоцитами и макрофагами в процессе иммунного ответа. Они обладают самым высоким полиморфизмом, который определяет антигенную индивидуальность каждого человека. Это свойство антигенов HLA, а также способность каждой антиген-презентирующей клетки (АПК) экспрессировать несколько разных молекул главного комплекса гистосовместимости обеспечивают возможность презентации T-клеткам множества самых различных антигенных пептидов [13, 14].
Наличие отцовских антигенов у плода позволяет рассматривать его как аллотрансплантат по отношению к материнскому организму. Какими бы ни были иммунологические причины ранних репродуктивных потерь, сам процесс иммунологического отторжения эмбриона на уровне патофизиологии стандартен – реакция отторжения эмбриона-«полуаллогенного трансплантата» сочетает некоторые черты цитотоксической и воспалительной форм клеточного иммунного ответа. Она реализуется с участием как CD8+, так и CD4+ Т-лимфоцитов. Первые являются основными эффекторными клетками, ответственными за гибель клеток эмбриона; вторые обеспечивают развитие иммунного воспаления, способствующего его гибели через нарушение трофики и активацию факторов врожденного иммунитета [15].
Афферентное звено иммунного ответа на «объект атаки» состоит из двух параллельных путей, приводящих к активации CD4+ и CD8+ Т-лимфоцитов. Показано, что Т-клетки могут распознавать белковые продукты экспрессии HLA с помощью двух разных механизмов (рис. 1) — прямого и непрямого, опосредованного через презентацию аутологичными АПК. Прямое распознавание HLA-антигенов чаще реализуется при активации CD8+Т-клеток. Формирующиеся эффекторные Т-клетки обоих типов (Thl-клетки и цитототоксические Т-лимфоциты) поступают в циркуляцию и в результате экспрессии на их поверхности хемокиновых рецепторов мигрируют в очаги воспаления, всегда сопутствующего трансплантации, и инициируют реакции, приводящие к отторжению ткани. Наряду с этим в трансплантат мигрируют естественные киллеры, а также воспалительные клетки, прежде всего макрофаги. Цитотоксические клетки обоих типов осуществляют цитолиз по перфориновому и Fas-зависимому механизмам. Дополнительный вклад в отторжение аллотрансплантатов вносит IFN-ɣ, выделяемый цитотоксическими клетками обоих типов.

HLA I класса в патогенезе ранних репродуктивных потерь
В настоящее время активно ведутся работы, исследующие роль экспрессии генов HLA I класса (G, E, C) в патогенезе ранних репродуктивных потерь. В этих работах, проведенных за последние 20 лет, наглядно продемонстрирована корреляционная связь при нарушении имплантации, переносе эмбриона, полученного от донорских ооцитов, влиянии на развитие плаценты в зависимости от уровня экспрессии HLA I класса и белковых продуктов его экспрессии.
Гены HLA I класса демонстрируют высокий полиморфизм, обладают центральными функциями при процессинге и представлении антигена, ингибируют рецептор NK-клеток, что приводит к снижению иммунного ответа на границе мать–плод и обеспечивают иммунную толерантность к плоду со стороны материнского организма, могут индуцировать смещение от провоспалительного Th1 ответа к клеточноопосредованному ответу Th2, оказывая таким образом положительное влияние на процесс имплантации и плацентации, развитие плода, течение и исходы беременности [16].
Иммуноглобулин-подобные рецепторы и натуральные киллеры (KIR2DS1+uNKs), которые стимулируются плодовыми HLA-C2, могут образовывать растворимые продукты, включая гранулоцитарно-макрофагальный колониестимулирующий фактор, который усиливает миграцию первичных трофобластных клеток [17]. Рядом авторов [18] была продемонстрирована связь между совместной экспрессией KIR2DS1 и HLA-C2 и ПНБ на ранних сроках гестации. Также отмечено, что особую роль в успехе наступления беременности оказывает экспрессия гена HLA-E. Экспрессия аллели HLA-Е*01:01/01:01 ассоциирована с повышением риска ПНБ, а наличие аллели HLA-Е*01:03, наоборот, ассоциировано с понижением данного риска [19].
Белковые продукты экспрессии генов HLA-G
Среди всех продуктов экспрессии HLA I класса HLA-G является наиболее ярким представителем среди неклассических белков Ib класса, которые экспрессируются на клетках трофобласта, демонстрируя низкий полиморфизм и ограниченное распределение в тканях. Главные отличия HLA-G – это ограниченное распространение в тканях, низкий уровень полиморфизма, наличие 7 различных изоформ – структур, которые образуются в результате альтернативного сплайсинга, а также способность оказывать супрессивное действие на иммунокомпетентные клетки [20]. HLA-G экспрессируется тканеспецифично клетками цитотрофобласта, клетками плаценты, амниона [21] и в некоторых здоровых тканях взрослого человека, такими, как тимус, роговица глаза, в эпителиальных клетках бронхов и в поджелудочной железе, в мезенхимальных стволовых клетках, моноцитах [22]. Растворимая форма HLA-G также обнаружена в плазме крови, спинномозговой жидкости [23], злокачественном асците, плевральном выпоте и сперме [24].
За последнее время было проведено большое количество исследований, свидетельствующих о том, что HLA-G экспрессируется в опухолевых клетках, а также доказано, что экспрессия данных белковых продуктов ассоциирована с другими патологическими состояниями, такими как вирусные инфекции [25], повторные репродуктивные потери на ранних сроках, аутоиммунные заболевания, последствия трансплантации органов и воспалительные заболевания.
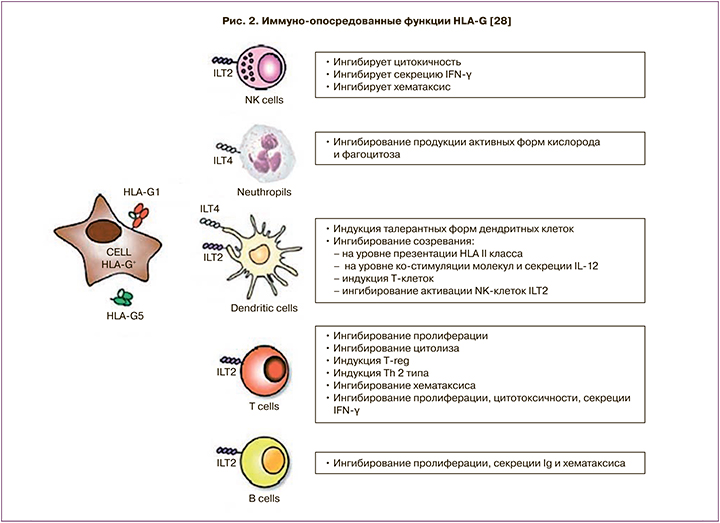
Экспериментальные данные свидетельствуют о способности растворимых белковых продуктов гена HLA-G локально подавлять иммунную систему, однако конкретный молекулярный механизм данного воздействия до сих пор не изучен (рис. 2). Показана способность белка HLA-G модулировать пролиферацию клеток иммунной системы, а также силу и специфичность иммунного ответа. HLA-G отличается от классических HLA генов меньшим количеством полиморфных аллелей; так, например, HLA-B имеет 1431 аллель, HLA-C — 569 аллелей [26]. Международный проект ImMunoGeneTics позволил идентифицировать 51 аллель гена HLA-G [27].
Наиболее распространенные аллели: HLA-G*0101, HLA-G*0102, HLA-G*0103, HLA-G*0104, HLA-G*0105N, HLA-G*0106 и HLA-G*0107. По данным Nardi F.S. и соавт. [29], частота аллеля HLA-G* 01:03:01 была достоверно выше у пар с неудачей при переносе эмбриона по сравнению с фертильными парами. Положительная корреляционная прямая была выявлена у супружеских пар с 14-bp полиморфизмом гена HLA-G ins(rs66554220) и риском неудачи ЭКО[30]; также в трех метаанализах отмечена ассоциация между 14-bp полиморфизмом гена HLA-G ins и ПНБ (особенно у женщин с 3 самопроизвольными выкидышами в анамнезе и более) [31, 32].
Полиморфные изменения гена затрагивают как кодирующие, так и некодирующие области гена. Полиморфизм 725 С/G в промоторной области гена и 3741*ins/del 14 п.н. (пар нуклеотидов) в 3’ нетранслируемой области гена влияют на уровень экспрессии гена и стабильность мРНК; тогда как аллели G*0101, G*0102, G*0103, G*0104, G*0105N, G*0106, G*0107 кодируют продукты, имеющие разный аминокислотный состав. Для некоторых из аллелей выявлена достоверная ассоциация с повышением риска развития патологий, связанных с нарушением иммунного ответа. Можно выделить неблагоприятные генотипы, которые, по-видимому, вносят весомый вклад в развитие мультифакторных заболеваний [33, 17].
Аллель G*0101 рассматривается как один из наиболее древних аллелей данного гена, так как аллель G*0101 обнаружен еще у обезьян шимпанзе [34]; появление других аллелей (G*0102-0107) сводится непосредственно к видовой эволюции человека.
Еще один аллельный вариант в некодирующей части гена HLA — инсерция/делеция 14 п.н. в положении 3741 (rs66554220) достаточно активно изучается. Аллель с инсерцией 14 п.н. ассоциирован с пониженным количеством матричной РНК HLA-G, и более низкими концентрациями растворимых и мембран-ассоциированных форм HLA-G [35]. В настоящее время установлена ассоциация аллеля 3741*ins с развитием осложнений после трансплантации органов и тканей, с болезнью Крона и язвенным колитом, артритом, преэклампсией и ПНБ [26, 36].
Популяционные особенности экспрессии HLA-G
В результате проведенной работы Аленичева А.С. и соавт. [34] установлены статистически значимые различия в частотах аллелей G*0101-0107 между популяцией Северо-Западного региона России в сравнении с рядом других популяций. В результате исследования была разработана система праймеров для мультиплексной аллельспецифической полимеразной цепной реакции и определены особенности распределения аллелей гена HLA-G у жителей Северо-Западного региона России. Сравнительный анализ распределения частот аллелей между популяционной выборкой Северо-Западного региона России и популяциями других стран показал сходство между популяциями Дании, Германии и северо-запада России по аллелям G*0101-G0107 и полиморфизму 3741del14/ins14. Авторами был сделан вывод: вклад аллелей влияет на относительную приспособленность популяции, действие определенного аллеля тонко регулирует иммунную реакцию путем модуляции на уровне связывания с рецепторами и влияния на стабильность матричной РНК. Данное явление свидетельствует о том, что распределение частот аллелей эволюционно подбиралось для оптимальной работы гена HLA-G в популяции.
Регуляция экспрессии HLA-G
Экспрессия HLA-G зависит от сочетания факторов транскрипции, миРНК и факторов окружающей среды.
Регуляция HLA-G количественно и качественно отличается от регуляции других HLA класса I. Использованная модель трансгенных мышей продемонстрировала, что экспрессия HLA-G в клетках цитотрофобласта зависит от фрагмента ДНК длиной 250 п.н., которая расположена в 1,1-kb перед стартовым кодоном трансляции HLA-G. Данная последовательность ДНК отсутствует в классической HLA класса I и может действовать в качестве предполагаемой локус-контролирующей области (locuscontrol region, LCR). Сообщается о транскрипционных факторах ATF1/CREB1/c-jun, которые связывают 5’-регуляторную последовательность гена HLA-G и способны контролировать и регулировать его экспрессию.
Посттранскрипционная регуляция: роль миРНК
Накопленные данные свидетельствуют о том, что в дополнение к факторам транскрипции микроРНК (миРНК) могут выступать в качестве ключевых регуляторов экспрессии HLA. Они представляют собой молекулы, которые способны подавлять экспрессию генов посредством индукции деградации РНК при связывании со специфическими 3’сайтами матричной РНК. Однако их функция должна быть пересмотрена в свете сообщений о том, что в зависимости от клеточного контекста миРНК может переключаться от супрессии в сторону активации или даже выполнять в ядре неизвестные ранее функции.
Ориентировочно в человеческом организме существует более 1000 миРНК [37]. По последним оценкам, в настоящее время до 30% человеческих генов могут регулироваться посредством миРНК, и генетические изменения миРНК, вероятно, могут лежать в основе большего количества заболеваний, чем предполагается.
МиРНК и HLA-G
Существует определенная группа миРНК, состоящая из миРНК-148a, миРНК-148b и миРНК-152, целью которых является 3›-регион HLA-G. Это семейство миРНК способно подавлять экспрессию генов данного главного комплекса гистосовместимости, что приводит к функциональным последствиям. В своей работе Zhu и соавт. продемонстрировали, что 3›-нетранслируемая область матричной РНК HLA-G (3›-UTR), экспрессируемая в клеточных линиях трофобласта человека JEG-3, содержит сайт связывания, который обратно комплементарен миРНК-152 [38]. Manaster и соавт. показали, что в физиологических условиях уровни экспрессии этих миРНК в плаценте являются низкими по сравнению с другими тканями, тогда как уровни матричной РНК HLA-G – высокими, что предполагает возможную обратную связь между этими молекулами.
При наличии патологических процессов в организме отмечается высокий уровень вышеперечисленных миРНК, тогда как уровень HLA-G достоверно снижен. Данное явление наблюдалось в плаценте у беременных с внутрипеченочным холестазом [39] и при преэклампсии. Повышение экспрессии миРНК-152 в плаценте беременных с преэклампсией по сравнению с нормальными плацентами может нарушать способность HLA-G защищать плод от атак со стороны иммунных клеток организма путем снижения экспрессии HLA-G. Другая миРНК – миРНК-133a – регулирует экспрессию HLA-G. Доказано, что эта миРНК снижает экспрессию HLA-G при ПНБ [40]. Напротив, в образцах первичного колоректального рака, в которых уровни HLA-G являются высокими, активность миРНК-133a была значительно снижена. Регуляторные сети состоят из транскрипционных факторов и миРНК. Созданные ими прямые и обратные связи определяют поведение клеток иммунной системы и влияют на иммунитет в целом.
Заключение
Таким образом, генам HLA I класса (G, E, C) отведена ведущая роль в наступлении и успешном пролонгировании беременности, имплантации, плацентации и развитии плода. Белковые продукты экспрессии генов HLA I класса определяют ответ организма на чужеродные ткани, осуществляют контроль взаимодействия всех иммунокомпетентных клеток организма, распознавание своих и чужеродных (в том числе измененных собственных) клеток, запуск и реализацию иммунного ответа: при процессе имплантации, при трансплантации чужеродных органов и тканей, при аутоиммунных заболеваниях, при развитии онкологических заболеваний. В качестве модулятора иммунной системы гены HLA I класса оказывают протективное действие на всех стадиях имплантации, опосредованно влияя на различные звенья иммунной системы, ответственной за взаимоотношения системы мать–плод – двух генетически различных организмов.



