Human papillomavirus genotyping results of human papillomavirus vaccinated women of reproductive age
Objective. To determine the most common types of human papillomavirus (HPV) involved in the neoplastic transformation of the cervical epithelium in HPV vaccinated women of reproductive age. Materials and methods. The study included 3 groups with 141 patients. Group 1 (HPV vaccinated) included 62 patients (average age of 31 years). This group was divided into 2 subgroups: patients vaccinated before sexual activity (n=26, 41.9%) were enrolled in subgroup 1a and women vaccinated after sexual activity (n=36, 58.06%) were in subgroup 1b. Group 2 included patients with the cytological diagnosis of high-grade squamous intraepithelial lesions (HSIL) (n=42), group 3 included patients negative for intraepithelial lesion or malignancy (NILM) (control group, n=37). A comprehensive clinical and laboratory examination was carried out, including HPV typing, cytological examination. Results. Among them, 76 (53.9%) patients were HPV-positive: 100% in the HSIL group, 54% in the NILM group and 22.5% in the vaccinated group (38.8% in subgroup 1b and 0% in subgroup 1a). HPV vaccine types were statistically significantly less identified in group 1 (vaccinated) – 6.4%, compared to group 2 (HSIL) (29.7%) (p<0.05). In group 1 the most common type of HPV was HPV 56 (4.2%), in groups 2 and 3, HPV type 16 was the most common one with 21.9% and 4.96%, respectively. Conclusion. HPV of group 1 (carcinogenic) A9 and A7 were significantly less common in patients of group 1 compared to unvaccinated patients of groups 2 and 3.Gusakov K.I., Nazarova N.M., Frankevich V.E., Starodubtseva N.L., Burmenskaya O.V., Prilepskaya V.N., Sukhikh G.T.
Keywords
Human papillomaviruses (HPV) are a large group of viruses (more than 100 types), some of them have proven carcinogenic activity. If oncogenic HPV types are persistent in the body, they can cause various diseases; the most dangerous of them are anogenital malignancies. As HPV is epitheliotropic, the virus cannot be detected in the blood. HPV affects the epithelium of the genital tract, anal canal, upper respiratory tract, and oral cavity. After its attachment to the cell, penetration into the nucleus, replication, and incorporation of viral DNA into the DNA of the cell, there is an impairment of the mitochondrial pathway of apoptosis, and the process of cell differentiation and adhesion. When certain types of HPV persist in the body, they can cause a wide range of diseases, including precancerous diseases of the anogenital region and cancer of the cervix, vulva, vagina, and anus [1]. According to the International Agency for Research on Cancer (IARC), more than 500,000 new cases of HPV-associated breast cancer are registered annually in the world, its incidence is 13.1 per 100,000 population, and the mortality rate is 6.9 per 100,000 population [2, 3]. According to the data of P. Herzen Moscow Oncology Research Institute (2019) [4], cervical cancer occurs in 2.8% of cases among the total number of cancers (in both sexes), and it takes the 5th place (5.2%) in the structure of oncological pathologies among women after cancer of breast, skin, uterus, and colon; at the same time, malignant neoplasms of the reproductive organs have the largest share (39.1%). The average age of patients with a first-time diagnosis of cervical cancer in Russia (2018) is 52.2 years. The increase in the incidence of cervical cancer from 2008 to 2018 was 24.93%, 22.57 per 100,000 people in 2018 compared to 18.1 per 100,000 people in 2008. Due to the absence of specific treatment for HPV, the only effective method for preventing cervical cancer and other dangerous HPV-associated diseases is the prevention of HPV infection. Nowadays, three HPV vaccines have been registered and are actively used in the world for this purpose: bivalent, four-valent and nine-valent. The maximum effectiveness of HPV vaccination is achieved when children and adolescents are involved in the program before sexual debut. As a rule, children and adolescents aged 9–14 years are the primary target group [5, 6].
 However, the distribution of HPV types involved in the development of neoplastic transformation in women previously vaccinated against HPV has not been studied so far.
However, the distribution of HPV types involved in the development of neoplastic transformation in women previously vaccinated against HPV has not been studied so far.
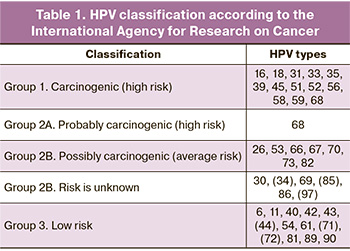
In this paper, we used the IARC classification, which identifies the following HPV groups: carcinogenic (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59), probably carcinogenic (68) and possibly carcinogenic (26, 30, 34, 53, 66, 67, 69, 70, 73, 82, 85, 97) (Table 1) [7–9].
The aim of the study is to determine the most common HPV types involved in the neoplastic transformation of the cervical epithelium in HPV vaccinated women of reproductive age.
Materials and Methods
This study analyzed the results of examination of patients who presented to the outpatient department in the V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow, in 2017–2020. The study included three groups with 141 patients. Group 1 consisted of vaccinated patients who received all three doses of the quadrivalent vaccine (n=62), aged 19 to 45 years, the average age was 31 years. This group was divided into two subgroups: patients vaccinated before sexual activity (n=26, 41.9%) were enrolled in subgroup 1a and women vaccinated after sexual activity (n=36, 58.06%) were in subgroup 1b. Group 2 included patients with the cytological diagnosis of high-grade squamous intraepithelial lesions (HSIL) (n=42), group 3 included patients negative for intraepithelial lesion or malignancy (NILM) (control group, n=37, aged 18 to 45, the average age was 31 years).
There were the following criteria for inclusion in group 1a: reproductive age (18–45 years), regular menstrual cycle, HPV vaccination with a quadrivalent vaccine (Gardasil) administered prior to sexual activity, three doses per one year, and the time from the last dose of the vaccine is at least three years. Patients of group 1b met the following criteria: reproductive age (18–45 years), regular menstrual cycle, HPV vaccination with a quadrivalent vaccine (Gardasil) after the onset of sexual activity, three doses per one year, the time from the last dose of the vaccine is at least three years.
Inclusion criteria for group 2 were reproductive age (18–45 years), regular menstrual cycle, cytological diagnosis of HSIL. Patients in group 3 met the following criteria: reproductive age (18–45 years), regular menstrual cycle, and cytological diagnosis of NILM. The exclusion criteria for all groups were pregnancy, lactation, inflammation in the acute stage, impaired function of the kidneys, liver, lungs in the decompensation stage, and neuropsychiatric diseases. All patients underwent HPV typing and liquid cytology.
Real-time polymerase chain reaction was used for identifying HPV 21, and the assessment of viral load was conducted using logarithms. The classification of detected HPV types was carried out in accordance with the IARC classification. For sampling a material, the following method was used: the material was collected from the transformation zone by rotational movements of the brush (5 turns). After collecting the material, it was placed in a container together with a preservative and delivered to the laboratory for the collection and storage of biomaterial at the V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow.
The material for cytological examination (liquid-based cytology) was collected using a special cervical brush with ecto- and endocervical component Cervex-Brush. The central part of the brush was inserted into the cervical canal, the lateral bristles were in contact with the vaginal part of the cervix. For collecting the material, 5 turns of the brush were made clockwise. After collecting the material, the removable part of the brush was placed in a stabilizing solution. The results of the cytological study were evaluated in accordance with the Bethesda system [10].
Statistical analysis
The initial data processing was performed using the OpenOffice®, STATISTICA software package. Categorical data were described using an absolute number (N) and percentages of the total number of patients in the group (P) as N (P%). To compare the two groups by categorical features, the Pearson chisquare test was used; a p value of <0.05 was considered as statistically significant.
Results and Discussion
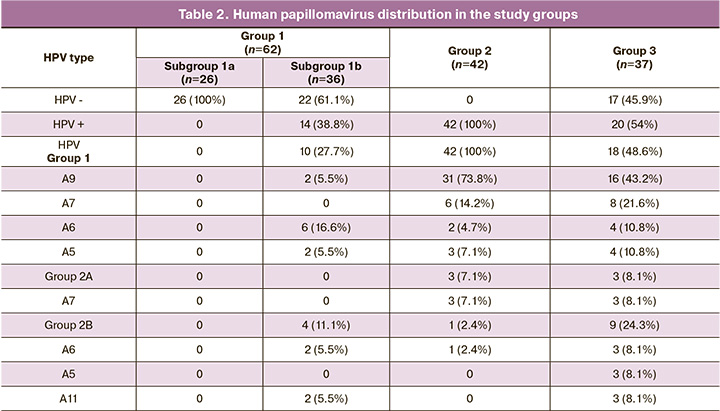
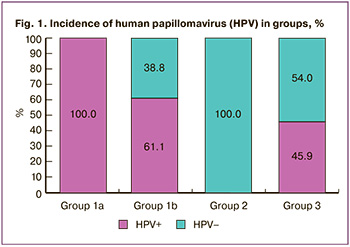 All patients of subgroup 1a were HPV-negative (Fig. 1). According to the cytological study, no cervical pathology was detected in the group of vaccinated women. HPV was detected in 14 patients (38.8%) in subgroup 1b. HPV group 1 («carcinogenic») was detected in eight patients (22.2%): two patients had HPV A9 (HPV 16), six patients had HPV A6 (HPV 56), and two patients had HPV A5 (HPV 51). Four patients (11.1%) were diagnosed with HPV group 2B («possibly carcinogenic»): two patients (5.5%%) had HPV A6 (HPV 53), and two patients had HPV A11 (HPV 73). It should be noted that two patients were diagnosed with vaccine HPV 16 type.
All patients of subgroup 1a were HPV-negative (Fig. 1). According to the cytological study, no cervical pathology was detected in the group of vaccinated women. HPV was detected in 14 patients (38.8%) in subgroup 1b. HPV group 1 («carcinogenic») was detected in eight patients (22.2%): two patients had HPV A9 (HPV 16), six patients had HPV A6 (HPV 56), and two patients had HPV A5 (HPV 51). Four patients (11.1%) were diagnosed with HPV group 2B («possibly carcinogenic»): two patients (5.5%%) had HPV A6 (HPV 53), and two patients had HPV A11 (HPV 73). It should be noted that two patients were diagnosed with vaccine HPV 16 type.
All patients in group 2 (HSIL) were HPV-positive, and HPV group 1 was found in 42 (100%) patients: HPV A9 was detected in 31 (73.8%) patients, HPV A7 was detected in six (14.2%) cases, HPV A6 was detected in two (4.7%) cases, and HPV A5 was detected in three (7.1%) cases. HPV group 2A («probably carcinogenic») was detected in three (7.1%) patients; there was HPV group 2B in one (2.4%) case.
In the NILM group, 20 patients (54%) were HPV-positive. HPV group 1 was determined in 18 (48,6%) patients: HPV A9 was detected in 16 (43.2%) patients (7 patients with HPV 16 (18.9%), 5 patients with HPV 31 (13.5%), 3 patients with HPV 35 (8.1%), 1 patient with HPV 52 (2.7%)); HPV A7 was detected in 8 (21.6%) cases (2 patients with HPV 18 (5.4%), 4 patients with HPV 39 (10.8%), and 2 patients with HPV 45 (5.4%); HPV A6 (HPV 56) was detected in 4 (10.8%) cases, and HPV A5 (HPV 51) was also revealed in 4 (10.8%) cases.
Among 141 patients, 76 (53.9%) women were HPV-positive. In the HSIL group there were 100% of HPV-positive patients, in the NILM group there were 54%, and in the vaccinated group there were 22.5% of patients (38.8% in subgroup of those who were vaccinated after sexual activity and 0% in subgroup of patients who were vaccinated before sexual activity).
The study conducted by M. Paz-Zulueta et al. [11] showed that the incidence of high-risk HPV (in unvaccinated patients) in the general population is 38.49%. The results of the study show that there is no cross-protection.
HPV group 1 («carcinogenic») was detected in 10 cases in the group of vaccinated patients (all cases were in the subgroup of those vaccinated after the initiation of sexual activity), in 42 cases in the HSIL group, and in 18 cases in the NILM group. HPV group 1 was significantly lower in group 1b (22.2%) than in group 2 (100%, p<0.001) and group 3 (48.6%, p=0.027, p<0.05).
The study by E. Jeannot et al. [12] included 409 women aged 18-31 years, 69% of them were vaccinated with a quadrivalent vaccine. HPV types 16 or 18 were detected in 7.2% of unvaccinated patients and in 1.1% of patients who were vaccinated. However, the researchers found no statistically significant data regarding cross-protection against other HPV types. All three doses of the vaccine were not received by 28% of the vaccinated patients. In the study there was no division of patients into those who were vaccinated before and after the initiation of sexual activity. Our results indicate that the vaccine induces neutralizing antibodies against HPV phylogenetic types belonging to groups A9 and A7 in vaccinated women.
HPV group 2A («probably carcinogenic») was not detected in any of the two subgroups of vaccinated patients; these types were found in 3 cases (7.1%) in the HSIL group, and in 3 cases (8.1%) in the NILM group.
HPV group 2B («possibly carcinogenic») was identified in 4 cases in the vaccinated patients (all cases were found in the subgroup of women vaccinated after the initiation of sexual activity); there was one case in the HSIL group (there were no statistically significant differences from the vaccinated group: p=0.17, p>0.05), and there were 9 cases in the NILM group (no statistical correlation was found: p= 0.22, p>0.05).
HPV group A9 was statistically significantly less common in subgroup 1b (5.5%) than in group 2 (HSIL) (73.8%) (p<0.001), and A7 was not found in the vaccinated group.
HPV vaccine types were significantly less common (6.4%) in patients of group 1 than in patients of group 3 (29.7%) (p=0.003, p<0.05).
H. Shilling et al. [13] included 362 patients in the study: 65.6% of them received all three doses of the vaccine, 9.8% received less than three, and 24.7% were not vaccinated. The prevalence of four vaccine types (6, 11, 16, 18) was 6.4%, regardless of vaccination status. The prevalence of vaccine types in the subgroup of those vaccinated after the initiation of sexual activity in our study was 11.1%.
In group 1 (vaccinated patients), the most common HPV type was HPV 56 (9.6%), in groups 2 and 3 it was HPV 16 (69% in group 2 and 18.9% in group 3).
According to the IARC classification, high-risk HPV (phylogenetic groups A9: 16, 18, 31, 33, 35, 52, 58; A7: 39, 45; A5: 51, A6: 56) was detected in 10 cases (27.7%) in the group of vaccinated patients, while in the HSIL group, high-risk HPV was detected in 42 cases (100%) (Table 2). The incidence of high-risk HPV was significantly lower in the group of vaccinated patients (p<0.001).
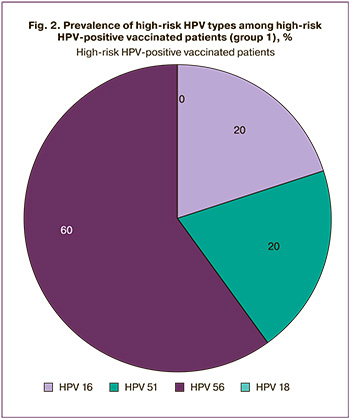 High-risk HPV of non-vaccinated types (non-HPV 16/18) was detected in 8 cases (12.9%) in the group of vaccinated patients. In the group of HSIL, high-risk HPV of non-vaccinated types was detected in 22 cases (52.4%). The incidence of high-risk HPV of non-vaccinated types was significantly less frequent in the vaccinated group than in the HSIL group (p<0.001). Among the vaccinated patients with high-risk HPV, the incidence of non-vaccinated types was 80% (8 out of 10 patients): 60% – HPV 51, 20% – HPV 56 (Fig. 2).
High-risk HPV of non-vaccinated types (non-HPV 16/18) was detected in 8 cases (12.9%) in the group of vaccinated patients. In the group of HSIL, high-risk HPV of non-vaccinated types was detected in 22 cases (52.4%). The incidence of high-risk HPV of non-vaccinated types was significantly less frequent in the vaccinated group than in the HSIL group (p<0.001). Among the vaccinated patients with high-risk HPV, the incidence of non-vaccinated types was 80% (8 out of 10 patients): 60% – HPV 51, 20% – HPV 56 (Fig. 2).
High-risk HPV was significantly less common among vaccinated patients than in the patients of the NILM group (p<0.001).
The obtained data suggest that HPV type 16 which prevails in general population is replaced by other types of high-risk HPV (A6 HPV type 56 (9.6%), A5 HPV type 51 (3.2%)) in vaccinated patients.
To date, HPV genotyping (type 21) has not been performed in vaccinated women. As a rule, vaccine types are evaluated [11, 12]. Moreover, some studies demonstrate that the low effectiveness of HPV vaccines in preventing cervical intraepithelial neoplasia (CIN) 3+ is due to the lack of division into groups of those vaccinated before and after the initiation of sexual activity [14]. Despite the fact that the two-dose vaccination scheme was approved for some countries in 2014 by the strategic advisory group of WHO experts in vaccination of girls under 15 years of age [15], the data on the concentration of antibodies against HPV 16 and 18 after two doses of the vaccine were obtained only for a period of 2 years after the moment of vaccination [16]. In our study, all patients in the group of vaccinated patients received all three doses of the quadrivalent vaccine.
Thus, the results of the study demonstrate effective protection of vaccination against infection and precancerous cervical lesions caused by HPV genotypes that can be and cannot be the part of the vaccine as a cross-protection (phylogenetic group A9 and A7). It should also be noted that HPV group 2B («possibly carcinogenic») was identified mainly in the group of vaccinated patients; therefore, the participation of this type HPV in the formation of the neoplastic process of the cervix should not be excluded.
Conclusion
In the HSIL group there were 100% of HPV-positive patients, in the NILM group there were 54%, and in the vaccinated group there were 22.5% of patients (38.8% in subgroup of those who were vaccinated after sexual activity and 0% in subgroup of patients who were vaccinated before sexual activity). HPV vaccine types were significantly less common (6.4%) in vaccinated patients than in patients of group NILM (29.7%) (p=0.003, p<0.05). HPV group 1 («carcinogenic») A9 and A7 were significantly less common in patients of group 1 (who were vaccinated) compared to unvaccinated patients of groups 2 and 3. The most common HPV type in vaccinated patients was HPV 56 (9.6%), in groups 2 and 3 it was HPV 16 (69% in group 2 and 18.9% in group 3).
References
- Сычева Е.Г., Назарова Н.М., Бурменская О.В., Прилепская В.Н., Трофимов Д.Ю., Сухих Г.Т. Персистенция ВПЧ высокого онкогенного риска и другие молекулярно-генетические предикторы развития цервикальных интраэпителиальных неоплазий. Акушерство и гинекология. 2018; 12: 104-10. [Sycheva E.G., Nazarova N.M., Burmenskaya O.V., Prilepskaya V.N., Trofimov D.Yu., Sukhikh G.T. High-risk human papillomavirus persistence and other molecular genetic predictors for cervical intraepithelial neoplasias. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2018; 12: 104-10.
- WHO. Global Cancer Observatory. Lyon: International Agency for Research on Cancer; March 2019.
- Bruni L., Albero G., Serrano B., Mena M., Gómez D., Muñoz J., Bosch F.X., de Sanjosé S. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human papillomavirus and related diseases in Russian Federation. Summary Report 17. June 2019.
- Каприн А.Д., Старинский В.В., Петрова Г.В., ред. Злокачественные новообразования в России в 2018 году (заболеваемость и смертность). М.: МНИОИ им. П.А. Герцена – филиал ФГБУ "НМИРЦ" Минздрава России. 2019. Available at: http://www.oncology.ru/service/statistics/malignant_tumors/2018.pdf [Kaprin A.D., Starinsky V.V., Petrova G.V., ed. Malignant neoplasms in Russia in 2018 (Morbidity and mortality). 2019. Electronic resource. (in Russian)].
- Human Papillomavirus (HPV) Vaccine. Merck Sharp; Dohme. August 2019. Available at: https://www.msdmanuals.com/home/infections/immunization/human-papillomavirus-hpv-vaccine.
- FDA approves expanded use of Gardasil 9 to include individuals 27 through 45 years old. U.S. Food & Drug Administration.5 October 2018.
- ARC Monographs on the Evaluation of Carcinogenic Risks to Humans. IARC Monographs, Volume 100 (B). 2012.
- de Villiers E.M. Cross-roads in the classification of papillomaviruses. Virology. 2013; 445(1-2): 2-10. https://dx.doi.org/10.1016/j.virol.2013.04.023.
- Arbyn M., Tommasino M., Depuydt C., Dillner J. Are 20 human papillomavirus types causing cervical cancer? J. Pathol. 2014; 234(4): 431-5. https://dx.doi.org/10.1002/path.4424.
- Nayar R., Wilbur D. The Bethesda system for reporting cervical cytology. Definitions, criteria, and explanatory notes. January 2015. https://dx.doi.org/10.1007/978-3-319-11074-5.
- Paz-Zulueta M., Álvarez-Paredes L., Rodríguez Díaz J.C., Parás-Bravo P., Andrada Becerra M.E., Rodríguez Ingelmo J.M. et al. Prevalence of high-risk HPV genotypes, categorised by their quadrivalent and nine-valent HPV vaccination coverage, and the genotype association with high-grade lesions. BMC Cancer. 2018; 18(1): 112. https://dx.doi.org/10.1186/s12885-018-4033-2.
- Jeannot E., Viviano M., de Pree C., Amadane M., Kabengele E., Vassilakos P., Petignat P. Prevalence of vaccine type infections in vaccinated and non-vaccinated young women: HPV-IMPACT, a Self-Sampling Study. Int. J. Environ. Res. Public Health. 2018; 15(7): 1447. https://dx.doi.org/10.3390/ijerph15071447.
- Shilling H., Murray G., Brotherton J.M.L., Hawkes D., Saville M., Sivertsen T. et al. Monitoring human papillomavirus prevalence among young Australian women undergoing routine chlamydia screening. Vaccine. 2020; 38(5): 1186-93. https://dx.doi.org/10.1016/j.vaccine.2019.11.019.
- Lehtinen M., Lagheden C., Luostarinen T., Eriksson T., Apter D., Harjula K. et al. Ten-year follow-up of human papillomavirus vaccine efficacy against the most stringent cervical neoplasia end-point-registry-based follow-up of three cohorts from randomized trials. BMJ Open. 2017; 7(8): e015867. https://dx.doi.org/10.1136/bmjopen-2017-015867.
- World Health Organization. Wkly Epidemiol. Rec. 2014; 21.
- D'Addario M., Redmond S., Scott P., Egli-Gany D., Riveros-Balta A.X., Henao Restrepo A.M., Low N. Two-dose schedules for human papillomavirus vaccine: Systematic review and meta-analysis. Vaccine. 2017; 35(22): 2892-901. https://dx.doi.org/10.1016/j.vaccine.2017.03.096.
Received 24.04.2020
Accepted 31.08.2020
About the Authors
Kirill I. Gusakov, MD, pre-PhD, FSBI «V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology» Ministry of Healthcare of the Russian Federation. Tel.: +7(495)438-14-03. E-mail: kigusakov@gmail.com. 117997, Russian Federation, Moscow, Oparin str., 4.Niso M. Nazarova, MD, senior research fellow, FSBI «V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology»
Ministry of Healthcare of the Russian Federation. Tel.: +7(495)438-14-03. E-mail: grab2@yandex.ru. 117997, Russian Federation, Moscow, Oparin str., 4.
Vladimir E. Frankevich, PhD, Head of Department of Systems Biology in Reproduction, FSBI «V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology» Ministry of Healthcare of the Russian Federation. Tel.: +7(495)438-0788 (2198). E-mail: v_frankevich@oparina4.ru.
117997, Russian Federation, Moscow, Oparin str., 4.
Nataliia L. Starodubtseva, PhD, Head of Laboratory of Proteomics of Human Reproduction, FSBI «V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology» Ministry of Healthcare of the Russian Federation. Tel.: +7(916)463-98-67. E-mail: n_starodubtseva@oparina4.ru.
117997, Russian Federation, Moscow, Oparin str., 4.
Olga V. Burmenskaya, PhD, senior researcher, Laboratory of Molecular Genetic Methods, FSBI «V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology» Ministry of Healthcare of the Russian Federation. Tel.: +7(495)438-22-92. E-mail: o_bourmenskaya@oparina4.ru.
117997, Russian Federation, Moscow, Oparin str., 4.
Vera N. Prilepskaya, PhD, Professor, Deputy Director for Science, FSBI «V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology» Ministry of Healthcare of the Russian Federation. Tel.: +7 (495) 4381403. E-mail: vprilepskaya@mail.ru. 117997, Russian Federation, Moscow, Oparin str., 4.
Gennady T. Sukhikh, Academician of the Russian Academy of Sciences, PhD, Professor, Director of the FSBI «V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology» Ministry of Healthcare of the Russian Federation. Tel: +7(495)438-18-00. E-mail: g_sukhikh@oparina4.ru.
117997, Russian Federation, Moscow, Oparin str., 4.
For citation: Gusakov K.I., Nazarova N.M., Frankevich V.E., Starodubtseva N.L., Burmenskaya O.V., Prilepskaya V.N., Sukhikh G.T. Human papillomavirus genotyping results of human papillomavirus vaccinated women of reproductive age.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2020; 9: 114-119 (in Russian)
https://dx.doi.org/10.18565/aig.2020.9.114-119



