Reproductive function of women with rheumatoid arthritis: the effect of the disease and methotrexate therapy on the serum level of anti-Müllerian hormone
Aim. To investigate ovarian reserve using anti-Müllerian hormone (AMH) as a marker of ovarian reserve in patients with rheumatoid arthritis (RA) and infertility, who received or did not receive methotrexate (MTX) therapy.Perminova S.G., Vlasova G.A., Kosheleva N.M.
Materials and methods. The observational study comprised 72 patients with infertility. Of them, patients with RA (n=32) constituted the study group, and patients without RA (n=40) made up the control group. Patients with RA were stratified into subgroups based on whether or not they received MTX. The level of AMH was evaluated concerning RA duration and activity, as well as the age at initiation of MTX therapy, dosage, and treatment duration.
Results. There was a statistically significant decrease in the level of AMH in RA patients compared with patients with infertility only, and MTX therapy was associated with a reduction in ovarian reserve. No statistically significant differences in the levels of AMH were found regarding RA duration and activity, as well as the total dose and duration of MTX therapy.
Conclusions. Patients with RA have lower serum AMH levels. MTX therapy is associated with a more significant decrease in AMH levels. The age at initiation of MTX therapy is negatively correlated with the AMH level.
Keywords
Autoimmune diseases commonly affect young women during their reproductive years, and many of them are concerned about preserving their reproductive potential. Rheumatoid arthritis (RA) is one of the most common autoimmune diseases worldwide. In Russia, according to epidemiological studies, about 800,000 people suffer from RA [1]. As is known, about a third of patients with RA diagnosed before the first pregnancy has reduced fertility [2]. Clowse M. et al. [3] reported that among women with RA and systemic lupus erythematosus (SLE), who were interested in having children, 55% with RA and 64% with SLE had fewer children than originally planned. Decreased fertility in women with rheumatic diseases and, in particular, with RA, was reported in several publications in 2011-2016. [4, 5]. It should be noted that in patients with RA, the first pregnancy occurred at a later age than in healthy women [5]. Both autoimmune disease and drug therapy can have a negative effect on the reproductive system [6]. This adverse effect may be attributed to the following mechanisms:
- Impaired interaction of pro-inflammatory mediators. The impact of high RA disease activity on fertility could be mediated via inflammatory mediators, since many cytokines and growth factors play an essential role in the physiology of reproduction, in particular, in the process of implantation [7]. Still, their role in reducing fertility in patients with systemic inflammatory diseases is not fully understood [8].
- The use of non-steroidal anti-inflammatory drugs (NSAIDs), which may interfere with ovulation, implantation, and placentation through inhibition of prostaglandin synthesis [9]. Selective cyclooxygenase-2 inhibitors are thought to suppress ovulation more than non-selective NSAIDs. However, these findings are based on small sample studies [10].
- The use of prednisone may disrupt the function of the hypothalamic-pituitary-ovarian (HPO) axis. It has been shown that long-term treatment with glucocorticoids in therapeutic doses may inhibit luteinizing hormone (LH) secretion, disrupt ovulation, and also prolong the time for pregnancy to occur [11].
Along with NSAIDs and glucocorticoids, many RA patients receive methotrexate (MTX) as first-line therapy. MTX is a cytotoxic antimetabolite drug classified as an antimetabolite agent. MTX has been shown to have a negative effect on rapidly dividing cells, which include ovarian or endometrial cell populations. Damage to these cells, whether transient or permanent, can interfere with subsequent fertility, especially in women with already compromised reproductive function due to the underlying disease or the use of other drugs, or concomitant gynecological diseases [12]. Several studies have investigated the effect of MTX on reproductive function and ovarian reserve. According to Brouwer J. et al. [13], serum levels of anti-Müllerian hormone (AMH) in 72 women with recent-onset RA were comparable to those of healthy controls. The AMH levels in RA patients, who were prescribed MTX at a mean dosage of 20 mg/week for 6months were similar to those who did not receive MTX [14].
However, according to Henes M. et al. [15], serum AMH levels were significantly lower in patients with rheumatic diseases (RA, Behcet’s disease, and spondyloarthritis) compared with healthy women of comparable age. Another study investigating the ovarian response to controlled ovarian stimulation demonstrated that patients receiving MTX for 18 months had a significantly weaker ovarian response to stimulation than women in the control group [12].
Therefore, a considerable lack of research addressing the effect of RA activity on reproductive function and MTX therapy on the ovarian reserve in female RA patients experiencing reproductive problems became the rationale for conducting this study.
This study aimed to investigate serum levels of AMH as a marker of ovarian reserve in patients with RA and infertility, who received or did not receive MTX therapy.
Materials and methods
The observational study comprised 72 patients with infertility managed at the V.I. Kulakov NMRC for OG&P of Minzdrav of Russia in 2016-2019. Of them, patients with RA (n = 32) constituted the study group, and patients without RA (n = 40) made up the control group. As the study was a pilot study, the sample size was determined by the number of patients with a given diagnosis, identified during the specified period. The selection of patients in the study and control groups was carried out using the matched-pair method. Patients of the study and control groups were comparable in age, duration, and type of infertility.
The age of RA patients (study group) was 36 [33; 39] years, age at the onset of the disease was 29 [24; 34] years, and the median duration of RA was 4.0 [3; 11] years. At the time of the study, 6 (18.8%) patients with RA were in remission; 13 (40.6%), 10 (31.3%), and 3 (9.3%) patients had a low, medium, and high disease activity, respectively. The clinical stage of the disease was estimated as early, advanced, and late in 7 (21.9%), 18 (56.2%), and 7 (21.9%) patients, respectively. Rheumatoid factor (RF) and antibodies to the cyclic citrullinated peptide (CCP) were detected in 22 (68.8%) and 25 (78.1%) patients. In the study group, 19 (59.4%) women received MTX administered either orally [11 (34.4%)] or subcutaneously and intramuscularly [8 (28.1%)] at a dose of 10 to 25 mg/week.
The median MTX dose was 15 [15; 20] mg/week; the duration of MTX therapy on the day of inclusion in the study was 18.7 [1; 15] months.
All patients underwent a comprehensive examination at the V.I. Kulakov NMRC for OG&P; patients of the study group were also observed at the V.A. Nasonova Research Institute of Rheumatology. All study participants signed an informed consent to participate in the study. The criteria for inclusion in the study group were as follows: a verified diagnosis of RA and infertility, age from 18 to 40 years. Non-inclusion criteria were inaccurate diagnosis of RA, age over 40, the use of other (except MTX) cytotoxic drugs, surgical interventions on the ovaries. Criteria for inclusion in the control group were infertility, age from 18 to 40, no somatic comorbidities, or a history of surgical interventions on the ovaries.
AMH concentration was measured using a standard magnetic particle-based chemiluminescence enzyme immunoassay. The normal values of AMH were 1.0– 10.6 ng/ml. AMH levels less than 1 ng/ml indicated women with diminished ovarian reserve.
Statistical analysis was performed using Statistica 10 software (StatSoft Inc., USA). Quantitative variables were tested for normality of distribution. Categorical variables were compared by the Chi-square test (analysis of contingency tables). Continuous variables were compared between groups with the unpaired Student t-test. Variables not showing normal distribution were compared by the Mann-Whitney U test and the Kruskal-Wallis test. Quantitative variables were expressed as the median (Me) and the quartiles Q1 and Q3. Qualitative variables were summarized as counts and percentages. Correlation analysis was conducted by calculating Spearman’s rank correlation coefficients. Odds ratios (OR) and 95% confidence intervals (CI) for the reduction in ovarian reserve were calculated depending on the presence of the underlying disease and MTX intake. Differences between the groups were considered statistically significant at p<0.05.
Results
Analysis of clinical and anamnestic data showed that patients in the study and control groups were comparable in age (Me 36 and 35 years; p = 0.594) and duration of infertility (Me 3 and 4.5 years; p = 0.120). Primary (53.1 and 42.5%) and secondary (46.9 and 42.5%) infertility occurred in similar proportions of patients in the two groups (p = 0.369). There were no significant differences in the proportions of tubal-peritoneal (21.9 and 32.5%, p = 0.317) and male (31.3 and 37.5%, p = 0.579) infertility factors in the two groups. Also, in both groups, there were no differences in the age at menarche (Me 12.9 and 11.4 years, p = 0.692) and the number of spontaneous pregnancies (from 1 to 4) in patients with secondary infertility (p = 0.503) (Table 1).
AMH concentration was statistically significantly lower in the study group (2.1 ng/ml) than in the control group (2.73 ng/ml), p = 0.043). The proportion of patients with diminished ovarian reserve (AMH level less than 1.0 ng/ml) was significantly higher in patients with RA (25 and 5% in the study and control group, respectively, p = 0.015).
There were no significant differences in median AMH levels between RA patients with a disease duration of ≤ 5 years [2.8 (0.7; 3.8)] ng/ml and > 5 years [1.8 (0.9; 2 5)] ng/ml (p = 0.213). Serum levels of RF and CCP did not significantly affect AMH level. In 22 (68.7%) patients with positive RF and 10 (31.3%) RF seronegative patients, the median AMH levels were 2.4 [1.0; 3.7] ng/ml and 1.8 [0.7; 2.6] ng/ml, respectively (p = 0.309). In 25 (78.1%) CCP positive patients and 7 (21.9%) CCP negative patients, median AMH levels were 2.3 [0.9; 3.7] ng/ml and 2.1 [0.7; 2.6] ng/ml, respectively, (p = 0.412).
To clarify the effect of MTX therapy on AMH level, patients of the study group were stratified into subgroups based on whether or not they received MTX. The first subgroup included 19 infertile RA patients who received MTX therapy. The second subgroup consisted of 13 infertile RA patients who did not receive MTX. Both subgroups were comparable in age, duration, and level of disease activity (p > 0.05). A comparative assessment of AMH levels depending on the use of MTX is presented in Table 2.
There were no statistically significant differences in AMH levels between the two subgroups (p1-2 = 0.788). RA patients not receiving MTX and patients in the control group did not differ significantly in the levels of AMH (p2-K = 0.160). RA patients treated with MTX tended to have lower AMH levels than patients in the control group; however, there was no statistically significant difference (p1-K = 0.074), which may be due to a small sample size.
The decrease in the ovarian reserve (AMH level less than 1.0 ng/ml), depending on the presence of RA and MTX therapy was estimated by calculating ORs with 95% CI (Table 3).
A diminished ovarian reserve was more common among patients with RA [8/32 (25%)] than in patients of the control group [2/40 (5%)], OR 3.52, 95% CI [0.96–12.81], p = 0.063. RA patients not receiving MTX therapy were significantly more likely to have a diminished ovarian reserve [5/13 (38%)] than patients in the control group [2/40 (5%)], OR 5.63, 95% CI [1.22-26.05], p = 0.031. Patients with a history of MTX therapy did not demonstrate a higher incidence of a decrease in AMH level compared with patients in the control group. RA patients receiving and not receiving MTX therapy did not differ significantly in the proportions of diminished ovarian reserve ([3/19 (16%)] vs. [5/13 (38%)]), (OR 2.3, 95% CI [0.49-11.27], p = 0.427).
There were no statistically significant correlations between the AMG levels and RA activity, as well as between the dose and duration of MTX therapy (p > 0.05).
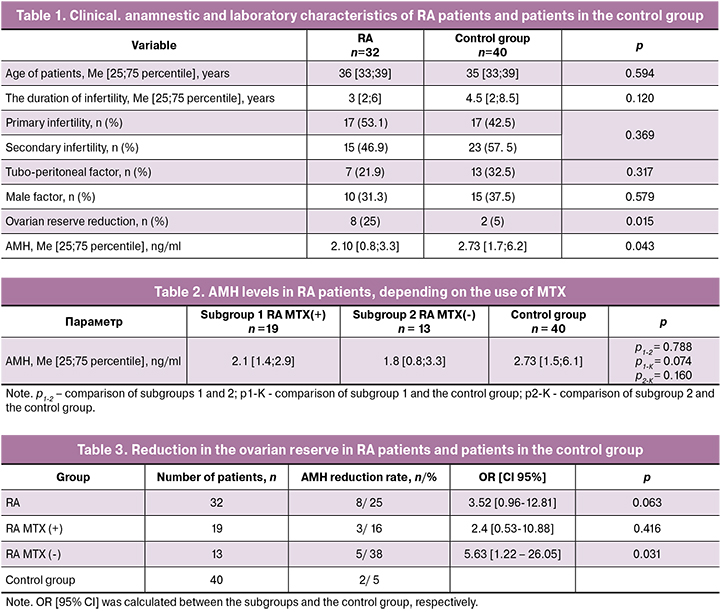
The serum level of AMH is known to be the most objective marker of ovarian reserve, which decreases with age. To identify the effect of MTX on the ovarian reserve, an assessment of the level of AMH in the subgroups, as well as in the whole group of RA patients, was performed. A correlation analysis between AMH levels and the age of RA patients (patients were comparable in the age in all compared groups) showed the most pronounced decrease in AMH in the subgroup of patients taking MTX compared with the subgroup of RA patients not receiving MTX and compared with all RA patients, regardless of the therapy received (rs = -0.563) (p < 0.05) (Fig. 1, a, b, c).
Discussion
A decrease in fertility in patients with RA has been reported in several studies [2–5]; however, a significant effect of a particular factor or group of factors has not been established. This study analyzed the impact of disease activity and MTX therapy on the level of AMH and the ovarian reserve.
AMH is one of the key quantitative markers for reproductive function and ovarian reserve. The serum AMH level is quite stable throughout the entire menstrual cycle and correlates with the residual follicular pool in reproductive-age women [16]. Current literature shows inconsistent data regarding levels of AMH in female RA patients receiving and not receiving MTX therapy [12-14].
The presented study analyzed the impact of RA and MTX therapy on the level of AMH. AMH concentration was significantly lower in patients with RA compared with somatically healthy women (2.1 ng/ml vs. 2.73 ng/ ml, p = 0.043). The duration and level of RA activity, the presence of RF and CCP antibodies did not significantly affect the level of AMH, which is consistent with data from other researchers [14].
Another parameter evaluated in this study was the use of MTX, the first-line treatment for RA. RA patients treated with MTX tended to have lower AMH levels than patients in the control group (2.1 ng/ml vs. 2.73 ng/ml, p = 0.074). However, there was no statistically significant difference, possibly due to the small sample size.
Patients with RA were more likely to have diminished ovarian reserve (8/32, 25%) than patients of the control group (2/40, 5%), OR 3.52, 95% CI [0.96–12.81], p = 0.063. At the same time, the diminished ovarian reserve was significantly more often detected in patients on MTX therapy than in patients who did not receive MTX. These findings lend support for further studies sufficiently powered to explore the impact of MTX therapy on ovarian reserve.
Correlation analysis revealed a more pronounced decrease in AMH in the subgroup of RA patients on MTX therapy, compared with RA patients who did not receive MTX, and compared with RA patients regardless of treatment (rs = -0.563) (p < 0, 05). Thus, the age-related decrease in AMH was more pronounced in patients with a history of MTX therapy. A negative correlation of the AMH levels with age at the initiation of MTX therapy was observed (rs = -0.459) (p < 0.05), which suggests that MTX therapy in later reproductive age is associated with a more pronounced decrease in ovarian reserve. The impact of MTX on the ovarian reserve is likely to be similar to that of cyclophosphamide (CYC). Thus, according to Manger K. et al., among SLE patients receiving CYC, premature ovarian failure was observed in less than 50% of patients younger than 30 and about 60% of patients from 30 to 40 years [17].
Conclusion
The findings of the present study demonstrated that RA and MTX therapy were associated with a decrease in the level of AMH and, accordingly, diminished ovarian reserve. RA patients comprise a large group of patients with autoimmune diseases who often receive cytotoxic therapy, which is also employed to treat cancer. In cancer patients, cytotoxic drugs are used in large dosages that significantly affect the patients’ reproductive function. In autoimmune diseases, the same medications are used in lower doses, which is not always associated with a sharp decrease in reproductive function. However, long-term exposure to cytotoxic drugs, along with the immune-inflammatory process, may also negatively impact the fertility of these patients.
Patients with already compromised reproductive function and diminished ovarian reserve who are due to receive MTX should be advised to consider preserving their reproductive material or realizing their reproductive potential at an earlier age.
References
- Насонов Е.Л., ред. Ревматология. Российские клинические рекомендации. М.: «ГЭОТАР-Медиа», 2017. 456 с. [Nasonov Ye.L., red. Revmatologiya. Rossiyskiye klinicheskiye rekomendatsii. M., «GEOTAR-Media», 2017. 456 s.(In Russian)].
- Jawaheer D., Zhu J.L., Nohr E.A., et al. Time to pregnancy among women with rheumatoid arthritis. Arthritis Rheum. 2011; 63(6): 1517–21. doi: 10.1002/art.30327.
- Clowse M., Chakravarty E., Costenbader K. H., Chambers C., Michaud, K. Effects of infertility, pregnancy loss and patient concerns on family size of women with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Care Res. (Hoboken). 2012; 64(5): 668–74. doi: 10.1002/acr.21593.
- Brouwer J., Hazes J.M., Laven J.S., Dolhain R.J. Fertility in women with rheumatoid arthritis: influence of disease activity and medication. Ann. Rheum. Dis. 2015; 74(10):1836-41. doi: 10.1136/annrheumdis-2014-205383.
- Brouwer J., Fleurbaaij R., Hazes J.M., Dolhain, R.J., Laven J. S. Subfertility in rheumatoid arthritis is often unexplained or caused by anovulation. Arthritis Care Res (Hoboken). 2017; 69(8): 1142–9. doi: 10.1002/acr.23124.
- Andreoli L., Bertsias G.K., Agmon-Levin N., Brown S., Cervera R., Costedoat-Chalumeau N., et al. EULAR recommendations for women’s health and the management of family planning, assisted reproduction, pregnancy and menopause in patients with systemic lupus erythematosus and/or antiphospholipid syndrome. Ann. Rheum. Dis. 2017; 76(3): 76–85. doi:10.1136/annrheumdis-2016-209770.
- Van Sinderen M., Menkhorst E., Winship A., Cuman C., Dimitriadis E.Preimplantation human blastocyst-endometrial interactions: the role of inflammatory mediators. Am J Reprod Immunol 2013; 69(5): 427–40. doi: 10.1111/aji.12038.
- Ince-Askan H., Dolhain R.J. Pregnancy and rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2015; 29(4-5): 580–96. doi: 10.1016/j.berh.2015.07.001.
- Micu M.C., Micu R., Ostensen M. Luteinized unruptured follicle syndrome increased by inactive disease and selective cyclooxygenase 2 inhibitors in women with inflammatory arthropathies. Arthritis Care Res. 2011; 63(9):1334-8. doi: 10.1002/acr.20510.
- Mendonca L.L., Khamashta M.A., Nelson-Piercy C., Hunt B.J., Hughes G.R.Non-steroidal anti- inflammatory drugs as a possible cause for reversible infertility. Rheumatology. 2000; 39(8): 880–2.
- Saketos M., Sharma N., Santoro N.F. Suppression of the hypothalamic-pituitary-ovarian axis in normal women by glucocorticoids. Biol Reprod. 1993; 49(6): 1270–6.
- McLaren J.F., Burney R.O., Milki A.A., Westphal L.M., Dahan M.H., Lathi R.B. Effect of methotrexate exposure on subsequent fertility in women undergoing controlled ovarian stimulation. Fertil Steril. 2009; 92: 515–9. doi: 10.1016/j.fertnstert.2008.07.009
- Brouwer J., Laven, J.S., Hazes J.M., Schipper I., Dolhain R.J. Levels of serum anti-Mullerian hormone, a marker for ovarian reserve, in women with rheumatoid arthritis. Arthritis Care Res. (Hoboken) 2013; 65(9): 1534-8. doi: 10.1002/acr.22013.
- Hill M.J., et al. Ovarian reserve and subsequent assisted reproduction outcomes after methotrexate therapy for ectopic pregnancy or pregnancy of unknown location. Fertil. Steril. 2014; 101(2): 413–9. doi: 10.1016/j.fertnstert.2013.10.027.
- Henes M., et al. Ovarian reserve alterations in premenopausal women with chronic inflammatory rheumatic diseases: impact of rheumatoid arthritis, Behcet’s disease and spondyloarthritis on antiMullerian hormone levels. Rheumatology (Oxford) 2015; 54(9): 1709–12. doi: 10.1093/rheumatology/kev124.
- Sowers M., et al. Anti-Müllerian hormone and inhibin B variability during normal menstrual cycles. Fertil. Steril. 2010; 94(4): 1482–6. doi: 10.1016/j.fertnstert.2009.07.1674.
- Manger K., Wildt L., Kalden J.R., Manger B. Prevention of gonadal toxicity and preservation of gonadal function and fertility in young women with systemic lupus erythematosus treated by cyclophosphamide: the PREGO-Study. Autoimmunity reviews. 2006; 5(4):269–272.
Received 04.02.2020
Accepted 07.02.2020
About the Authors
Svetlana G. Perminova, Associate Professor, Dr.Med.Sci., Leading Researcher at the 1st Department of Gynecology, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.Tel.: +7(916)2021687. E-mail perisvet@list.ru
117997 Russia, Moscow, Ac. Oparina str. 4.
Galina A. Vlasova, Ph.D. Student at the 1st Department of Gynecology, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Tel.: +7(906)0794449. E-mail: galinavlasova089@gmail.com
117997 Russia, Moscow, Ac. Oparina str. 4.
Nadezhda M. Kosheleva, Ph.D., Senior Researcher at the Laboratory of Vascular Rheumatology, V.A. Nasonova Research Institute of Rheumatology.
Tel.: +74951092910. E-mail: nadkosheleva@yandex.ru 115522 Russia, Moscow, Kashirskoye Shosse 34A.
For citation: Perminova S.G., Vlasova G.A., Kosheleva N.M. Reproductive function of women with rheumatoid arthritis: the effect of the disease and methotrexate therapy on the serum level of anti-Müllerian hormone.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2020; 4: 104-110 (In Russian).
https://dx.doi.org/10.18565/aig.2020.4.104-110



