Reproductive function of patients with lymphoproliferative diseases after completion of chemotherapy
Objective: The objective of this study was to investigate the state of ovarian reserve in patients with Hodgkin's lymphoma and non-Hodgkin's lymphoma after completion of polychemotherapy (PCT). The study also aimed to identify factors influencing the recovery or loss of reproductive function and determine the long-term prospects for reproductive function.Biryukova A.M., Antukh I.E., Nazarenko T.A., Khokhlova S.V., Martirosyan Ya.O., Tumyan G.S., Shpirko V.O.
Materials and methods: The study included 247 women with lymphoproliferative diseases, including Hodgkin's lymphoma and non-Hodgkin's lymphoma. The patients were divided into two groups: group I (n=194), consisting of those who returned to their menstrual cycle after treatment completion, and group II (n=53), consisting of those diagnosed with premature ovarian failure. Anamnestic, clinical, and reproductive characteristics of the patients were assessed. The state of the ovarian reserve was compared in terms of AMH, FSH concentrations and antral follicle count in the ovaries before and after PCT. These parameters were compared between the groups of patients who did and did not have their menstrual cycles restored after treatment completion.
Results: Among patients with Hodgkin's lymphoma, the frequency of menstrual cycle recovery and loss of ovarian function was 79.68% and 20.32%, respectively. In patients with non-Hodgkin's lymphoma, the frequencies were 75% and 25%, respectively. Restoration of the menstrual cycle occurred on average 3 months after completion of chemotherapy in both types of lymphoproliferative diseases. One hundred forty-nine (60.32%) women who planned a pregnancy became pregnant, and 46.56% (115) gave birth to healthy children. Predictive factors for the restoration of the menstrual cycle after treatment completion were the woman's age being less than 28.5 years and AMH levels above 2.45 ng/ml. PCT had a negative effect on ovarian function, with the ovarian reserve indicators decreasing twofold compared to the initial values. This decrease predicted premature depletion of ovarian function within 2.5–10 years, depending on the initial parameters of the ovarian reserve.
Conclusion: It is important to determine the baseline ovarian reserve in young women with lymphoma when planning PCT to predict the recovery or loss of reproductive function after treatment completion. For patients with initially reduced ovarian reserve and women receiving high-dose PCT, preliminary collection and cryopreservation of oocytes/embryos are advisable due to the high risk of a sharp decline and loss of ovarian function.
Authors' contributions: Biryukova A.M. – material collection and processing, verification of important content; Antukh I.E. – review of the literature on the topic of the article, concept and design of the study, material collection and processing, text writing, editing; Nazarenko T.A. – concept and design of the study, paper editing, review of impotrant content, approval of the final version of the paper; Khokhlova S.V. – material collection and processing, verification of important content, approval of the final version of the article; Martirosyan Ya.O. – literature review on the topic of the article, text writing, editing, checking important content; Tumyan G.S. – concept and designof the study, approval of the final version of the article; Shpirko V.O. – literature review on the topic of the article, material collection and processing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Biryukova A.M., Antukh I.E., Nazarenko T.A., Khokhlova S.V., Martirosyan Ya.O., Tumyan G.S., Shpirko V.O. Reproductive function of patients
with lymphoproliferative diseases after completion of chemotherapy.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (7): 66-73 (in Russian)
https://dx.doi.org/10.18565/aig.2023.79
Keywords
Oncofertility is an emerging interdisciplinary field in obstetrics and gynecology that focuses on providing fertility preservation options for patients with cancer. Currently, it is receiving significant attention and study from physicians and researchers, particularly for young cancer patients [1–4]. Among various malignancies, lymphoproliferative diseases, including Hodgkin's lymphoma and non-Hodgkin's lymphoma, rank second in terms of incidence following breast cancer [5, 6].
Polychemotherapy (PCT) is the primary treatment for this patient category. Although it offers a high probability of curing the disease, it also carries a substantial risk of causing a rapid decline in reproductive function [7–10]. Experts generally agree that the recovery or loss of reproductive function depends on several factors, such as the patient's age, ovarian reserve status at the time of disease diagnosis, disease stage, gonadotoxicity of treatment, and the patient's reproductive plans following treatment. For instance, researchers have observed that in patients with Hodgkin's lymphoma above the age of 35 years, the level of anti-Müllerian hormone (AMH) only recovers up to 37% of the initial values one year after PCT [11]. In 70% of patients who undergo highly toxic treatment regimens, the AMH level falls below the detectable limit within three years [12, 13]. Furthermore, delayed pregnancy reduces the reproductive potential of women.
Despite the available research evidence, no models have been developed to assess and predict the state of ovarian reserve after treatment completion or to determine the optimal strategy for preserving reproductive function or reproductive material in young women with lymphoproliferative diseases.
This study aimed to assess the ovarian reserve in patients with Hodgkin's lymphoma and non-Hodgkin's lymphoma after completing PCT, identify the factors influencing the restoration/loss of reproductive function, and determine the long-term prospects of reproductive function.
Materials and methods
Of the 247 patients, 187/247 (75.7%) and 60/247 (24.3%) had Hodgkin's lymphoma and non-Hodgkin's lymphoma, respectively. The mean age of the patients was 23.69±6.6 years. The distribution of patients with Hodgkin's lymphoma according to disease stage is shown in Figure 1.
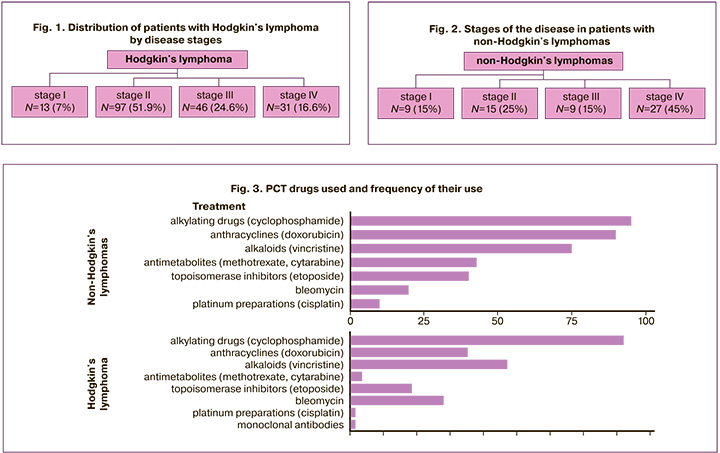
Figure 2 shows the distribution by stages of the disease in patients with non-Hodgkin's lymphomas.
All patients underwent PCT. The drugs used and their frequency of use are shown in Figure 3.
High-dose PCT with high gonadotoxicity and subsequent transplantation of hematopoietic stem cells were performed in 33 patients. Iatrogenic loss of reproductive function was recorded in 100% of the cases.
Anamnestic, clinical and reproductive characteristics of the patients were assessed. The state of the ovarian reserve was compared based on the concentrations of anti-Müllerian hormone (AMH), follicle-stimulating hormone (FSH), and the antral follicle count in the ovaries before and after PCT. A comparative assessment of these parameters was carried out between the groups of patients who restored and did not restore their menstrual cycle after completion of treatment. The presence of ovulation at the restoration of menstruation and pregnancy rates were evaluated in patients who were interested in pregnancy.
Statistical analysis
The GraphPad Prism statistical software package (GraphPad Software, USA) was used for statistical analysis and plotting. The generalized D'Agostino–Pearson test was used to determine the normality of the distribution. Normally distributed data were presented as the mean (standard deviation) and compared using a t-test. Nonparametric data are presented as median (interquartile range) and were compared using the Mann–Whitney U test. Qualitative data are presented as counts (n) and percentages, and were compared using Fisher's exact test. Differences were considered statistically significant at p<0.05.
Results
PCT was performed by oncohematologists according to existing standards; however, among the standard schemes, there is variability in the use of individual drugs. Given this fact, for the purpose of calculation convenience, it was decided to assign conditional “scores” to chemotherapy regimens based on the existing scientific literature data on the degree of gonadotoxicity of each drug [14–16]. Each drug in the chemotherapy regimen was assigned a conditional score. Thus, different chemotherapy regimens were assigned from 0 to 5 points, which made it possible to distinguish three degrees of gonadotoxicity of the drugs used: 0–1 – low, 2–3 – moderate, 4–5 – high. Patients received 3 to 20 courses of PCT with a total conditional toxicity of up to 45 points for the entire period of treatment (mean 14.68 points for non-Hodgkin's lymphomas and 23.65 points for Hodgkin’s lymphoma). Among 194/247 women (78.5%), the menstrual cycle was restored after the end of treatment in 53/247 patients (21.5%) amenorrhea was diagnosed. The average recovery period of the menstrual cycle was three months. Of the 247 women planning pregnancy, 149 (60.32%) became pregnant, and 115 (46.56%) gave birth to healthy children.
A comparative analysis of the baseline characteristics of patients who did and did not restore their menstrual cycle after completion of treatment was carried out. The results are presented in Table 1.
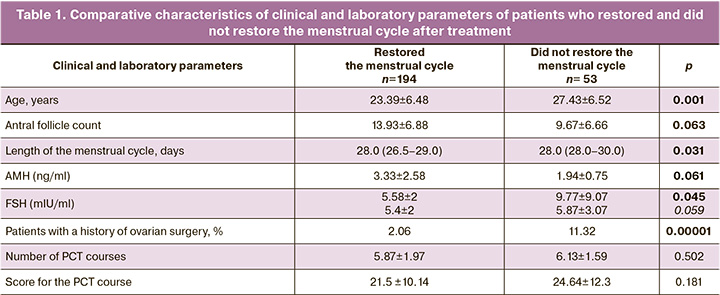
The data obtained showed a significant difference in the baseline ovarian reserve, AMH levels, and antral follicle counts. Patients who did not restore their menstrual cycle were older, although they belonged to the group of women of active reproductive age; they also had higher baseline FSH levels, lower AMH levels, and fewer antral follicles. In contrast, the groups did not differ in terms of the degree of gonadotoxicity induced by PCT.
It has been shown that the main predictors of recovery or loss of reproductive function after completion of treatment are the age of the patient (Fig. 4) and the level of AMH (Fig. 5).
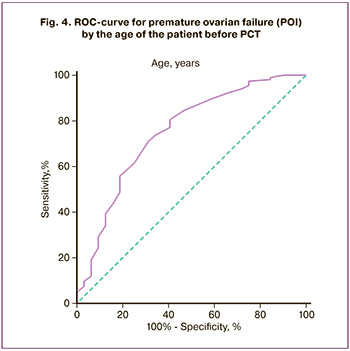
A linear logistic regression model was developed to predict POI development based on patient age and AMH levels. The variables used in this equation are listed in Table 2. The area under the curve for this model was 0.833 (95% CI 0.720–0.946). With a cutoff threshold of the model value less than -1.480, the sensitivity of the model was 65.2%, and the specificity was 93.3% (Fig. 6).
The data obtained made it possible to predict a high probability of loss of reproductive function in women after PCT at an initial age of over 28.5 years and an AMH level below 2.45 ng/l, with a moderate gonadotoxicity of the therapy.
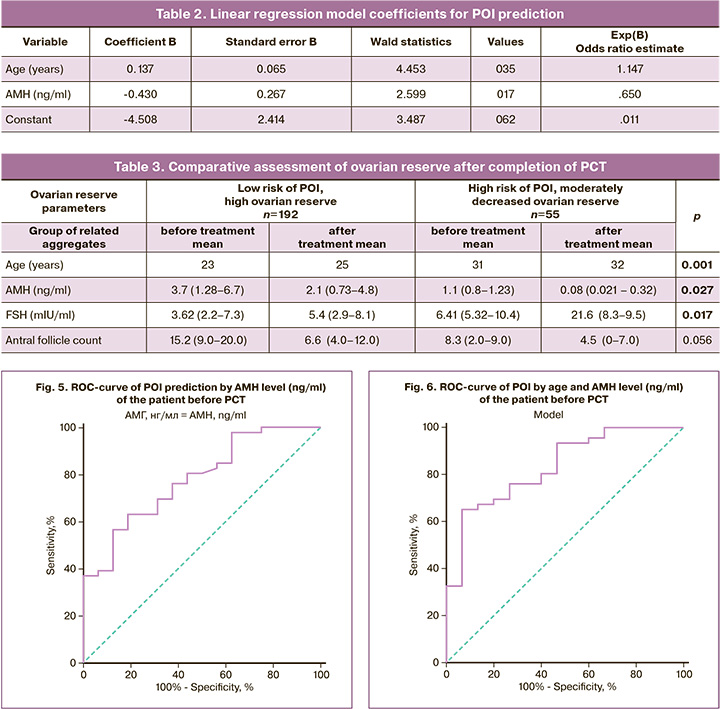
To assess the impact of PCT on the state of the ovarian reserve in patients with a restored menstrual cycle, we compared the main characteristics before and after therapy. The results are presented in Table 3.
The present data showed a decrease in ovarian reserve in patients with both low and high baseline reserves. The AMH values decreased by almost two times, the FSH levels increased, and the number of antral follicles in the ovaries decreased by more than two times, although the patients were of active reproductive age.
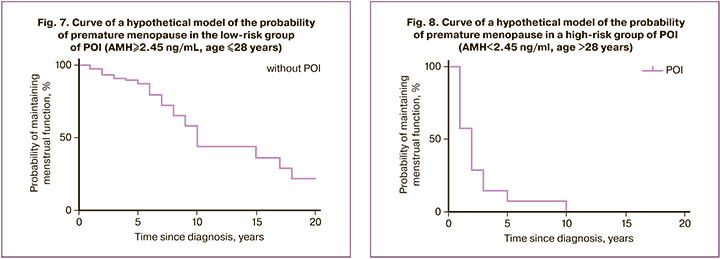
The data obtained made it possible to predict the loss or sharp decrease in reproductive function in patients who underwent chemotherapy for 2.5–10 years. At the same time, with an initially reduced ovarian reserve, this period can correspond to 2.5–5 years, and with an initially high ovarian reserve, 7–10 years (Fig. 7, 8).
Discussion
Specialists are undoubtedly interested in preserving and restoring the reproductive function of young women with cancer. Lymphoproliferative diseases, particularly Hodgkin's lymphoma and non-Hodgkin's lymphomas, are prevalent among oncological diseases. These diseases often manifest at a young age, require chemotherapy, and have a good prognosis for cure [6, 17–19]. Therefore, it is crucial to address the preservation of reproductive function and childbearing in this patient group is crucial [15, 20]. In this study, the group of patients with Hodgkin's lymphoma and non-Hodgkin's lymphoma ranked second in terms of referral, totaling 247 patients. All patients were young, with an average age of 23.69 ± 6.6 years. Of these, 78.5% recovered their menstrual cycle, 149 women who desired pregnancy became pregnant, and 21.5% experienced persistent amenorrhea and premature ovarian failure. The primary objectives of this study were to identify prognostic criteria for the restoration/loss of reproductive function and to determine the impact of chemotherapy on the ovarian reserve of patients. These findings will enable the implementation of effective evidence-based measures to ensure childbearing in this patient group.
Literature data suggest that age and the initial state of the ovarian reserve play a significant role in predicting the restoration of reproductive function in patients undergoing chemotherapy [21, 22]. However, there is a lack of convincing and substantiated recommendations. In our study, we aimed to evaluate the effects of chemotherapy on ovarian function and introduce a scoring assessment to standardize the results regarding the degree of gonadotoxicity of the treatment. According to our data, all patients received chemotherapy with a moderate degree of gonadotoxicity, and the restoration/loss of reproductive function was not dependent on the type and duration of treatment among those who did or did not recover their menstrual cycles. However, the use of high-dose chemotherapy in 33 patients prior to hematopoietic stem cell transplantation resulted in premature ovarian insufficiency. Our data identified age ≤ 28.5 years and AMH level ≥ 2.45 ng/ml before treatment as crucial prognostic factors. We also attempted to assess the ovarian reserve in patients who had a restored menstrual cycle before and after therapy. We observed a nearly 2-fold decrease in AMH levels, an increase in FSH levels, and a reduction of more than 2 times in the number of antral follicles in the ovaries, despite the patients being in the active reproductive age range. These findings demonstrate the negative effect of chemotherapy on the ovarian reserve of patients. Moreover, our mathematical model reliably predicts that patients with initially moderate/reduced ovarian reserve indicators may experience a loss or significant decline in reproductive function after 2.5–5 years, whereas those with initially high ovarian reserve may experience this decline within 7–10 years. The literature also suggests an early onset of menopause in women undergoing chemotherapy [9]. The data obtained from our study can guide the development of management strategies for young women with lymphomas who wish to have children in the future.
Conclusion
It is essential to assess the initial state of the ovarian reserve in young women diagnosed with lymphoma when planning chemotherapy to predict recovery or loss of reproductive function after treatment completion. For patients with an initially reduced ovarian reserve and those receiving high-dose chemotherapy, it is advisable to consider oocyte/embryo cryopreservation before treatment to mitigate the risk of a rapid decline or loss of ovarian function. Chemotherapy leads to a decrease in ovarian reserve among patients who restore their menstrual cycle after treatment. There is a risk of a sharp decline in ovarian function and the development of premature ovarian insufficiency within 2.5–10 years, depending on the initial state of the ovarian reserve. Therefore, the preliminary cryopreservation of reproductive material is recommended for patients who are not currently planning pregnancy.
References
- Каприн А.Д., Старинский В.В., Петрова Г.В., ред. Злокачественные новообразования в России в 2018 году (заболеваемость и смертность). М.: МНИОИ им. П.А. Герцена – филиал ФГБУ «НМИЦ радиологии» Минздрава России; 2019. 250с. [Kaprin A.D., Starinsky V.V., Petrova G.V., eds. Malignant neoplasms in Russia in 2018 (morbidity and mortality). Moscow: P.A. Herzen Moscow State Medical Research Institute, Branch of the Federal State Budgetary Institution "NMIC of Radiology" of the Ministry of Health of Russia; 2019. 250p. (in Russian)].
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J. Clin. 2019; 69(1): 7‐34. https//dx.doi.org/10.3322/caac.21551.
- Ruggeri M., Pagan E., Bagnardi V., Bianco N., Gallerani E., Buser K. et al. Fertility concerns, preservation strategies and quality of life in young women with breast cancer: Baseline results from an ongoing prospective cohort study in selected European Centers. Breast. 2019; 47: 85‐92. https//dx.doi.org/10.1016/j.breast.2019.07.001.
- Kreuser E.D., Hetzel W.D., Billia D.O., Thiel E. Gonadal toxicity following cancer therapy in adults: significance, diagnosis, prevention and treatment. Cancer Treat. Rev. 1990; 17(2-3): 169‐75. https//dx.doi.org/10.1016/0305-7372(90)90043-f.
- Smith K.L., Gracia C., Sokalska A., Moore H. Advances in fertility preservation for young women with cancer. Am. Soc. Clin. Oncol. Educ. Book. 2018; 38: 27‐37. https//dx.doi.org/10.1200/EDBK_208301.
- Kim S.S., Donnez J., Barri P., Pellicer A., Patrizio P., Rosenwaks Z. et al.; ISFP Practice Committee. Recommendations for fertility preservation in patients with lymphoma, leukemia, and breast cancer. J. Assist. Reprod. Genet. 2012; 29(6): 465‐8. https//dx.doi.org/10.1007/s10815-012-9786-y.
- Bedaiwy M.A., Abou-Setta A.M., Desai N., Hurd W., Starks D., El-Nashar S.A. et al. Gonadotropin-releasing hormone analog cotreatment for preservation of ovarian function during gonadotoxic chemotherapy: a systematic review and meta-analysis. Fertil. Steril. 2011; 95(3): 906‐14.e144.https//dx.doi.org/10.1016/j.fertnstert.2010.11.017.
- Martinez F.; International Society for Fertility Preservation–ESHRE–ASRM Expert Working Group. Update on fertility preservation from the Barcelona International Society for Fertility Preservation-ESHRE-ASRM 2015 expert meeting: indications, results and future perspectives. Fertil. Steril. 2017; 108(3): 407‐15.e11. https//dx.doi.org/10.1016/j.fertnstert.2017.05.024.
- Sutcliffe S.B. Cytotoxic chemotherapy and gonadal function in patients with Hodgkin's disease. Facts and thoughts. JAMA. 1979; 242(17): 1898-9.
- Behringer K., Wildt L., Mueller H., Mattle V., Ganitis P., van den Hoonaard B. et al. No protection of the ovarian follicle pool with the use of GnRH-analogues or oral contraceptives in young women treated with escalated BEACOPP for advanced-stage Hodgkin lymphoma. Final results of a phase II trial from the German Hodgkin Study Group. Ann. Oncol. 2010; 21(10): 2052‐60.https//dx.doi.org/10.1093/annonc/mdq066.
- Fréour T., Barrière P., Masson D. Anti-müllerian hormone levels and evolution in women of reproductive age with breast cancer treated with chemotherapy. Eur. J. Cancer. 2017; 74: 1‐8. https//dx.doi.org/10.1016/j.ejca.2016.12.008.
- Behringer K., Thielen I., Mueller H., Goergen H., Eibl A.D., Rosenbrock J. et al. Fertility and gonadal function in female survivors after treatment of early unfavorable Hodgkin lymphoma (HL) within the German Hodgkin Study Group HD14 trial. Ann. Oncol. 2012; 23(7): 1818‐25.https//dx.doi.org/10.1093/annonc/mdr575.
- Tan S.J., Lee L.J., Tzeng C.R., Wang C.W., Hsu M.I., Chen C.H. Targeted anti-apoptosis activity for ovarian protection against chemotherapy-induced ovarian gonadotoxicity. Reprod. Biomed. Online. 2014; 29(5): 612‐20.https//dx.doi.org/10.1016/j.rbmo.2014.07.014.
- Paluch-Shimon S., Cardoso F., Partridge A.H., Abulkhair O., Azim H. Jr, Bianchi-Micheli G. et al. ESO-ESMO 4th International Consensus Guidelines for Breast Cancer in Young Women (BCY4). Ann. Oncol. 2020; 31(6): 674-96.https//dx.doi.org/10.1016/j.annonc.2020.03.284.
- Blumenfeld Z., Avivi I., Linn S., Epelbaum R., Ben-Shahar M., Haim N. Prevention of irreversible chemotherapy-induced ovarian damage in young women with lymphoma by a gonadotrophin-releasing hormone agonist in parallel to chemotherapy. Hum. Reprod. 1996; 11(8): 1620‐6.https//dx.doi.org/10.1093/oxfordjournals.humrep.a019457.
- Ассоциация онкологов России. Клинические рекомендации. Рак молочной железы. 2017. [Association of Oncologists of Russia. Clinical recommendations. Breast cancer. 2017. (in Russian)].
- Peccatori F.A., Azim H.A. Jr, Orecchia R., Hoekstra H.J., Pavlidis N., Kesic V. et al. Cancer, pregnancy and fertility: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013; 24(Suppl. 6): vi160‐70. https//dx.doi.org/10.1093/annonc/mdt199.
- Blumenfeld Z., Dann E. GnRH agonist for the prevention of chemotherapy-induced ovarian failure in lymphoma. J. Clin. Oncol. 2013; 31(29): 3721.https//dx.doi.org/10.1200/JCO.2012.47.8222.
- Demeestere I., Brice P., Peccatori F.A., Kentos A., Dupuis J., Zachee P. et al. No evidence for the benefit of gonadotropin-releasing hormone agonist in preserving ovarian function and fertility in lymphoma survivors treated with ghemotherapy: final long-term report of a prospective randomized trial. J. Clin. Oncol. 2016; 34(22): 2568‐74. https//dx.doi.org/10.1200/JCO.2015.65.8864.
- von Wolff M., Germeyer A., Liebenthron J., Korell M., Nawroth F. Practical recommendations for fertility preservation in women by the FertiPROTEKT network. Part II: fertility preservation techniques. Arch. Gynecol. Obstet. 2018; 297(1): 257‐67. https//dx.doi.org/10.1007/s00404-017-4595-2.
- Behringer K., Breuer K., Reineke T., May M., Nogova L., Klimm B. et al. Secondary amenorrhea after Hodgkin's lymphoma is influenced by age at treatment, stage of disease, chemotherapy regimen, and the use of oral contraceptives during therapy: a report from the German Hodgkin's Lymphoma Study Group. J. Clin. Oncol. 2005; 23(30): 7555‐64. https//dx.doi.org/10.1200/JCO.2005.08.138.
- Bildik G., Akin N., Senbabaoglu F., Sahin G.N., Karahuseyinoglu S., Ince U. et al. GnRH agonist leuprolide acetate does not confer any protection against ovarian damage induced by chemotherapy and radiation in vitro. Hum. Reprod. 2015; 30(12): 2912‐25. https//dx.doi.org/10.1093/humrep/dev257.
Received 28.03.2023
Accepted 04.05.2023
About the Authors
Almina M. Biryukova, PhD, deputy chief accountant for clinical work at the Scientific Clinical Center of ART named after Frederik Paulsen, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, a_birukova@oparina4.ru, 4 Akademika Oparina str., Moscow, Russia, 117997.Irina E. Antukh, postgraduate student at the Scientific Clinical Center of ART named after Frederik Paulsen, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(916)089-08-09, nika06@inbox.ru, 4 Akademika Oparina str., Moscow, Russia, 117997.
Tatiana A. Nazarenko, Dr. Med. Sci., Рrofessor, Head of the Department at the Scientific Clinical Center of ART named after Frederik Paulsen, Director of the Institute of Reproductive Medicine, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, t_nazarenko@oparina4.ru, 4 Akademika Oparina str., Moscow, Russia, 117997.
Svetlana V. Khokhlova, Dr. Med. Sci., Head of the Department of Anticancer Drug Therapy, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, s_hohlova@oparina4.ru, 4 Akademika Oparina str., Moscow, Russia, 117997.
Yana O. Martirosyan, PhD, Researcher at the Scientific Clinical Center of ART named after Frederik Paulsen, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(925)124-99-99, ya_martirosyan@oparina4.ru, 4 Akademika Oparina str., Moscow, Russia, 117997.
Gayane S. Tumyan, Dr. Med. Sci., Head of the Chemotherapy Department for Hematologic Malignancies, N.N. Blokhin National Medical Research Center of Oncology, Ministry of Health of Russia, 24 Kashirskoye Shosse, Moscow, Russia, 115522.
Valeria O. Shpirko, oncologist at the Chemotherapy Department for Hematologic Malignancies, N.N. Blokhin National Medical Research Center of Oncology,
Ministry of Health of Russia, 24 Kashirskoye Shosse, Moscow, Russia, 115522.



