Пик заболеваемости раком эндометрия (РЭ) приходится на возраст старше 50 лет, однако в настоящее время существует тенденция к омоложению больных РЭ [1]. Возможности органосохраняющего лечения начального РЭ находятся в центре внимания ведущих отечественных и зарубежных онкологов (рис. 1), что обусловлено увеличением количества заболевших молодых женщин, а также тем, что в силу различных социально-экономических причин значительная часть женщин откладывают рождение даже первого ребенка на возрастной период старше 35 лет [1–3] (.
 Наиболее распространенными факторами риска развития РЭ у молодых женщин являются увеличение индекса массы тела (ИМТ), нерегулярные менструальные циклы. Риск развития РЭ увеличивается в 22 раза у женщин моложе 45 лет с ИМТ выше 35. Также молодые женщины могут иметь генетическую предрасположенность к развитию заболевания. Женщины с синдромом Линча или наследственным неполипозным раком толстой кишки (HNPCC) подвергаются повышенному риску развития РЭ, толстой кишки и яичников. Некоторые авторы предполагают, что риск для этих женщин составляет до 34% [3].
Наиболее распространенными факторами риска развития РЭ у молодых женщин являются увеличение индекса массы тела (ИМТ), нерегулярные менструальные циклы. Риск развития РЭ увеличивается в 22 раза у женщин моложе 45 лет с ИМТ выше 35. Также молодые женщины могут иметь генетическую предрасположенность к развитию заболевания. Женщины с синдромом Линча или наследственным неполипозным раком толстой кишки (HNPCC) подвергаются повышенному риску развития РЭ, толстой кишки и яичников. Некоторые авторы предполагают, что риск для этих женщин составляет до 34% [3].
Учитывая ведущую роль в возникновении данного заболевания избыточного влияния на ткань эндометрия эстрогенов и дефицита прогестерона, гормональная терапия начального РЭ заключается в назначении высоких доз гестагенов, воздействие которых приводит к атрофическим изменениям в эндометрии и ремиссии заболевания, на фоне чего возможно проведение терапии, направленной на восстановление репродуктивной функции [4, 5].
История самостоятельной гормональной терапии РЭ берет начало в 50-х гг. прошлого столетия, когда начали использовать гестагены для лечения патологии эндометрия. Первым отечественным онкогинекологом, успешно применившим гестагены для лечения высокодифференцированной аденокарциномы эндометрия, является Ян Владимирович Бохман, который в 1985 г. успешно вылечил пациентку от данной патологии, и впоследствии пациентка смогла забеременеть.
Ранее в терапии начальных форм РЭ чаще всего применялся 12,5% масляный раствор 17 оксипрогестерон-капроната, медроксипрогестерон ацетат в высоких дозировках или мегестрол 160 мг/сут до достижения полной атрофии эндометрия и под контролем морфологического исследования состояния эндометрия каждые 3 месяца. Эффективность такой терапии достигала 80%, однако она достаточно тяжело переносится женщинами, страдающими от побочных метаболических эффектов данной терапии. С 90-х гг. прошлого века начали использовать агонисты гонадотропин-рилизинг-гормона (аГнРГ) в сочетании с прогестинами. Дальнейшее совершенствование методик консервативного лечения РЭ привело к разработке комбинированного метода лечения начального РЭ с применением гестагенов в виде левоноргестрелсодержащей внутриматочной гормональной системы (ВМС) и ингибирующего воздействия аГнРГ на секрецию гонадотропных гормонов гипофиза.
Установка ВМС позволяет контролировать состояние эндометрия путем проведения пайпель-биопсии без извлечения ВМС. Что позволяет осуществлять длительную терапию гестагенами у пациенток, не планирующих беременность в настоящее время. По данным отечественных авторов, эффективность такой терапии при РЭ составляет более 80% [5]. Несмотря на длительную историю консервативного лечения, до настоящего времени в мире нет четких рекомендаций о дозах, продолжительности, пути введения и последующем лечении прогестинами.
Проспективные исследования показали, что левоноргестрел-высвобождающая ВМС связана с большей регрессией гистологии, более низкими показателями рецидивов и более низкими показателями гистерэктомии [6] .
Метаанализ данных о качестве жизни показал превосходство ВМС, высвобождающих левоноргестрел, по сравнению с пероральными прогестеронами, с уменьшением прибавки в весе, нарушений сна, головных болей и расстройств настроения. Рецидив может возникать в 50% случаев [6], но второй цикл лечения прогестероном был связан с хорошим уровнем ответа у 89% пациентов [7]. Своевременное обращение к репродуктивному эндокринологу имеет решающее значение для минимизации времени до зачатия, поскольку у этих пациенток, как правило, есть другие факторы, которые могут способствовать бесплодию, например, ожирение, синдром поликистозных яичников (СПКЯ) и возраст. Кроме того, появляются доказательства использования метформина у больных РЭ. В ретроспективном исследовании с участием 985 пациентов изучали больных диабетом и РЭ и разделили их на две группы в зависимости от того, принимали ли они метформин или нет [8]. Те, кто принимал метформин, имели большую общую выживаемость после поправки на возраст, клиническую стадию, степень, химиотерапию, лучевую терапию и наличие гиперлипидемии. После завершения репродуктивной функции рекомендуется проведение гистерэктомии.
Недавно был проведен метаанализ 28 исследований, в которых участвовали 1038 женщин с диагнозом РЭ на ранней стадии, или комплексная атипичная гиперплазия [9]. Результаты показали, что консервативное лечение прогестинами имело суммарный показатель полного ответа (CR) 71%. Обобщенные результаты показали, что 34% женщин забеременели, однако только 20% из них родили живых новорожденных. Общий показатель CR для женщин, использующих ВМС, составил 76%, а общий показатель рецидивов составил 9%. У женщин, применявших левоноргестрелсодержащую ВМС, беременности отмечены в 18%, а 14% родили живых новорожденных. У пациенток, использующих прогестины + ВМС, суммарный показатель CR составил 87%; среди этих пациенток 40% забеременели, а 35% родили живых новорожденных [9].
Приведенные данные свидетельствуют о необходимости четкой формулировки показаний к консервативной терапии РЭ и настроя пациентки на лечение, так как при применении хирургической тактики вероятность прогрессирования при 1 стадии РЭ не превышает 5% [10].
Важным аспектом лечения является восстановление регулярного менструального цикла после окончания гормональной терапии ввиду продолжающихся ановуляторных циклов, что вновь может приводить к гиперэстрогении и возникновению рецидива заболевания [4, 11].
Клиническое наблюдение
Пациентка Т., 33 лет, впервые обратилась в клинику с жалобами на обильные менструации. Проведены гистероскопия, раздельное диагностическое выскабливание эндометрия и эндоцервикса. Получено гистологическое заключение: в полости матки высокодифференцированная эндометриоидная аденокарцинома (рис. 2, 3). Учитывая настоятельное желание пациентки сохранить фертильность, отсутствие беременностей и родов в анамнезе, репродуктивный возраст, начато лечение медроксипрогестерона ацетатом в течение 6 месяцев (500 мг в день 3 месяца, далее 500 мг через день 3 месяца) с контрольным диагностическим выскабливанием через 6 месяцев и ежемесячным ультразвуковым контролем на фоне лечения. Далее пациентка принимала комбинированные оральные контрацептивы (КОК) в течение 1 года, контрольное ультразвуковое исследование (УЗИ) органов малого таза и биопсия эндометрия проводились каждые 3 месяца.

На этом фоне через год и 6 месяцев после лечения диагностирован рецидив заболевания. Пациентке проведено комплексное обследование, включавшее раздельное выскабливание, гистероскопию, УЗИ с цветовым допплеровским картированием, магнитно-резонансная томография (МРТ) органов малого таза с внутривенным контрастированием. Гистологическое заключение: высокодифференцированная эндометриоидная аденокарцинома. Иммуногистохимическое исследование: реакция с антителами к рецепторам эстрогена положительна в 90% ядер опухолевых клеток, интенсивное окрашивание, реакция с антителами к рецепторам прогестерона положительна в 97%. Начат курс консервативной гормональной терапии: медроксипрогестерона ацетат+аГнРГ в течение 6 месяцев. После достижения атрофии эндометрия, подтвержденной данными контрольного исследования (УЗИ, гистероскопия, гистологическое заключение), пациентка принимала КОК и в течение некоторого времени к врачу не обращалась.
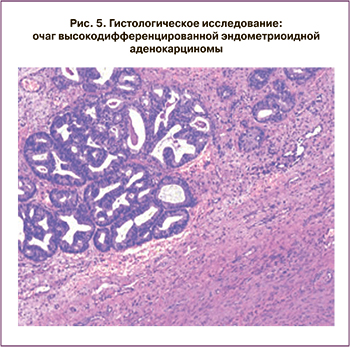
Следующий рецидив был диагностирован через год после окончания курса консервативной гормональной терапии (рис. 4, 5). Учитывая настоятельное желание пациентки, было вновь принято решение после дообследования начать проведение органосохраняющего консервативного лечения. Была установлена левоноргестрелсодержащая ВМС, и пациентка получала аГнРГ в течение 6 месяцев. Далее, после контрольного выскабливания и морфологического подтверждения отсутствия опухоли в соскобе из полости матки, пациентка принимала КОК в течение 6 месяцев.
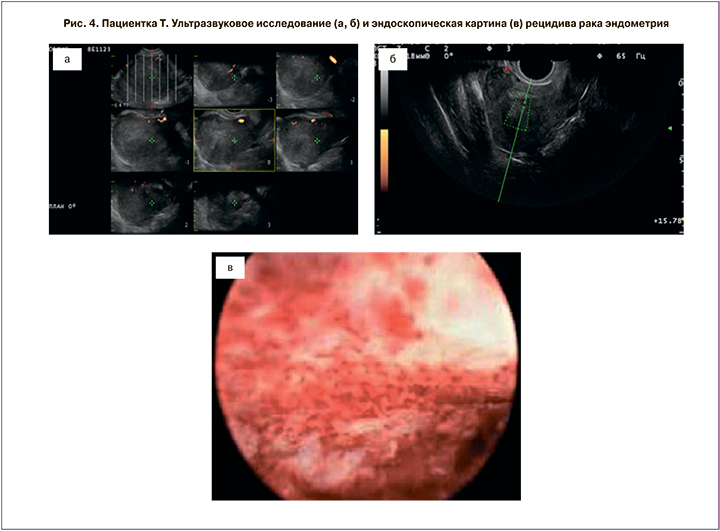
 Пациентка находилась под тщательным динамическим контролем (выполняли УЗИ органов малого таза и биопсию эндометрия каждые 3 месяца), несмотря на это через 2 года 3 месяца диагностирован повторный рецидив РЭ. Пациентке было предложено хирургическое лечение, от которого она категорически отказалась, после чего вновь установлена левоноргестрелсодержащая ВМС+антиэстрогены 20 мг+индол-3-карбинол 6 месяцев. В настоящее время пациентка планирует беременность с использованием вспомогательных репродуктивных технологий (ВРТ). После окончания противорецидивного лечения прошло более года. В настоящее время без прогрессирования.
Пациентка находилась под тщательным динамическим контролем (выполняли УЗИ органов малого таза и биопсию эндометрия каждые 3 месяца), несмотря на это через 2 года 3 месяца диагностирован повторный рецидив РЭ. Пациентке было предложено хирургическое лечение, от которого она категорически отказалась, после чего вновь установлена левоноргестрелсодержащая ВМС+антиэстрогены 20 мг+индол-3-карбинол 6 месяцев. В настоящее время пациентка планирует беременность с использованием вспомогательных репродуктивных технологий (ВРТ). После окончания противорецидивного лечения прошло более года. В настоящее время без прогрессирования.
Таким образом, консервативное лечение начального РЭ, а также рецидивов РЭ у пациенток, желающих сохранить фертильность в репродуктивном возрасте, возможно, и следует проводить, но лишь под контролем специалистов, имеющих опыт такого лечения и при тщательном соблюдении следующих условий:
- стадия заболевания IА (T1АN0M0);
- высокая дифференцировка опухоли;
- отсутствие признаков инвазивного роста опухоли в миометрий,
- подтвержденное данными УЗИ с допплерографией и МРТ с внутривенным контрастированием;
- отсутствие данных за распространение опухоли на ткань яичников, тазовых лимфатических узлов и признаков отдаленного метастазирования (подтвержденные данными исследований);
- репродуктивный возраст пациентки;
- настойчивое желание пациентки сохранить фертильность;
- чувствительность опухоли к гормональной терапии, по данным иммуногистохимического исследования (ER+ и PR+) (не обязательно) [1];
- информированность пациентки о возможных вариантах лечения и потенциальных рисках;
- информированное согласие о планируемом лечении;
- настрой пациентки на длительную терапию и соблюдение рекомендаций врача по срокам лечения и дальнейшего тщательного наблюдения.
Анализ данных литературы свидетельствует о том, что поддерживающее лечение низкими дозами прогестинов в циклическом режиме или прогестинсодержащей ВМС связано с более низким риском рецидива РЭ [7, 11, 12].
Заключение
Пациентки, желающие отложить беременность, должны продолжать поддерживающее лечение до тех пор, пока они не будут готовы забеременеть. Напротив, женщины, планирующие беременность, должны попытаться сделать это сразу же, как только будет достигнут полный ответ на лечение, потому что беременность также связана с уменьшением риска рецидива РЭ, вследствие воздействие высоких концентраций собственного прогестерона во время беременности.
Таким образом, следует отметить, что щадящее консервативное лечение для сохранения фертильности является высокоэффективным у молодых пациенток с IA стадией G1 первичной и рецидивной эндометриоидной аденокарциномы эндометрия. Такой вид лечения безопасен и эффективен при четком соблюдении показаний, а также приверженности пациентки назначенной терапии.



