Genital prolapse in women of reproductive age: structural changes in the pelvic floor support system
Remneva O.V., Ivanyuk I.S., Gal'chenko A.I., Semenikhina N.M.
Objective: This study aimed to examine the structural characteristics of pelvic floor muscles using ultrasound and histological examinations.
Materials and methods: In this study, 31 patients with POP-Q grade I–II genital prolapse and 30 women without pelvic organ prolapse were evaluated using pelvic floor ultrasound. The histological study involved examining muscle tissue samples from the m. levator ani obtained during the surgical correction of severe genital prolapse in 10 women.
Results: Among patients with genital prolapse, there was a significant decrease in the height of the perineal tendon center as well as the thickness of the bulbocavernosus and puborectalis muscles (p<0.05). The study group also exhibited an increase in the area of the levator fissure and levator-urethra gap. Histological examination of five m. levator ani samples revealed varying degrees of fibrosis and a decrease in the cross-sectional area of the muscle fibers.
Conclusion: Pathognomonic signs of genital prolapse include pelvic floor dysfunction and disruption of the integrity and structure of muscle fibers, characterized by the replacement of normal tissue with fibrosis and dystrophic changes.
Authors' contributions: Ivanyuk I.S., Remneva O.V. – conception and design of the study; Ivanyuk I.S., Gal’chenko A.I., Semenikhina N.M. – material collection and processing; Ivanyuk I.S. – statistical analysis, drafting of the manuscript; Remneva O.V. – editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the Altai SMU
(Ref. No: 11 of 24.12.2021).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Remneva O.V., Ivanyuk I.S., Gal'chenko A.I., Semenikhina N.M.
Genital prolapse in women of reproductive age: structural changes in the pelvic floor support system.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (7): 81-86 (in Russian)
https://dx.doi.org/10.18565/aig.2024.97
Keywords
Pelvic organ prolapse refers to the displacement of the pelvic organs from their normal anatomical position. It can cause symptoms such as urinary incontinence, defecation disorders, pelvic pain, sexual dysfunction, and sensation of a foreign object in the vagina. The prevalence of this condition is difficult to determine, as not all women seek medical help owing to the sensitive nature of the problem. Patients typically seek medical attention when noticeable anatomical changes affect their daily lives. Severe cases of genital prolapse (GP) require surgical treatment; however, long-term follow-up studies have shown complication rates of up to 42% with the use of synthetic implants. Additionally, the rate of disease recurrence after surgical correction can reach up to 36%. The cause of pelvic organ prolapse is believed to be damage and weakness of the ligamentous structures that support the pelvic floor. Extensive research has been conducted to understand the etiology and pathogenesis of this condition.
The severity of pelvic organ prolapse and the condition of the genital fissure can be assessed through visual and bimanual examinations, perineometer measurements, analysis of questionnaires, and patient complaints. Typically, a diagnosis is made when significant changes are present that significantly affect a woman's quality of life. Approximately 30% of cases of first-degree prolapse are undiagnosed during routine gynecological examinations.
Ultrasound examination of pelvic floor (PF) structures is a highly informative and noninvasive diagnostic method. Modern devices equipped with three- and four-dimensional reconstruction modes allow visualization of anatomical structures and assessment of biomechanics and prolapse degree in the cine loop mode. Ultrasound diagnostics offer advantages such as low cost, accessibility, safety, and the ability to dynamically monitor treatment progress.
Evaluation of several indicators is necessary to diagnose PF conditions. These indicators include the height of the perineal tendon center (normal, 10 mm or more), thickness of the bulbocavernosus muscles (normal, 10 mm or more), symmetry, and the thickness and structure of the puborectalis muscle (normal, 7 mm or more). The levator-urethral interval (LUI) was also assessed to rule out defects in the levator ani muscle. A value of ≥ 25 mm is considered an ultrasound indication of a levator ani defect. The gap between the branches of the levator ani muscle has the largest potential hernial opening in the human body. An area value of ≥25 cm2 during the Valsalva maneuver is regarded as "stretching." The degree of levator fissure stretching is closely related to prolapse, its symptoms, and risk of disease recurrence.
Although GP has been extensively studied, its incidence has not decreased. This may be due to a lack of complete understanding of the pathogenesis of the disease and prevention methods. However, it has been established that damage to musculoligamentous structures occurs early in this condition, highlighting the need for further investigation into changes in the supporting structures of the PF.
The objective of this study was to examine the structural characteristics of the pelvic floor muscles using ultrasound and histological examinations.
Materials and methods
The study was conducted in 2022 at the Department of Obstetrics and Gynecology, Altai State Medical University, Ministry of Health, Russia. The study included echographic visualization of the PF structures and histological examination. For ultrasound evaluation of the PF condition, the study group included 31 patients with grade I-II GP according to the POP-Q classification, determined during a comprehensive perineologic examination. The control group consisted of 30 women without pelvic organ prolapse. The ages of the patients ranged from 24 to 45 years, with a median age of 37 years in both groups.
The study was reviewed and approved by the Research Ethics Committee of Altai SMU (Ref. No: 11 of 24.12.2021).
The criteria for inclusion in the study group were age 18–45 years, presence of grade I–II pelvic organ prolapse, and history of vaginal birth. The criteria for inclusion in the control group included women aged 18–45 years, history of vaginal delivery, and absence of GP. Exclusion criteria were exacerbation of chronic diseases, malignant neoplasms of the pelvic organs, and psychoneurological disorders.
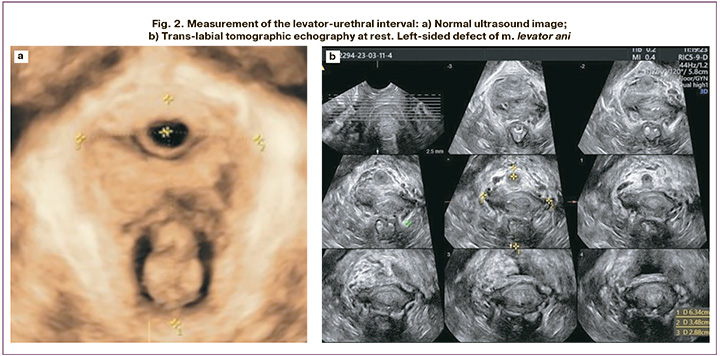
Ultrasound examination was performed using an expert-class Voluson E8 device with an intracavity volumetric sensor RIC5-9-D. During the examination, the patients were in a supine position with their knees and hips bent, and their hips abducted. To obtain an ultrasound image of the studied PF structures, scanning was performed via transperineal access using the B-mode and volumetric reconstruction mode (3D, 4D). The sensor was installed without applying pressure on the soft tissue of the perineum to obtain more reliable results. During the study, the height of the tendon center, thickness of the bulbocavernosus and puborectalis muscles, length of the LUI at rest, and area of the hiatus during the Valsalva maneuver were measured. To evaluate the levator ani muscle, a tomographic scanning mode (slice-by-slice) was used to obtain eight axial slices with a slice interval of 2.5 mm. To measure the LUI, the first caliper was placed in the area of the midpoint of the urethral cross-section, and the second at the most medial point of the levator attachment to the inferior ramus of the pubic bone (Fig. 2). LUI values of 25 mm or more were considered defects of the m. levator ani.
For histological examination, biopsies were obtained from the medial part of the left levator ani. Tissue samples were obtained during a combined surgical procedure (Manchester operation) in patients aged 37–45 years with grade III GP. The diagnosis was established using a comprehensive perineologic examination, including a visual assessment of the external genitalia and genital fissure at rest, during straining, during a cough test, bimanual vaginal examination, and ultrasound examination to assess the condition of the pelvic floor and exclude a defect or separation of the m. levator ani. The biopsy material was fixed in 10% neutral formalin. The specimens were then passed through multiple grafts of isopropyl alcohol, embedded in paraffin, and then embedded in histologic blocks. Sections of 7 μm thickness were cut on a Thermo Fisher HM 525 rotary microtome. Standard staining of the resulting sections was performed using hematoxylin-eosin and a commercial Malori kit (Biovitrum).
The morphological examination of the resulting specimens was performed using a Zeiss Primo Star microscope. The cross-sectional area and percentage of muscle fiber fibrosis were evaluated at ×200 and ×400 magnification in 10 fields of view in the Image Tool 3.0, using the Cout and Tag tools.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 26. Normality of the distribution of continuous variables was tested using the Shapiro–Wilk test. Non-normally distributed variables were compared using the non-parametric Mann–Whitney test. The median (Me) and interquartile range (Q1, Q3) were calculated for each group. Normally distributed variables were compared using the Student's t-test, and the sample mean and standard deviation M (SD) were used for descriptive statistics. Differences were considered statistically significant at p<0.05.
Results and discussion
The results of B-mode ultrasound showed a statistically significant decrease in the thickness of the bulbocavernosus and puborectalis muscles in patients with GP compared to women in the control group (p<0.05) (Fig. 1a, b). Domestic scientists have shown that thinning of the puborectalis muscle to less than 7 mm is a sign of connective tissue dysplasia [5]. This category of women is at risk of GP recurrence after surgical correction with autologous tissues. There was also a tendency towards a decrease in the height of the tendon center in patients in the study group (p=0.05) (Table). The obtained results are confirmed by data of other authors [10, 11].
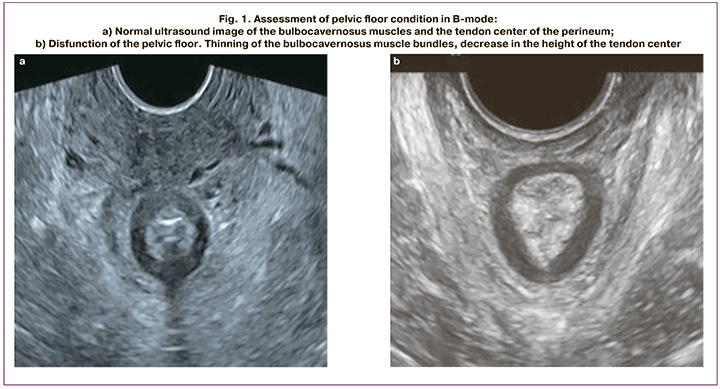
According to the results of our study, there was a statistically significant increase in the levator hiatus area in women with GP compared to the control group (p<0.05), which is comparable with the data described by other authors.
The results of assessing the length of LUI showed a higher value in the study group (p<0.05). In three women with GP, the length of the LUI was > 25 mm, which is a sign of damage to the levator ani muscle (Fig. 2b). The data obtained allowed us to consider the injury of the m. levator ani as one of the causes of pelvic organ prolapse. Other authors have confirmed the role of this muscle integrity disruption in patients with prolapse, including recurrent prolapse after surgical correction [2, 12, 13].
A significant amount of research has been conducted on PF muscle morphology. PF consists primarily of smooth and skeletal muscles, which are interconnected in complex ways, particularly in the area just in front of the anal canal. The bundles of the levator ani muscle are one of the main structural components that support the PF and prevent GP [14, 15]. Performing a pathomorphological study of the PF muscles presents certain difficulties that are associated with the quality of the biopsy material obtained and the degenerative processes occurring in the muscles of women with PF dysfunction. Dystrophic changes in muscle fibers lead to a decrease in the fiber density of striated muscles, making it challenging to detect the corresponding tissue in muscle biopsies [16]. In our study, muscle tissue samples were obtained from 10 women, all of whom had grade III GP. Striated muscle tissue was found in 5 samples (50%). Fibrosis of varying severities was observed in each case. Minor fibrosis (1–25%) was found in one patient, moderate fibrosis (25–50%) in two patients, and severe fibrosis (> 50%) in two patients. Patients with severe fibrosis had a disease duration of > 7 years, whereas those with mild and moderate fibrosis had a disease duration of ≤ 3 years. Researchers from the Peoples' Friendship University of Russia established a relationship between the duration of prolapse and degree of fibrosis [17]. According to our study, the cross-sectional area of the muscle bundles ranged from 2.2 to 4.1 μm². Russian authors have reported that the cross-sectional area of the fiber in healthy women is 3.4–5.2 µm² [16].
Recent studies have highlighted the role of not only skeletal muscles but also smooth muscles in supporting PF [15]. Smooth muscles form a connecting structure between the pelvic viscera and levator ani muscle, transmitting the force of the skeletal levator muscles to the pelvic viscera [18]. Therefore, changes in the structure and function of a muscle can disrupt the entire PF support system.
Vaginal delivery is the primary cause of PF muscle damage is vaginal delivery [19]. The levator ani muscle is injured by excessive stretching and pressure exerted by the fetal head as it passes through the birth canal. If the regeneration processes are disrupted, normal tissue is replaced by fibrous tissue, which not only leads to structural changes, but also impairs the contractility of the PF muscles [20]. Fibrosis is the final step in a complex chain of processes that occur following tissue damage. As the duration of the disease affects the severity of fibrotic changes in the levators, it is important to diagnose structural disorders of these muscles early and initiate measures to prevent severe GP.
Conclusion
Dystrophic changes and replacement of normal tissue with fibrosis occur in PF muscle fibers. In women with GP, there is a decrease in the height of the tendon center of the perineum, the thickness of the bulbocavernosus and puborectalis muscle bundles, and an increase in the area of the levator hiatus and LUI compared to healthy patients. Evaluating these parameters is necessary for the early diagnosis of PF disorders and for implementing timely measures to prevent severe GP.
References
- Mangir N., Roman S., Chapple C.R., MacNeil S. Complications related to use of mesh implants in surgical treatment of stress urinary incontinence and pelvic organ prolapse: infection or inflammation? World J. Urol. 2020; 38(1): 73-80. https://dx.doi.org/10.1007/s00345-019-02679-w.
- Friedman T., Eslick G.D., Dietz H.P. Risk factors for prolapse recurrence: systematic review and meta-analysis. Int. Urogynecol. J. 2018; 29(1): 13-21. https://dx.doi.org/10.1007/s00192-017-3475-4.
- Song C., Wen W., Pan L., Sun J., Bai Y., Tang J. et al. Analysis of the anatomical and biomechanical characteristics of the pelvic floor in cystocele. Acta Obstet. Gynecol. Scand. 2023; 102(12): 1661-73. https://dx.doi.org/10.1111/aogs.14657.
- Weintraub A.Y., Glinter H., Marcus-Braun N. Narrative review of the epidemiology, diagnosis and pathophysiology of pelvic organ prolapse. Int. Braz. J. Urol. 2020; 46(1): 5-14. https://dx.doi.org/10.1590/S1677-5538.IBJU.2018.0581.
- Чечнева М.А., Буянова С.Н., Попов А.А., Краснопольская И.В. Ультразвуковая диагностика пролапса гениталий и недержания мочи у женщин. М.: МЕДпресс-информ; 2016. 136c. [Chechneva M.A., Buyanova S.N., Popov A.A., Krasnopol'skaya I.V. Ultrasound diagnosis of genital prolapse and urinary incontinence in women. Moscow: MEDpress-inform; 2016. 136p.(in Russian)].
- Dietz H.P. Ultrasound in the assessment of pelvic organ prolapse. Best Pract. Res. Clin. Obstet. Gynaecol. 2019; 54: 12-30. https://dx.doi.org/10.1016/j.bpobgyn.2018.06.006.
- Dietz H.P., Garnham A.P., Guzmán Rojas R. Is it necessary to diagnose levator avulsion on pelvic floor muscle contraction? Ultrasound Obstet. Gynecol. 2017; 49(2): 252-6. https://dx.doi.org/10.1002/uog.15832.
- Dietz H.P. Pelvic floor ultrasound: a review. Clin. Obstet. Gynecol. 2017; 60(1): 58-81. https://dx.doi.org/10.1097/GRF.0000000000000264.
- Liu Z., Sharen G., Wang P., Chen L., Tan L. Clinical and pelvic floor ultrasound characteristics of pelvic organ prolapse recurrence after transvaginal mesh pelvic reconstruction. BMC Womens Health. 2022; 22(1): 102.https://dx.doi.org/10.1186/s12905-022-01686-1.
- Оразов М.Р., Хамошина М.Б., Геворгян Д.А. Диагностическая эффективность трансперинеальной сонографии в верификации мышечно-фасциальных дефектов тазового дна. Клинический случай. Гинекология. 2022; 24(3): 219-22. [Orazov M.R., Khamoshina M.B., Gevorgyan D.A. Diagnostic effectiveness of transperineal sonography in the verification of musculofascial defects of the pelvic floor. A clinical case. Gynecology. 2022; 24(3): 219-22.(in Russian)]. https://dx.doi.org/10.26442/20795696.2022.3.201673.
- Токтар Л.Р., Оразов М.Р., Геворгян Д.А., Арютин Д.Г., Маркина Я.В., Достиева Ш.М., Маслюков И.А. Трансперинеальное ультразвуковое исследование в диагностике несостоятельности тазового дна. Акушерство и гинекология: новости, мнения, обучение. 2020; 8(3) Приложение: 75-9. [Toktar L.R., Orazov M.R., Gevorgyan D.A., Aryutin D.G., Markina Ya.V., Dostieva Sh.M., Maslyukov I.A. Transperineal ultrasound examination in the diagnosis of pelvic floor failure. Оbstetrics and Gynecology: News, Opinions, Training. 2020; 8(3) Supplement: 75-9. (in Russian)].https://dx.doi.org/10.24411/2303-9698-2020-13912.
- Handa V.L., Blomquist J.L., Roem J., Muñoz A., Dietz H.P. Pelvic floor disorders after obstetric avulsion of the levator ani muscle. Female Pelvic Med. Reconstr. Surg. 2019; 25(1): 3-7. https://dx.doi.org/10.1097/SPV.0000000000000644.
- Shi W., Guo L. Risk factors for the recurrence of pelvic organ prolapse: a meta-analysis. J. Obstet. Gynaecol. 2023; 43(1): 2160929. https://dx.doi.org/10.1080/01443615.2022.2160929.
- Baramee P., Muro S., Suriyut J., Harada M., Akita K. Three muscle slings of the pelvic floor in women: an anatomic study. Anat. Sci. Int. 2020; 95(1): 47-53. https://dx.doi.org/10.1007/s12565-019-00492-4.
- Muro S., Akita K. Pelvic floor and perineal muscles: a dynamic coordination between skeletal and smooth muscles on pelvic floor stabilization. Anat. Sci. Int. 2023; 98(3): 407-25. https://dx.doi.org/10.1007/s12565-023-00717-7.
- Ищенко А.И., Александров Л.С., Никонов А.П., Горбенко О.Ю., Чушков Ю.В. Патоморфологические основы тазового пролапса. Медицина и экология. 2013; 4: 32-9. [Ishchenko A.I., Aleksandrov L.S., Nikonov A.P.,Gorbenko O.Yu., Chushkov Yu.V. Pathomorphological bases of pelvic prolapse. Medicine and Ecology. 2013; (4): 32-9. (in Russian)].
- Лологаева М.С., Токтар Л.Р., Оразов М.Р., Арютин Д.Г., Михалёва Л.М., Мидибер К.Ю., Геворгян Д.А., Хованская Т.Н. Морфологические особенности m. levator ani при генитальном пролапсе. Доктор.Ру. 2020; 19(6): 70-8. [Lologaeva M.S., Toktar L.R., Orazov M.R., Aryutin D.G., Mikhaleva L.M., Midiber K.Yu., Gevorgyan D.A., Khovanskaya T.N. Morphology of the levator ani in patients with genital prolapse. Doctor.Ru. 2020; 19(6): 70-8. (in Russian)]. https://dx.doi.org/10.31550/1727-2378-2020-19-6-70-78.
- Kato M.K., Muro S., Kato T., Miyasaka N., Akita K. Spatial distribution of smooth muscle tissue in the female pelvic floor and surrounding the urethra and vagina. Anat. Sci. Int. 2020; 95(4): 516-22. https://dx.doi.org/10.1007/s12565-020-00549-9.
- Иванюк И.С., Ремнёва О.В., Федина И.Ю., Гальченко А.И., Мельник М.А., Трухачёва Н.В. Факторы риска дисфункции тазового дна у женщин репродуктивного возраста. Бюллетень медицинской науки. 2023; 1: 43-52. [Ivanyuk I.S., Remneva O.V., Fedina I.Yu., Gal’chenko A.I., Mel’nik M.A., Trukhacheva N.V. Risk factors for pelvic floor dysfunction in reproductive-age women. Bulletin of Medical Science. 2023; (1): 43-52. (in Russian)]. https://dx.doi.org/10.31684/25418475-2023-1-43.
- Mukund K., Subramaniam S. Skeletal muscle: a review of molecular structure and function, in health and disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2020; 12(1): e1462. https://dx.doi.org/10.1002/wsbm.1462.
Received 19.04.2024
Accepted 13.06.2024
About the Authors
Ol'ga V. Remneva, Dr. Med. Sci., Professor, Head of the Department of Obstetrics and Gynecology, Altai State Medical University, Ministry of Health of Russia,40, Lenin Ave., Barnaul, 656038, Russia, +7(913)250-02-80, rolmed@yandex.ru, https://orcid.org/ 0000-0002-5984-1109
Irina S. Ivanyuk, PhD Student at the Department of Obstetrics and Gynecology, Altai State Medical University, Ministry of Health of Russia,
40, Lenin Ave., Barnaul, 656038, Russia, Ivanukirina@yandex.ru, https://orcid.org/ 0000-0002-6895-7103
Anzhelika I. Gal'chenko, PhD, Associate Professor at the Department of Obstetrics and Gynecology, Altai State Medical University, Ministry of Health of Russia,
40, Lenin Ave., Barnaul, 656038, Russia, https://orcid.org/ 0000-0003-3013-7764
Natalya M. Semenikhina, PhD, Associate Professor at the Department of Anatomy, Altai State Medical University, Ministry of Health of Russia,
40, Lenin Ave., Barnaul, 656038, Russia, https://orcid.org/ 0000-0003-4954-1716
Corresponding author: Ol'ga V. Remneva, rolmed@yandex.ru



