Prediction of preeclampsia on the couts of CD-16 negative monocytes
Objective of the study. To investigate the relative counts of peripheral blood monocyte subsets in women with normal pregnancy and pre-eclampsia.Boris D.A., Volgina N.E., Krasnyi A.M., Tyutyunnik V.L., Kan N.E.
Materials and methods. 48 patients were involved to the study. The main group included 36 pregnant women separated on 2 subgroups, 20 pregnant women with mild pre-eclampsia and 12 pregnant women with severe pre-eclampsia. The comparison group included 16 pregnant women with a physiological course of pregnancy. The leukocyte fraction was isolated from peripheral blood samples, the ratio of classical (CD14++CD16+HLA-DR+), intermediate (CD14++CD16–HLA-DR+) and non-classical (CD14+CD16++HLA-DR+) monocytes was determined using flow cytofluorimetry. The relative counts of monocyte subsets (%) was determined how the number of monocytes in each subsets to the total number of monocytes.
Results. The study of peripheral blood monocyte showed that, the relative counts of classical CD16-negative monocytes in peripheral blood is inversely correlated with the severity of pre-eclampsia. The counts of classical monocytes in the control group was 56,5% in women with mild pre-eclampsia 40,65% р=0,01, in women with severe pre-eclampsia 17,4% р=0,001. ROC analysis showed very good diagnostic value when comparing control group and mild pre-eclampsia AUC=0,81 (95% CI 0,63-0,98) sensitivity 86%, specificity 80% and excellent diagnostic value when comparing control group and severe pre-eclampsia AUC=0,96 (95% CI 0,87-1) sensitivity 83%, specificity 100%. The counts of intermediate and non-classical monocytes was increased in the groups with mild pre-eclampsia and severe pre-eclampsia, while significant differences were observed only when comparing severe pre-eclampsia with the control group in both subsets.
Conclusion. The study showed a significant discrepancy in the counts of classical monocytes between the control group and mild pre-eclampsia – 15.9%, and between the control group and severe pre-eclampsia – 55.1%. The findings can be used in the diagnosis of pre-eclampsia in early pregnancy in risk groups.
Keywords
Preeclampsia (PE) is an important global medical and social problem, and one of the major causes of maternal morbidity and mortality [1, 2]. Over the past decades, knowledge in the field of pathogenesis of PE has expanded sufficiently. It is considered that the development of this disease is associated with an imbalance of the systemic inflammatory response that occurs during pregnancy. It is also associated with immune tolerance to the semiallogeneic fetus (or allogeneic in case of egg donation) [3, 4].
Our previous study of a wide range of cytokines in the peripheral blood showed that monocyte-derived cytokines, namely interleukin-10 (IL-10) and interleukin-6 (IL-6), have the most significant differences in case of PE [4]. Monocytes are multifunctional cells that play a key role in homeostasis, immune response and tissue repair [5]. According to the International Union of Immunological Societies classification, there are three subclasses of monocytes divided by their phenotypic receptors. Based on the level of expression of the lipopolysaccharide receptor (CD14) and Fcγ-III receptor (CD16), the following monocytes were identified: classical monocytes (CD14++CD16-), intermediate monocytes (CD14++CD16+), and non-classical monocytes (CD14+CD16++) [6]. Monocytes express leukocyte antigen-DR (HLA-DR), a molecule mediating antigen presentation to T cells; and a combination of surface markers CD14, CD16, and HLA-DR makes it possible to accurately distinguish monocytes from lymphocytes and other leukocytes.
Healthy people have approximately 80–90% of classical CD16-negative monocytes of all monocytes. The remaining 10–20% is divided between CD16-positive intermediate and CD16-positive non-classical monocytes [6]. In a normal pregnancy the number of classical monocytes decreases while the number of intermediate monocytes increases [7]. These changes may be caused by the work of innate immunity, which can be observed in organ transplantation. Particularly in kidney transplantation, a decrease in the number of CD16-negative monocytes and an increase in the CD16-positive monocytes of both subsets can be seen [8]. The ratio of monocyte subsets changes in several pathological conditions, such as inflammatory and infectious diseases [9] and coronary heart disease [10].
Although there are a lot of studies focusing on the peripheral blood monocytes in PE, the distinct distribution of monocytes into groups has not been done yet [11]. In our study we reviewed the relative counts of monocyte subsets in the peripheral blood of women diagnosed with mild PE, severe PE, and women with a normal pregnancy.
The aim of the study is to compare the relative counts of the peripheral blood monocyte subsets in women with PE and with a normal pregnancy.
Materials and Methods
The study, which included 48 pregnant women, was conducted at the outpatient clinic of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov, Moscow, Russia. Patients were divided into two groups: the main and the control. The main group had two subgroups: 1st subgroup involved 20 pregnant women with mild PE, 2nd subgroup involved 12 pregnant women with severe PE. The control group involved 16 women with a normal full-term pregnancy, without a burdened obstetric history. The diagnosis of mild/severe PE was based on the clinical picture, laboratory and instrumental methods of common diagnosis. This study was approved by the local ethics committee. All patients were informed about the purpose and methods of the study, and signed a written consent. Pregnant women at 22 to 40 weeks’ gestation, 18-45 years of age, without severe extragenital pathology, with mild or severe PE were eligible for inclusion in the study, the main group. Patients with Rh disease, exacerbation and/or chronic inflammatory diseases, autoimmune diseases, oncological diseases, fetal malformations were excluded from the study.
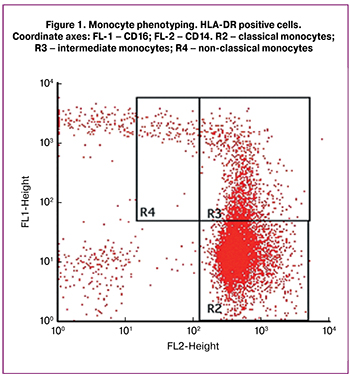 Venous blood samples were collected in the EDTA tubes, and then whole blood was layered onto Histopaque-1077 (Sigma-Aldrich, USA), and separated into components by 20-minute centrifugation at 400 g. The leukocyte ring cells were carefully isolated, and by two subsequent centrifugations the cells were washed from Histopaque. The leukocyte pellet was suspended in Facs Flow sheath fluid, and then each of the samples was divided into two 100-μl aliquots. An antibodies cocktail against IgG with phycoerythrin (PE), fluorescein (FITC), and allophycocyanin (APC) (Beckman Coulter, USA) of 10 μl each was added in one aliquot. Antibodies CD14-PE, CD16-FITC, and HLA-DR-APC (Beckman Coulter, USA) were added in the other aliquot. Antibody incubation lasted 30 minutes at 4oC. Further, the volume of aliquots was increased to 1.5 ml with Facs Flow. The analysis was performed on a flow cytometer (BD FACSCalibur, USA). Three subsets of monocytes were identified: classical monocytes (CD14++CD16−HLA-DR+), intermediate monocytes (CD14++CD16+HLA-DR+), and non-classical monocytes (CD14+CD16++HLA-DR+) (Figure 1).
Venous blood samples were collected in the EDTA tubes, and then whole blood was layered onto Histopaque-1077 (Sigma-Aldrich, USA), and separated into components by 20-minute centrifugation at 400 g. The leukocyte ring cells were carefully isolated, and by two subsequent centrifugations the cells were washed from Histopaque. The leukocyte pellet was suspended in Facs Flow sheath fluid, and then each of the samples was divided into two 100-μl aliquots. An antibodies cocktail against IgG with phycoerythrin (PE), fluorescein (FITC), and allophycocyanin (APC) (Beckman Coulter, USA) of 10 μl each was added in one aliquot. Antibodies CD14-PE, CD16-FITC, and HLA-DR-APC (Beckman Coulter, USA) were added in the other aliquot. Antibody incubation lasted 30 minutes at 4oC. Further, the volume of aliquots was increased to 1.5 ml with Facs Flow. The analysis was performed on a flow cytometer (BD FACSCalibur, USA). Three subsets of monocytes were identified: classical monocytes (CD14++CD16−HLA-DR+), intermediate monocytes (CD14++CD16+HLA-DR+), and non-classical monocytes (CD14+CD16++HLA-DR+) (Figure 1).
The relative counts (%) of monocytes were calculated as the ratio of the number of monocytes in each subset to the total number of monocytes.
Statistical processing. Results are presented as median of upper and lower quartiles Me (Q1; Q3) for nonparametric statistics. The statistical significance of the differences was measured by the Student’s t-test, with the normal distribution measured by the Shapiro-Wilk test, and the equality of variances measured by the Levene’s test. If these conditions were not met, the non-parametric Mann-Whitney U test was used. The comparison of groups by qualitative characteristics was performed using Fisher’s exact test. Differences were considered as statistically significant at p <0.05. For the comparison of three independent groups by the same quantitative characteristic, the analysis of variance (ANOVA) was used in a normal distribution of characteristic and equality of variances in the compared subsets; the Kruskal-Wallis rank test was used in distributions other than normal. When statistically significant differences were found in ANOVA or Kruskal-Wallis test, a posteriori pairwise comparisons were performed using Student’s t-test in the normal distribution or the Mann-Whitney U test in the non-normal distribution with Bonferroni correction in both cases. When comparing three groups for qualitative characteristics, the adjustment for multiple comparisons was also used. For evaluating the diagnostic efficiency of the model, ROC curve analysis was used. ROC curve analysis data are presented as area under the curve with a 95% confidence interval. The programs Attestat (Russia), Statistica 10.0 and OriginPro 8.5 (USA) were used for statistical processing of results and graphing.
Results and Discussion
The clinical and anamnestic data, significant complications of pregnancy are given in Table.
The age of women surveyed ranged from 18 to 45 years, and was 31 (30; 36), 32.5 (29.5; 37), 37.5 (34; 40.5) years, respectively. The gestational age at delivery was 39 (37.5; 33), 38 (39; 37), 38 (29; 36.25) weeks, respectively. The analysis of anthropometric data revealed no statistically significant differences between the groups. The analysis of the relationship of PE and the common risk factors identified the following conditions: age, clinically significant proteinuria, extragenital pathology, and cardiovascular disease (the most frequent). The analysis of fetal outcomes showed that chronic fetal hypoxia was more common in groups with mild and severe PE, but there was no statistically significant difference. We did not find the relationship between PE and the common risk factors such as: primiparity, multiparity with previous PE, interpregnancy intervals >10 years, excessive body weight/obesity, a family history, chronic kidney disease, collagenosis, diabetes mellitus, antiphospholipid syndrome.
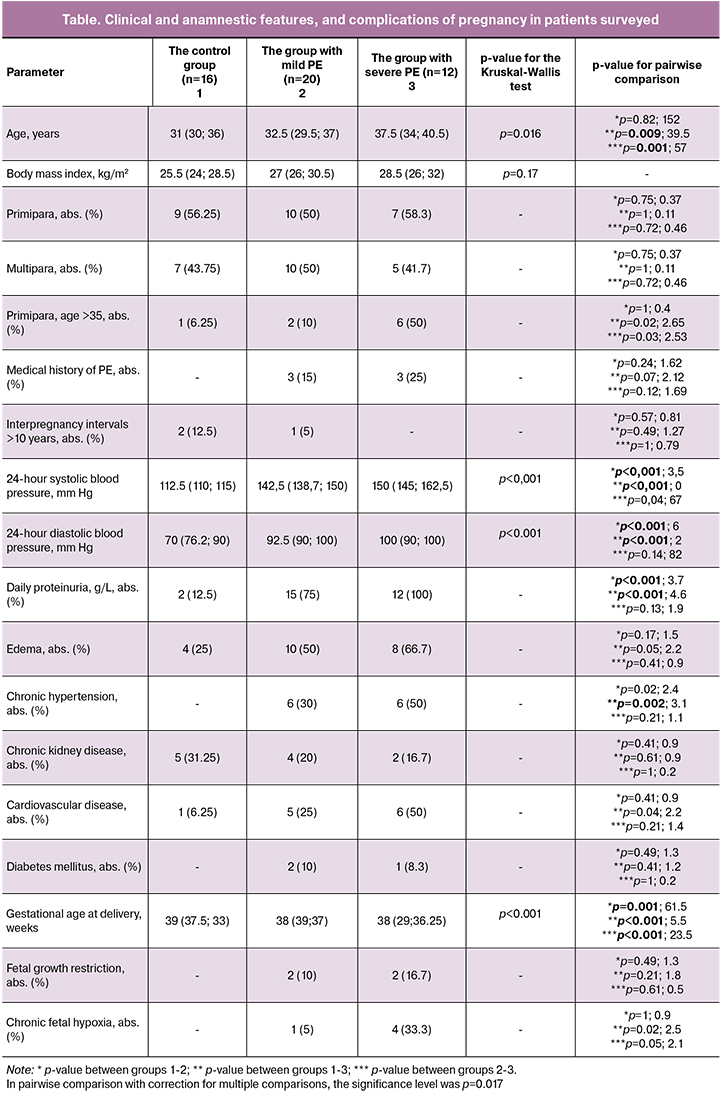
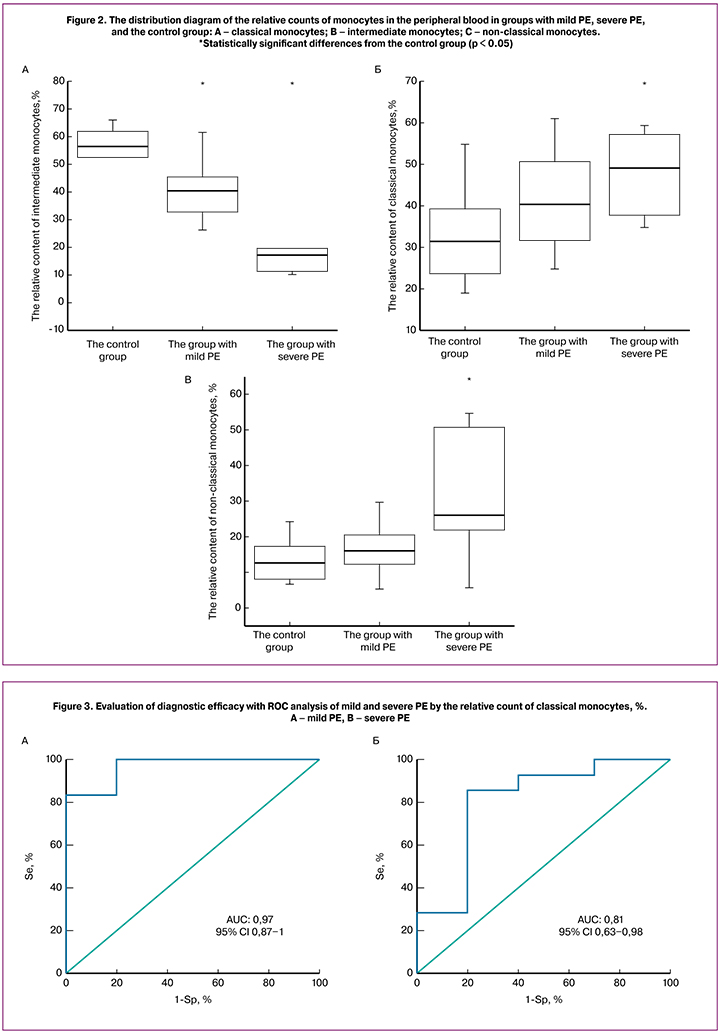
The counts (%) of classical, intermediate, and non-classical monocyte subsets of the total number of monocytes in the control group, the group with mild PE, and the group with severe PE are presented in Figure 2. A decrease in counts of classical CD16-negative monocytes in peripheral blood was observed in the groups with mild and severe PE compared with the control group (Figure 2A). Moreover, in the group with severe PE, the counts of classical monocytes were lower than in the group with mild PE. Thus, the relative counts of classical CD16-negative monocytes in peripheral blood inversely correlated with the severity of PE. The counts of classical monocytes in the control group were 56.55% (45.3; 12.6); in the group with mild PE – 40.65% (34.2; 60.8), p = 0.01; in the group with severe PE – 17.4% (53.2; 19.5), p = 0.001 (Figure 2A). Furthermore, ROC analysis showed a good diagnostic value when comparing the control group and the group with mild PE: AUC = 0.81 (95% CI: 0.63–0.98), sensitivity 86%, specificity 80% as well as an excellent diagnostic value when comparing the control group and the group with severe PE: AUC = 0.96 (95% CI: 0.87-1), sensitivity 83%, specificity 100% (Figure 3). The counts of intermediate and non-classical monocytes in the peripheral blood were increased in the groups with mild and severe PE. The counts of non-classical monocytes in the peripheral blood were as follows: in the control group – 12.65% (20.4; 22.7); in the group with mild PE – 16.05% (12.6; 16.7); in the group with severe PE – 26.05% (8.9; 44.7). The counts of intermediate monocytes in the peripheral blood were as follows: 31.45% (50.3; 39.2), 40.3% (32.2; 39) and 49.2% (25; 56.6), respectively. However, statistically significant differences were observed only when comparing the group with severe PE with the control group in both subsets (non-classical and intermediate) of monocytes (Figures 2B and C).
There are some studies on phenotyping monocytes in case of PE. B.N. Melgert et al. (2012) showed in their study that in PE the number of intermediate monocytes increases while the number of classical monocytes decreases, compared with a normal pregnancy [12]. Their study did not include the monocyte marker HLA-DR, and there was no division of PE into mild and severe. Hence, the relative count of classical monocytes in PE was a few percent less. The research of M.X. Tang et al. [13] on intermediate and non-classical monocytes showed that the counts of intermediate monocytes correlate with the severity of PE; however, this study did not include classical monocytes. These findings indirectly confirm our results. Furthermore, the study of the UK researchers E. Al-Ofi et al. [14] demonstrated an elevated count of non-classical monocytes in pregnancy complicated by PE compared with a normal pregnancy. The inconsistency of the results may be associated with the use of different methods and ethnic differences in the groups studied. Our study demonstrated a significant decrease in the counts of classical CD16-negative monocytes in the group with mild PE – by 15.9% and in the group with severe PE – by 55.1%, while the counts of both subsets of CD16-positive (non-classical and intermediate) monocytes increased. This may be caused by the reaction of the innate immunity of a pregnant woman to a semiallogeneic transplant (the fetus). The immune tolerance, which is observed in the normal pregnancy, is a state of unresponsiveness of the mother’s immune system to the developing fetus and placenta. The decrease in counts of classical CD16-negative monocytes caused by PE confirms the theory that PE development results from a lack of tolerance of the mother’s immune system to the fetus [3, 15].
Conclusion
Therefore, the findings of the study indicate the potential benefits of further research on monocytes in early pregnancy for the PE diagnosis.
References
- Савельева Г.М., Сухих Г.Т., Серов В.Н., Радзинский В.Е., ред. Акушерство. Национальное руководство. М.: ГЭОТАР-Медиа; 2015. 1080 с. [Savelyeva G.M., Sukhikh G.T., Serov V.N., Radzinsky V.E. Obstetrics. National leadership. М.: GEOTAR-Media; 2015. 1080 s. (in Russian)]
- Phipps E., Prasanna D., Brima W., Jim B. Updates in pathogenesis, definitions, and guidelines. Clin. J. Am. Soc. Nephrol. 2016; 11(6): 1102-13. https://doi.org/10.2215/CJN.12081115.
- Красный А.М., Кан Н.Е., Тютюнник В.Л., Ховхаева П.А., Волгина Н.Е., Сергунина О.А., Тютюнник Н.В., Беднягин Л.А. Окислительный стресс при преэклампсии и при нормальной беременности. Акушерство и гинекология. 2016; 5: 90-4. [Krasnyi A.M., Kan N.E., Tyutyunnik V.L., Khovkhaeva P.A., Volgina N.E., Sergunina O.A., Tyutyunnik N.V., Bednyagin L.A. Oxidative stress in preeclampsia and in normal pregnancy. Akusherstvo i ginekologiya/Obstetrics and Gynecology. 2016; (5): 90-94. (in Russian)] doi.org/10.18565/aig.2016.5.90-94.
- Красный А.М., Грачева М.И., Садекова А.А., Вторушина В.В., Балашов И.С., Кан Н.Е., Боровиков П.И., Кречетова Л.В., Тютюнник В.Л. Комбинированное исследование общей, фетальной ДНК, цитокинов в плазме крови матери при преэклампсии. Бюллетень экспериментальной биологии и медицины. 2017; 164(12): 686-91. [Krasnyi A.M., Gracheva M.I., Sadekova A.A., Vtorushina V.V., Balashov I.S., Kan N.E., Borovikov P.I., Krechetova L.V., Tyutyunnik V.L. Combined study of total, fetal DNA, cytokines in the mother’s blood plasma with preeclampsia. Bulletin of experimental biology and medicine. 2017; 164 (12): 686-691. (in Russian)]
- Wells C.A., Chalk A.M., Forrest A., Taylor D., Waddell N., Schroder K. et al. Alternate transcription of the Toll-like receptor signaling cascade. Genome Biol. 2006; 7(2): R10. https://doi.org/10.1186/gb-2006-7-2-r10.
- Ziegler-Heitbrock L., Ancuta P., Crowe S., Dalod M., Grau V., Hart D.N. et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010; 116(16): 74-80. https://doi.org/10.1182/blood-2010-02-258558.
- Faas M.M., Spaans F., De Vos P. Monocytes and macrophages in pregnancy and pre-eclampsia. Front. Immunol. 2014; 30(5): 298. https://doi.org/10.3389/fimmu.2014.00298.
- Vereyken E.J., Kraaij M.D., Baan C.C., Rezaee F., Weimar W., Wood K.J. et al. A shift towards pro-inflammatory CD16+ monocyte subsets with preserved cytokine production potential after kidney transplantation. PLoS One. 2013; 8(7): e70152. https://doi.org/10.1371/journal.pone.0070152.
- Janols H., Bredberg A., Thuvesson I., Janciauskiene S., Grip O., Wullt M. Lymphocyte and monocyte flow cytometry immunophenotyping as a diagnostic tool in uncharacteristic inflammatory disorders. BMC Infect. Dis. 2010; 10: 205. https://doi.org/10.1186/1471-2334-10-205.
- Nahrendorf M., Pittet M.J., Swirski F.K. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation. 2010; 121(22): 2437-45. https://doi.org/10.1161/CIRCULATIONAHA.109.916346.
- Faas M.M., De Vos P. Maternal monocytes in pregnancy and preeclampsia in humans and in rats. J. Reprod. Immunol. 2017; 119: 91-7. 10.1016/j.jri.2016.06.009.
- Melgert B.N., Spaans F., Borghuis T., Klok P.A., Groen B., Bolt A. et al. Pregnancy and preeclampsia affect monocyte subsets in humans and rats. PLoS One. 2012; 7(9): e45229. https://doi.org/10.1371/journal.pone.0045229.
- Tang M.X., Zhang Y.H., Hu L., Kwak‐Kim J., Liao A.H. CD 14++ CD 16+ HLA‐DR+ monocytes in peripheral blood are quantitatively correlated with the severity of pre‐eclampsia. Am. J. Reprod. Immunol. 2015; 74(2): 116-22. https://doi.org/10.1111/aji.12389.
- Al-ofi E., Coffelt S.B., Anumba D.O. Monocyte subpopulations from pre-eclamptic patients are abnormally skewed and exhibit exaggerated responses to Toll-like receptor ligands. PLoS One. 2012; 7(7): e42217. https://doi.org/10.1371/journal.pone.0042217.
- Gleicher N., Kushnir V.A., Barad D.H. Redirecting reproductive immunology research toward pregnancy as a period of temporary immune tolerance. J. Assist. Reprod. Genet. 2017; 34(4): 425-30. https://doi.org/10.1007/s10815-017-0874-x.
Received 09.12.21018
Accepted 20.02.2019
About the Authors
Boris Daiana A., postgraduate student of the 1 National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of the Ministry of Healthcare of Russian Federation. 117997, Moscow, Ac. Oparina, 4 str.).. Tel.: +7-915-081-89-97. E-mail: dayana_boris@mail.ru. ORCID ID 0000-0002-0387-4040Volgina Nadegda E., scientific researcher of the cytology laboratory of the 1National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of the Ministry of Healthcare of Russian Federation. 117997, Moscow, Ac. Oparina, 4 str. Tel.: +7-495-438-22-72. E-mail: n_volgina@oparina4.ru.
Krasnyi Aleksey M., PhD, the head of the cytology laboratory of the 1National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of the Ministry of Healthcare of Russian Federation; senior scientist, laboratory of evolutionary developmental biology, N.K. Koltzov Institute of Developmental Biology of Russian Academy of Sciences. Tel.: +7-495-438-22-72. E-mail: alexred@list.ru
117997, Moscow, Ac. Oparina, 4 str.; 19334, Moscow, Vavilova str. 26, Russia.
Tyutyunnik Victor L., PhD, MD, the head of the obstetric physiological department of the 1National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of the Ministry of Healthcare of Russian Federation. Tel.: +7-903-969-50-41. E-mail: tioutiounnik@mail.ru. Number Researcher ID B-2364-2015.ORCID ID 0000-0002-5830-5099. 117997, Moscow, Ac. Oparina, 4 str.
Kan Natalia E., PhD, MD, the head of the obstetric department of the 1National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of the Ministry of Healthcare of Russian Federation Tel.: +7-926-220-86-55. E-mail: kan-med@mail.ru. Number Researcher ID B-2370-2015. ORCID ID 0000-0001-5087-5946.117997, Moscow, Ac. Oparina, 4 str.
For citations: Boris D.A., Volgina N.E., Krasnyi A.M., Tyutyunnik V.L., Kan N.E. Prediction of pre-eclampsia on the couts of CD-16 negative monocytes. Akusherstvo i ginekologiya/ Obstetrics and Gynecology. 2019; (7): 49-55 (in Russian).
https://dx.doi.org/10.18565/aig.2019.7.49-55



