Prediction of neonatal complications based on quantitative proteome analysis in blood of pregnant women with fetal growth restriction
Volochaeva M.V., Tokareva A.O., Bugrova A.E., Brzhozovskiy A.G., Kukaev E.N., Tyutyunnik V.L., Kan N.E., Starodubtseva N.L.
Objective: The objective of the study was to investigate relationship between early neonatal complications and plasma proteome composition in pregnant women diagnosed with fetal growth restriction.
Materials and methods: This pilot case-control study included 40 pairs of "pregnant woman – newborn baby". Four groups were formed: Group I and group II were the main groups, group III and group IV were the comparison groups. Group I was comprised of women with early fetal growth restriction (FGR) (<32 weeks) (n=10 pairs); group II was comprised of women with late FGR (≥32 weeks) (n=10 pairs). Group III and group IV consisted of pregnant women, who delivered before and after 32 weeks (n=10 pairs/n=10 pairs) (the comparison group). Confirmation of the diagnosis of fetal growth restriction, as well as definition of normal body weight in the group of women with preterm births (before and after 32 weeks), postnatal assessment of weight and growth indicators in newborns (n=40) was performed according to the INTERGROWTH-21st centile charts that reflected the international consensus reached by the members of Neonatal Group. Quantitative analysis of 125 plasma proteins was performed using BAK 125 plasma proteomics kit (MRM Proteomics Inc., Montreal, Canada) by high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). Based on the support vector machine used for classification, predictive models for possible development of asphyxia and intraventricular hemorrhage in newborns were created.
Results: Based on the results of quantitative proteomic analysis of maternal plasma proteins, two prognostic models were developed. Model 1 (AUC=0.96), including the proteins α-1-acid glycoprotein 1, α-1-antichimotrypsin, α-1-β-glycoprotein, α-2-macroglobulin, antithrombin III, apolypoprotein A-IV, apolypoprotein С–II, apolypoprotein С-IV, carboanhydrase 1, CD5 antigen like protein, ceruloplasmin, clasterin, complement C3, complement component C9, complement factor H, transcortin, fibrinogen α-chain, fibrinogen β-chain, fibronectin, fibulin-1, heparin cofactor II, kallistatin, keratin, type II cytoskeletal 2 epidermal, pregnancy zone protein, prothrombin, ferotransferrin, vitamin К-depended protein S, vitamin К-depended protein Z, vitronectin as variables, with 92% sensitivity and 76% specificity will enable to detect the risks for intraventricular hemorrhage in newborns. Model 2 (AUC=0.83), including the proteins α-1-antichimotrypsin, apolypoprotein С–III, apolypoprotein D, β-2-glycoprotein 1, complement C1q subcomponent subunit C, complement component C9, kininogen-1, plasma protease C1 inhibitor, pregnancy zone protein, AMBP protein, prothrombin, vitronectin as variables with 67% sensitivity and 100% specificity, will enable to predict birth asphyxia.
Conclusion: Using the plasma proteome of pregnant women to predict the development of birth asphyxia and intraventricular hemorrhage in newborns in early neonatal period will improve the quality of medical care, as well as reduce neonatal morbidity and mortality in the group of infants with intrauterine growth restriction (IUGR).
Authors' contributions: Volochaeva M.V., Tokareva A.O., Bugrova A.E., Brzhozovskiy A.G., Kukaev E.N., Tyutyunnik V.L., Kan N.E., Starodubtseva N.L. – the concept and design of the study, data collection and analysis, literature review, processing and analysis of material on the topic of the study, writing the text of the manuscript, article editing.
Conflicts of interest: The authors have no conflict of interest to declare.
Funding: The study was carried out without any sponsorship.
Ethical Approval: The study was approved by the local Ethics Committee of the Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Volochaeva M.V., Tokareva A.O., Bugrova A.E., Brzhozovskiy A.G., Kukaev E.N., Tyutyunnik V.L., Kan N.E., Starodubtseva N.L. Prediction of neonatal complications based on quantitative proteome analysis
in blood of pregnant women with fetal growth restriction.
Akusherstvo i Gynecologiya/Obstetrics and Gynecology. 2024; (5): 64-73 (in Russian)
https://dx.doi.org/10.18565/aig.2024.37
Keywords
The current definition of fetal growth restriction includes slow fetal growth leading to low birth weight and body length of babies, who are below the 10th percentile for estimated birth weight [1, 2]. The main cause of fetal growth restriction is placental insufficiency manifested by poor placental function and inability to supply adequate oxygen and nutrients to the fetus leading to clinical manifestation of chronic fetal hypoxia and hypoglycemia. Adaptation of the fetal cardiovascular system occurs against the background of chronic hypoxia, and is assessed as increased cardiac output, and cerebral and adrenal blood flow according to Doppler parameters. This leads to high neonatal morbidity in infants with fetal growth restriction [3–7]. According to Kim F. et al., Misan N. et al., intraventricular hemorrhages (IVH) are most common in newborns with fetal growth restriction, who were born at > 32 weeks’ gestation, that increases the risk of cerebral palsy [8, 9]. Despite the fact that in the last decade the number of studies devoted to the pathogenesis of fetal growth restriction, as well as the number of biomarkers that can identify early-onset fetal growth restriction increased, the ability to predict and stratify the severity of both prenatal and postnatal complications in newborns with these pathologies is still missing. Under these circumstances, the studies of the proteome, given their simplicity and accuracy, can become a promising direction in predicting neonatal complications in infants.
The objective of the study was to investigate relationship between early neonatal complications and specific plasma proteome composition in pregnant women diagnosed with fetal growth restriction.
Materials and methods
This pilot case-control study included 40 pairs of "pregnant woman – newborn baby". The women gave birth at the Academician V.I. Kulakov National Medical Research Centre of Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia in the period from 2019 to 2021. To realize the objective of the study, four groups were formed – group I and group II (the main groups), group III and group IV (the comparison groups). Group I was comprised of women with early-onset fetal growth restriction (FGR) (<32 weeks) (n=10 pairs). Group II was comprised of women with late-onset FGR (≥32 weeks) (n=10 pairs). Group III and group IV consisted of pregnant women, who delivered before and after 32 weeks (n=10 pairs/n=10 pairs) (the comparison group). Individual selection of collected blood plasma samples from pregnant women in the group with fetal growth restriction and the comparison group was performed according to the criterion “pregnancy length at the time of delivery”. The group with fetal growth restriction included pregnant women aged 18–45 years, who had no severe somatic and gynecologic pathology, had singleton pregnancy and prenatal diagnosis of fetal growth restriction (according to the Delphi diagnostic criteria for FGR using ultrasound and Doppler parameters, and to the criteria of Clinical Guidelines of the Ministry of Health of Russia “Fetal growth restriction”), and have signed informed consent for participation in the study [10]. In the comparison group, fetal size was appropriated to the gestational age. The pregnant women with severe external genital pathology, multiple pregnancy, chromosomal abnormalities and fetal malformations were excluded from the study. The study was approved by the Ethical Committee.
Postnatal assessment of weight and growth parameters in newborns (n=40) was performed according to the INTERGROWTH-21st centile charts and the international consensus criteria for FGR reached by the members of Neonatal Group for prenatal confirmation of the diagnosis of fetal growth restriction, as well as normal weight in the comparison groups (before and after 32 week’s gestation) [11, 12]. Comprehensive assessment of the newborns’ condition was performed at the stage of hospitalization to the neonatal units. Quantification of 125 plasma proteins in pregnant women was performed using BAK 125 plasma proteomics kit (MRM Proteomics Inc., Montreal, Canada) by high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) using multiple reaction monitoring mode (MRM). Tandem mass-spectrometry MS/MS) data analysis was performed using QTRAP SCIEX6500+ system (SCIEX, Canada).
Statistical analysis
Statistical analysis was performed using software programs Statistica 12.6, IBM SPSS Statistics 21 and custom R scripts (version 4.2.1.), and additional packages, containing libraries ropls, e1071 and Proc, were used. Normal distribution of quantitative parameters was assessed using the Shapiro–Wilk test. Normal distribution was described using the arithmetic mean (M), standard deviation (SD) and the 95% confidence interval (95% CI). When distribution differed from normal, median (Me) and the lower and upper quartiles [Q1; Q3] were used. The categorical data were described using the absolute values and percentage. Comparison between three and more groups with normal distribution of quantitative variables was made using analysis of variance (ANOVA), Tukey's post-hoc test (for equal variances) or the Games-Howell test (for unequal variances). The Mann–Whitney U test was used to compare the difference between two groups for non-normal distribution of quantitative variables. The Kruskal–Wallis test was used to compare the difference between three or more groups with post-hoc Dunn’s test using the Holm correction. Comparison of percentages in the multifield contingency tables was performed using Pearson’s chi-squared test. Statistical significance was determined at p<0.05. To solve the task of predicting the development of intraventricular hemorrhages and asphyxia in newborns in the early neonatal period, the models based on support vector machine (SVM) were built, for which variable ranking with recursive feature elimination was performed [13]: variables with minimal scaled weights by absolute value were step-by-step excluded from the models, and the control at each stage was carried out using sliding mode control for each separate object to calculate the accuracy of the model. The model with a minimum number of variables and the highest possible accuracy was considered to be optimal. For adequate performance of SVM classifier, the samples with fetal growth restriction (early or late phenotype) as response variables were assigned the value of -1, the samples of the control group were assigned the value of 1. The prognostic value of the constructed models was characterized by sensitivity (Se) and specificity (Sp).
Results
Our previous study showed relationship between the maternal blood proteome and fetal growth restriction. Given high incidence of complications in the early neonatal period due to fetal growth restriction, investigation of relationship between the changes in the proteomic composition of the blood plasma in pregnant women and development of early neonatal complications, was of particular interest to determine the possibility of predicting them.
The infant’s condition in the early neonatal period, as well as further adaptation depends on gestational age for delivery and the severity of disorders present at birth, and therefore these indicators were used in analysis (Table 1). It should be noted that the infants in the group with fetal growth restriction had lower birth weight, and lower Apgar scores at 1 and 5 minutes (groups I and II) versus the comparison group (р<0.001, р=0.01 and p=0.02, respectively), this was due to inclusion criteria in the study. In group 1 (early-onset fetal growth restriction), the infants were most often born with extremely low body weight versus group II (late-onset fetal growth restriction) (р<0.05).
Analysis of the parameters of umbilical cord blood acid-base status at birth did not show significant differences between the groups in the levels of lactate and pH values (p=0.07 and p=0.59, respectively). However, in groups I and II the levels of glucose were lower than in the comparison groups (p=0.001).
Higher incidence rate of complications in the early neonatal period was found in the group with fetal growth restriction (IVH, respiratory distress syndrome (RDS), pulmonary hemorrhage, disseminated intravascular coagulation (DIC) and bleeding into the skin) (р=0.003). It should be noted that early neonatal mortality was registered significantly most often in Group 1 (early-onset fetal growth restriction). The clinical characteristics of the newborns under study is represented in Table 1.
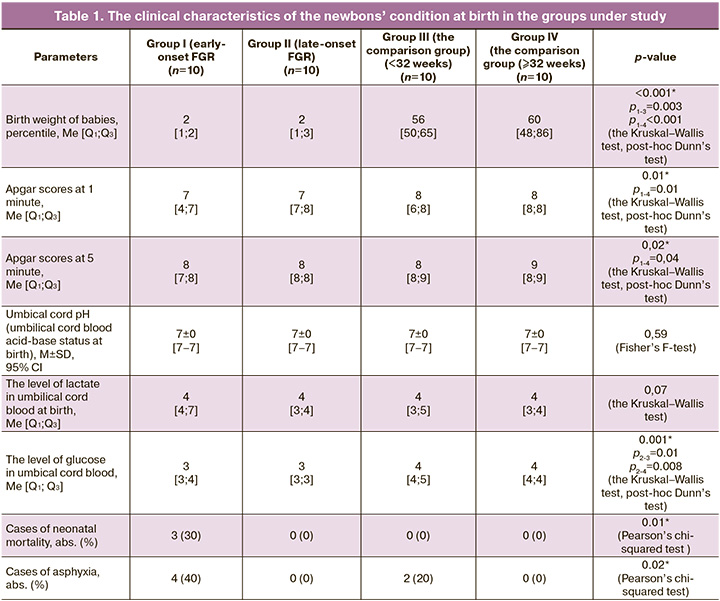
It should be noted that there was high incidence of asphyxia against the background of chronic hypoxia, and intraventricular hemorrhages in infants with fetal growth restriction in the early neonatal period. Based on the previous studies, proteome characteristics specific for early-onset fetal growth restriction were identified (high levels (p<0.05) of adiponectin, α-2-macroglobulin, gelsolin, pregnancy zone protein, S100 calcium binding protein A9 (S100A9), serum albumin, reduced levels (p<0.05) of apolipoprotein B-100, cathelicidin antimicrobial peptide (CAMP), complement component C7, tissue-type plasminogen activator), and for late-onset fetal growth restriction (high levels (p < 0.05) of apolipoprotein A-IV, biotinidase, fibrinogen gamma chain protein, gelsolin, glutathione peroxidase-3, plasminogen and retinol-binding protein 4, and reduced level (p<0.05) of cation-independent mannose-6-phosphate receptor) [14–17]. Correlation analysis showed correlations (p<0.05) between the above proteins and the clinical characteristics of the newborns: weight, Apgar score at 1 and 5 minutes, acid-base status (umbical cord рH, the levels of lactate and glucose) and the development of intraventricular hemorrhages, as well as the length of hospital stay (Fig. 1).
Statistically significant correlations were found between the changes in the blood plasma protein composition and development of intraventricular hemorrhages, as well as birth asphyxia, which according to the published data, most often occur in infants with fetal growth restriction [18].
It should be noted that prenatal prediction of the severity of fetal hypoxia leading to birth asphyxia in the group with fetal growth restriction is important, since birth asphyxia is directly related not only to the rationale for preterm labor decision-making, but also have an impact of the frequency of iatrogenic preterm birth and neonatal morbidity.
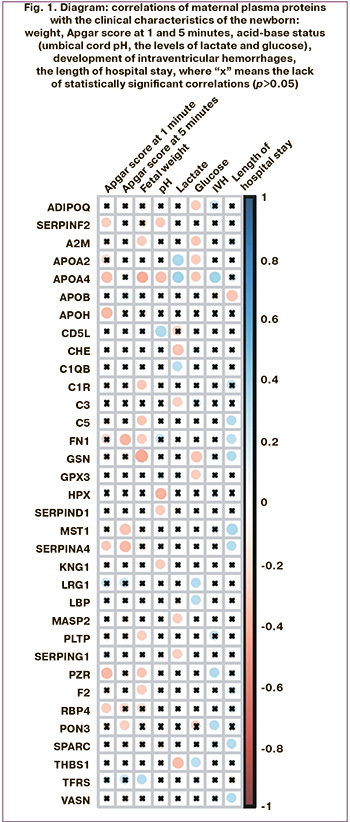
Given the obtained results of maternal plasma proteome analysis with puspose of prediction of these complications in newborns with fetal growth restriction in the early neonatal period, we have built 2 models using logistic regression (Fig. 2).
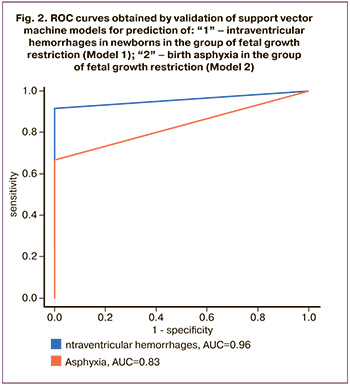
A correlation was found between the development of IVH and the levels of proteins – maternal plasma hemoglobin subunit alpha, S100A9, fibrinogen beta chain, serum paraoxonase/lactonase 3, Alpha-1-acid glycoprotein and lipopolysaccharide binding protein.
The model 1 for determination of the risk of intraventricular hemorrhage in newborns included alpha-1-acid glycoprotein 1 (ORM1), alpha 1-antichymotrypsin (SERPINA3), alpha-1-B glycoprotein (A1BG), alpha-2-macroglobulin (A2M), antithrombin III (SERPINC1), apolipoprotein A-IV (APOA4), apolipoprotein C-II (APOCII), apolipoprotein C-IV (APOC-IV), carbonic anhydrase 1 (CA1), CD5 antigen-like (CD5L), ceruloplasmin (CP), clusterin (CLU), complement C3 (C3), complement component 9 (C9), complement factor H (FH), transcortin (SERPINA6), fibrinogen alpha-chain (FGA), fibrinogen beta-chain (FGB), fibronectin (FN1), fibulin 1 (FBLN1), heparin cofactor 2 (SERPIND1 ), kallistatin (SERPINA4), keratin, type II cytoskeletal 2 epidermal (KRT2), pregnancy zone protein (PZP), prothrombin (F2), ferrotransferrin (Tf), vitamin K-dependent protein S (PROS), vitamin K-dependent protein Z (PROZ), vitronectin (VTN) (Table 2); and prediction model for intraventricular hemorrhage was developed (Fig. 2).

where Сx is protein X concentration.
Sensitivity and specificity of model 1 was 92% and 100%, respectively (Fig. 2).
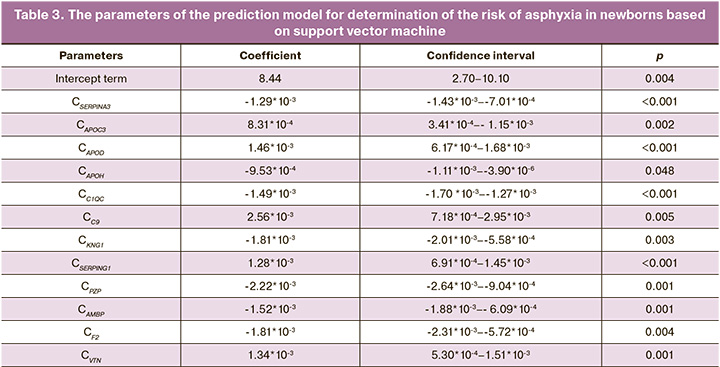
Model 2 for the prediction of asphyxia in the early neonatal period included alpha 1-antichymotrypsin (SERPINA3), apolipoprotein C-III (APOC-III), apolipoprotein D (ApoD), beta 2-Glycoprotein 1 (ApoH), complement C1q subcomponent subunit C (C1QC), complement component 9 (C9), kininogen 1 (KNG1), plasma protease C1 inhibitor (SERPING1), pregnancy zone protein (PZP), protein AMBP (AMBP), prothrombin (F2), vitronectin (VTN) (Table 3). Based on the levels of proteins, the model for the prediction of asphyxia was developed (Fig.2).
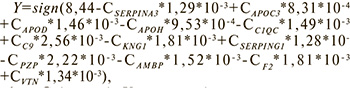
where Сx is protein X concentration.
Sensitivity of model 2 was 67%, specificity was 100% (Fig.2).
Discussion
Fetal growth restriction is one of the leading causes of the perinatal morbidity and mortality, and also contributes to the development of chronic diseases in the long term outcomes [19, 20]. This is primarily due to the development of perinatal asphyxia, difficulties in cardiopulmonary transition at birth, meconium aspiration, as well as the formation of persistent pulmonary hypertension against the background of the development of complications in the early neonatal period such as hypothermia, hypoglycemia, polycythemia, jaundice, necrotizing enterocolitis and sepsis [21]. Boghossian N.S. et al. and Malhotra A. et al. reported higher rates of necrotizing enterocolitis, retinopathy of prematurity, sepsis, and intraventricular hemorrhages in the group of babies diagnosed with fetal growth restriction [21, 22]. According to Hundscheid T.M. et al., high risk of perinatal asphyxia can be explained by placental dysfunction (insufficiency), leading to progressive chronic fetal hypoxia, which in some cases is combined with the structural characteristics of the umbilical cord (velamentous cord insertion) and the course of pregnancy against the background of the threating miscarriage (retroplacental hematomas, placental abruption) [23]. Given high risk of damaged brain tissue in infants with fetal growth restriction, timely prenatal prediction of severe birth asphyxia comes to the fore in modern perinatal medicine. Risk factor for perinatal brain damage in fetal growth restriction is formation of intraventricular hemorrhages. Association between “placental insufficiency – chronic fetal hypoxia” and abnormal umbilical cord blood flow was found in fetal growth restriction. According to a number of researchers, umbilical artery flow with absent or reversed end-diastolic velocity or abnormal ductus venosus blood flow (absent A-wave) are the predictors of intraventricular hemorrhages and possible death in the early neonatal period in this group of newborns [2, 18, 24]. According to Miller S.L. et al. and Kan N.E. et al., functional preservation of the brain activity is a specific reaction to placental insufficiency and chronic hypoxia, and changes in cerebral blood flow can indicate both the clinical severity of fetal growth restriction and can be associated prenatal neurodevelopmental disorders [2, 25]. Adequate, correct prediction of the development of intraventricular hemorrhages and severe birth asphyxia will make it possible to identify not only risk groups for this pathology in infants with fetal growth restriction, but also make a reasoned decision for preterm labor, which is directly related to reduced rates of iatrogenic preterm birth and the percentage of neonatal morbidity associated with the issue of prematurity.
Among differentially expressed proteins in fetal growth restriction, which were identified in our study, the proteins involved in hemostasis (including the process of platelet degranulation), as well as in regulation of the immunological processes, are of particular interest.
There are no clear markers for fetal growth restriction, which can help to predict the development of intraventricular hemorrhages both prenatally and postnatally. It is known that complement activation is closely related to increased platelet activity (extrinsic pathway of hemostasis). Hemostasis in fetal growth restriction has a number of pathological features associated with the fibrinolytic system, that affects the uteroplacental blood flow. The data obtained by us correlate with the data obtained by Gusar V. et al. They showed the relationship between the expression of microRNA 125b-5p (miR-125b-5p), which is involved in signaling pathways associated with angiogenesis, the response to hypoxia through activation of the HIF-1 signaling pathway, differentiation/vital function of neurons and genes associated with oxidative stress in the umbilical cord blood, and hemodynamic changes in the mother-placenta-fetus system, that has direct correlation with the development of intraventricular hemorrhages [26]. Our study showed that despite high activation levels of plateletes in the early phenotype of fetal growth restriction, there was decreased fibrinolysis and regulation of tissue plasminogen activator in the plasma. The imbalance in the fibrinolytic system can lead to excessive clot formation in early fetal growth restriction. Unlysed clots can damage the vascular wall, that leads not only to ischemia or placental abruption, but also potentially to the development of intraventricular hemorrhages both prenatally and postnatally.
Unforunately, Model 1 obtained in our study, for the time being does not help to differentiate intraventricular hemorrhage, which occurred prenatally or postnatally, but in future will make it possible to identify risk groups for development of this complication in fetal growth restriction.
To identify risk groups, the data on correlation of the proteome with the development of birth asphyxia in babies born with fetal growth restriction are of particular interest. It is known that the main cause of fetal growth restriction is placental insufficiency, resulting in the development of chronic fetal hypoxia, that leads to reduced growth velocity, redistribution of cerebral blood flow (reduction in brain volume and its structure) [18, 27]. Pathological values of resistance indices in the uteroplacental circulation leading to deterioration of the cardiovascular system, prematurity as a result of iatrogenicity and intracranial hemorrhage increase the risk of delayed psychomotor development and cerebral palsy. In turn, circulation changes are one of the factors regulating neuronal maturation, immune and endocrine interactions between the placenta and the fetus [28]. Our study found correlation of apolipoprotein A-IV with almost all clinical parameters in newborns in the main group (weight and Apgar scores at 1 and 5 minutes, severity of birth asphyxia, acid-base indicators at birth (umbilical artery pH, the levels of lactate and glucose), development of intraventricular hemorrhages, the length of hospital stay and life expectancy of newborns). In late-onset fetal growth restriction, secondary placental insufficiency often leads to higher fetal tolerance to hypoxia and more favorable outcomes, including lower rates of perinatal morbidity and mortality. The conclusions of our study correlate with the results of the study by Malhotra A. et al., who also marked a great practical and scientific interest in prenatal diagnostic strategy for detection of pathology of the nervous system in infants with fetal growth restriction [22]. The use of the proteomic profile obtained during the study (Model 1 and Model 2), as a minimally invasive method, will make it possible in future to predict and identify risk groups for the development of not only intraventricular hemorrhage, but also severe birth asphyxia in newborns with fetal growth restriction, that will also increase the effectiveness of assessing the severity brain injury associated with the outcomes of damage to the nervous system in this group of newborns.
Conclusion
Therefore, maternal plasma proteins can be proposed as prognostic markers for complications in the early neonatal period (asphyxia, intraventricular hemorrhages, hypoxic damage to the central nervous system) in newborns with diagnosed growth restriction, that will improve the quality of medical care from the point of view of modern perinatal medicine.
References
- Melamed N., Baschat A., Yinon Y., Athanasiadis A., Mecacci F., Figueras F. et al. FIGO (international Federation of Gynecology and obstetrics) initiative on fetal growth: best practice advice for screening, diagnosis, and management of fetal growth restriction. Int. J. Gynaecol. Obstet. 2021; 152(1): 3-57. https://dx.doi.org/10.1002/ijgo.13522.
- Miller S.L., Huppi P.S., Mallard C. The consequences of fetal growth restriction on brain structure and neurodevelopmental outcome. J. Physiol. 2016; 594: 807-23. https://dx.doi.org/10.1113/JP271402.
- Morsing E., Asard M., Ley D., Stjernqvist K., Marsál K. Cognitive function after intrauterine growth restriction and very preterm birth. Pediatrics. 2011; 127: e874-82. https://dx.doi.org/ 10.1542/peds.2010-1821.
- Гасанбекова А.П., Ломова Н.А., Долгополова Е.Л., Титова Е.В., Карапетян Т.Э., Рюмина И.И. Ранние и отдаленные последствия для новорожденных при синдроме задержки роста плода: данные ретроспективного исследования за 2019-2021 годы. Медицинский совет. 2023; 17(6): 172-9. [Gasanbekova A.P., Lomova N.A., Dolgopolova E.L., Titova E.V., Karapetyan T.E., Ryumina I.I. Early and long-term consequences for newborns with fetus growth retardation: retrospective study data for 2019-2021. Medical Council. 2023; 17(6): 172-9. (in Russian)]. https://dx.doi.org/10.21518/ms2022-002.
- Wang S.F., Shu L., Sheng J., Mu M., Wang S., Tao X.Y., Xu S.J., Tao F.B. Birth weight and risk of coronary heart disease in adults: a meta-analysis of prospective cohort studies. J. Dev. Orig. Health Dis. 2014; 5(6): 408-19. https://dx.doi.org/10.1017/S2040174414000440.
- Bygdell M., Ohlsson C., Lilja L., Celind J., Martikainen J., Rosengren A., Kindblom J.M. Birth weight and young adult body mass index for predicting the risk of developing adult heart failure in men. Eur. J. Prev. Cardiol. 2022; 29(6): 971-8. https://dx.doi.org/10.1093/eurjpc/zwab186.
- Якубова Д.И., Игнатко И.В., Меграбян А.Д., Богомазова И.М. Особенности течения беременности и исходы родов при различных фенотипах задержки роста плода. Акушерство и гинекология. 2022; 8: 54-62. [Yakubova D., Ignatko I.V., Megrabian A.D., Bogomazova I.M. Features of pregnancy course and delivery outcomes in various phenotypes of fetal growth restriction. Obstetrics and Gynecology. 2022; (8): 54-62. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.8.54-62.
- Kim F., Bateman D.A., Goldshtrom N., Sheen J.J., Garey D. Intracranial ultrasound abnormalities and mortality in preterm infants with and without fetal growth restriction stratified by fetal Doppler study results. J. Perinatol. 2023; 4(5): 560-7. https://dx.doi.org/10.1038/s41372-023-01621-8.
- Misan N., Michalak S., Kapska K., Osztynowicz K., Ropacka-Lesiak M. Blood-brain barrier disintegration in growth-restricted fetuses with brain sparing effect. Int. J. Mol. Sci. 2022; 23(20): 12349. https://dx.doi.org/10.3390/ijms232012349.
- Министерство Российской Федерации. Клинические рекомендации. Недостаточный рост плода, требующий предоставления медицинской помощи матери (задержка роста плода). М.; 2022. 71с. [Ministry of Health of the Russian Federation. Clinical guidelines. Insufficient growth of the fetus, requiring the provision of medical care to the mother (fetal growth retardation). Moscow; 2022. 71p. (in Russian)]. https://cr.minzdrav.gov.ru/schema/722_1
- Leite D.F.B., de Melo E.F. Jr, Souza R.T., Kenny L.C., Cecatti J.G. Fetal and neonatal growth restriction: new criteria, renew challenges. J. Pediatr. 2018; 203: 462-3. https://dx.doi.org/10.1016/j.jpeds.2018.07.094.
- Рюмина И.И., Байбарина Е.Н., Нароган М.В., Маркелова М.М., Орловская И.В., Зубков В.В., Дегтярев Д.Н. Использование международных стандартов роста для оценки физического развития новорожденных и недоношенных детей. Неонатология: новости, мнения, обучение. 2023; 11(2): 48-52. [Ryumina I.I., Baibarina E.N., Narogan M.V., Markelova M.M., Orlovskaya I.V., Zubkov V.V., Degtyarev D.N. The usage of the international growth standards to assess the physical development of newborn and premature children. Neonatology: News, Opinions, Training. 2023; 11(2): 48-52. (in Russian)]. https://dx.doi.org/10.33029/2308-2402-2023-11-2-48-52.
- Guyon I., Weston J., Barhill S., Vapnik V. Gene selection for cancer classification using support vector machines. Machine Learning. 2002; 46: 389-422. https://dx.doi.org/10.1023/a:1012487302797.
- Kononikhin A.S., Zakharova N.V., Semenov S.D., Bugrova A.E., Brzhozovskiy A.G., Indeykina M.I. et al. Prognosis of Alzheimer's disease using guantitative mass spectrometry of human blood plasma proteins and machine learning. Int. J. Mol. Sci. 2022; 23(14): 7907. https://dx.doi.org/10.3390/ijms23147907.
- Anwar M.A., Dai D.L., Wilson-McManus J., Smith D., Francis G.A., Borchers C.H. et al. Multiplexed LC-ESI-MRM-MS-based asay for identification of coronary artery disease iomarkers in human plasma. Proteomics Clin. Appl. 2019; 13(4): e1700111. https://dx.doi.org/10.1002/prca.201700111.
- Bhardwaj M., Gies A., Weigl K., Tikk K., Benner A., Schrotz-King P. et al. Evaluation and validation of plasma proteins using two different protein detection methods for early detection of colorectal cancer. Cancers (Basel). 2019; 11(10): 1426. https://dx.doi.org/10.3390/cancers11101426.
- Starodubtseva N.L., Tokareva A.O., Volochaeva M.V., Kononikhin A.S., Brhovosky A.S., Bugrova A.E. et al. Quantitative proteomics of maternal blood plasma in isolated intrauterine grow restriction. Int. J. Mol. Sci. 2023; 24(23): 16832. https://dx.doi.org/10.3390/ijms242316832.
- Malacova E., Regan A., Nassar N., Raynes-Greenow C., Leonard H., Srinivasjois R. et al. Risk of stillbirth, preterm delivery, and fetal growth restriction following exposure in a previous birth: systematic review and meta-analysis. BJOG. 2018; 125(2): 183-92. https://dx.doi.org/ 10.1111/1471-0528.14906.
- Kesavan K., Devaskar S.U. Intrauterine growth restriction: postnatal monitoring and outcomes. Pediatr. Clin. North Am. 2019; 66(2): 403-23. https://dx.doi.org/10.1016/j.pcl.2018.12.009.
- Шелехин А.П., Баев О.Р., Красный А.М. Сравнение течения и исходов беременностей, осложненных гипертензивными расстройствами. Акушерство и гинекология. 2023; 1: 41-7. [Shelekhin A.P., Baev O.R., Krasnyi A.M. Comparison of the course and outcomes of pregnancies complicated by hypertensive disorders. Obstetrics and Gynecology. 2023; (1): 41-7. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.248.
- Boghossian N.S., Geraci M., Edwards E.M., Horbar J.D. Morbidity and mortality in small for gestational age infants at 22 to 29 veeks' gestation. Pediatrics. 2018; 141(2): e20172533. https://dx.doi.org/10.1542/peds.20172533.
- Malhotra A., Allison B.J., Castillo-Melendez M., Jenkin G., Polglase G.R., Miller S.L. Neonatal morbidities of fetal growth restriction: pathophysiology and impact. Front. Endocrinol. (Lausanne). 2019; 10: 55. https://dx.doi.org/10.3389/fendo.2019.00055.
- Hundscheid T.M., Villamor-Martinez E., Villamor E. Association between endotype of prematurity and mortality: a systematic review, meta-analysis, and meta-regression. Neonatology. 2023; 120(4): 407-16. https://dx.doi.org/10.1159/000530127.
- Marsoosi V., Bahadori F., Esfahani F., Ghasemi-Rad M. The role of Doppler indices in predicting intra ventricular hemorrhage and perinatal mortality in fetal growth restriction. Med. Ultrason. 2012; 14(2): 125-32.
- Кан Н.Е., Леонова А.А., Тютюнник В.Л., Хачатрян З.В. Особенности нейрогенеза при задержке роста плода. Акушерство и гинекология. 2022; 11: 24-30. [Kan N.E., Leonova A.A., Tyutyunnik V.L., Khachatryan Z.V. Features of neurogenesis in case of fetal growth restriction. Obstetrics and Gynecology. 2022; (11): 24-30. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.11.24-30.
- Gusar V., Ganichkina M., Chagovets V., Kan N., Sukhikh G. MiRNAs Regulating oxidative stress: a correlation with doppler sonography of uteroplacental complex and clinical state assessments of newborns in fetal growth restriction. J. Clin. Med. 2020; 9(10): 3227. https://dx.doi.org/10.3390/jcm9103227.
- Baschat A.A. Neurodevelopment after fetal growth restriction. Fetal Diagn. Ther. 2014; 36(2): 136-42. https://dx.doi.org/10.1159/000353631.
- Baschat A.A. Neurodevelopment following fetal growth restriction and its relationship with antepartum parameters of placental dysfunction. Ultrasound Obstet. Gynecol. 2011; 37(5): 501-14. https://dx.doi.org/10.1002/uog.9008.
Received 15.02.2024
Accepted 02.04.2024
About the Authors
Maria V. Volochaeva, PhD, Senior Researcher at the Department of Regional Cooperation and Integration, Physician at the 1 Maternity Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(919)968-72-98, volochaeva.m@yandex.ru, https://orcid.org/0000-0001-8953-7952Alisa O. Tokareva, PhD, Specialist at the Laboratory of Clinical Proteomics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(495)531-44-44 (доб. 3113), alisa.tokareva@phystech.edu,
https://orcid.org/0000-0001-5918-9045
Anna E. Bugrova, PhD, Senior Researcher at the Laboratory of Proteomics of Human Reproduction, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4; Senior Researcher at the Laboratory of Neurochemistry, N.M. Emanuel Institute of Biochemical Physics of RAS, +7(495)531-44-44 (ex. 3113), a_bugrova@oparina4.ru
Alexander G. Brzhozovskiy, PhD, Senior Researcher at the Laboratory of Proteomics of Human Reproduction, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4; Junior Researcher at the Laboratory of Mass Spectrometry, Skolkovo Institute of Science and Technology, +7(495)531-44-44 (ex. 3113), agb.imbp@gmail.com
Evgenii N. Kukaev, PhD, Senior Researcher at the Laboratory of Clinical Proteomics, Academician V.I. Kulakov National Medical Research Center for Obstetrics,
Gynecology and Perinatology, Ministry of Health of Russia, 17997, Russia, Moscow, Ac. Oparina str., 4; Researcher, Semenov Federal Research Center for Chemical Physics, +7(495)531-44-44 (ex. 3113), e_kukaev@oparina4.ru, https://orcid.org/0000-0002-8397-35741
Victor L. Tyutyunnik, Professor, Dr. Med. Sci., Leading Researcher at the Center for Scientific and Clinical Research, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(903)969-50-41, tioutiounnik@mail.ru, Researcher ID: B-2364-2015, SPIN-code: 1963-1359, Authors ID: 213217, Scopus Author ID: 56190621500, https://orcid.org/0000-0002-5830-5099
Natalia E. Kan, Professor, Dr. Med. Sci., Deputy Director of Science, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(926)220-86-55, kan-med@mail.ru, Researcher ID: B-2370-2015,
SPIN-code: 5378-8437, Authors ID: 624900, Scopus Author ID: 57008835600, https://orcid.org/0000-0001-5087-5946
Natalia L. Starodubtseva, PhD, Head of the Laboratory of Clinical Proteomics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(495)531-44-44 (ex. 3113), n_starodubtseva@oparina4.ru,
https://orcid.org/0000-0001-6650-5915
Corresponding author: Maria V. Volochaeva, volochaeva.m@yandex.ru



