The role of magnetic resonance imaging and ultrasound diagnosis of fetal growth restriction in combination with pathological changes in fetal brain
Kulabukhova P.V., Bychenko V.G., Shmakov R.G.
Background: Fetal growth restriction (FGR) is a common complication of pregnancy and, in severe cases, leads to increased perinatal mortality, neonatal morbidity, and poor prognosis for life expectancy in patients with congenital malformations of the central nervous system and hypoxic-ischemic changes in the brain. Early detection of brain injury in IUGR enables to predict short-term and long-term outcomes for the development of the central nervous system, that currently remains a serious issue.
Objective: The aim of the study was to assess the role of ultrasound and MRI in diagnosis of FGR in combination with pathological changes in fetal brain.
Materials and methods: The retrospective study included 24 patients with suspected FGR. The mean age of patients (Me; Q1–Q3) was 33 (25–41) years, the average pregnancy length was 27.5 (20–35) weeks. The patients underwent simultaneous diagnostic US and MRI of the fetuses in the second and third trimester of pregnancy to assess fetal head circumference using percentile values of nomograms, and identify comorbidity, including the changes in fetal brain.
Results: No false positive results were found. MRI data and US imaging data were absolutely similar in nomograms for measurement of fetal brain volume using percentile method in 24 fetuses (100%) with FGR. Among them, FGR in combination with congenital diaphragmatic hernia was diagnosed in 3 fetuses (12.5%), and spina bifida in 1 fetus (4.2%). Comparison of two imaging techniques showed that false-negative results of ultrasound detection of malformation of the cortical plate and assessment of sulcation of the fetal brain were found in 3 fetuses (12.5%) versus 7 fetuses (29.2%) using MRI. Also, ultrasound imaging in diagnosing isolated unilateral cerebellar hypoplasia, showed false negative results in 2 fetuses (8.3%) versus false negative MRIs in 5 fetuses (20.9%).
Conclusion: The study showed that diagnostic ultrasound and MRI are comparable techniques in assessing biometry of the fetal brain using centile nomograms. However, MRI helps to perform more careful assessment of the concomitant pathology of the fetal brain.
Authors' contriburions: Kulabukhova P.V. – the concept and design of the study, material collection and processing, analysis of the obtained data, writing the text of the article; Bychenko V.G. – the concept and design of the article, editing the text of the article; approval of the final variant of manuscript; Shmakov R.G. – the concept and design of the article, editing the text of the article, approval of the final variant of manuscript. All authors have read and approved the final version of the article before publication and agreed to be responsible for all aspects of the study and ensure that they have considered and solved all issues related to the accuracy and academic integrity of all parts of the study.
Conflicts of interest: The authors confirm that they have no conflicts of interest to declare.
Funding: The study was conducted within the support of the State Assignment of the Ministry of Health of the Russian Federation [State Registration number 121040600408-4].
Ethical Approval: The study was approved by the local Ethics Committee of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data and associated images.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Kulabukhova P.V., Bychenko V.G., Shmakov R.G. The role of magnetic resonance imaging and
ultrasound diagnosis of fetal growth restriction in combination with pathological changes in fetal brain
Akusherstvo i Gynecologia/Obstetrics and Gynecology. 2024; (4): 51-58 (in Russian)
https://dx.doi.org/10.18565/aig.2023.55
Keywords
Fetal growth restriction (FGR) occurs in 10% of pregnancies all over the world. IUGR is associated with stillbirth, preterm birth and increased risk of neonatal complications in surviving newborns [1]. There are various causes of FGR including fetal, maternal and placental factors [2–4]. Placental insufficiency is the most important clinical factor leading to chronic hypoxia and hypoglycemia in normally developing fetus [5]. In turn, chronic fetal hypoxaemia and nutritional deficiencies directly reduce fetal growth rate, and hypoxia causes redistribution of cardiac output that protects brain and heart development as compared with other organs (so-called “brain-sparing” or cerebral redistribution). However, this does not guarantee fetal normal brain volume and development [6]. The specific pathology of central nervous system (CNS) in FGR is complex and differs both in preterm babies without FGR and in full-term infants exposed to severe chronic hypoxia [7]. Imaging studies of human FGR and postmortem studies, together with experimental studies of placental insufficiency in animals, describe reduction in total brain volume with loss of substructure of both gray matter and white matter, and disorganization of cortical structure [8]. In FGR, white matter in the brain is immature, with delayed maturation of oligodendrocyte progenitor cells [9], increased number of unmyelinated axons and thin myelin sheaths [10], the signs of astrogliosis and inflammation [11]. Very recently, it was found that structural brain changes in FGR are most pronounced in the cortico-striatal-thalamo-cortical (CSTC) pathway, that correlates with poor neurodevelopmental outcomes in young children born with FGR [12–14]. FGR is associated with increased risk of neurodevelopmental disorders, and the severity of impairment is associated with the severity of growth restriction and the timing of onset of FGR (early-onset or late-onset). Neurodevelopmental outcomes in infants born with early-onset FGR are worse compared with late-onset FGR [15]. In addition, preterm babies with FGR have increased risk of neonatal complications, such as pulmonary hypertension, metabolic disorders, and necrotizing enterocolitis, which in turn, may induce acute hypoxia/ischemia leading to serious brain injury [16]. Complicated and adverse outcomes in infants with FGR demonstrate the necessity for accurate assessment and early detection of brain injury to predict short-term and long-term outcomes for CNS development, that currently remains a major challenging issue [17, 18].
Thus, the aim of the study was to assess the role of ultrasound and MRI in diagnosis of FGR in combination with pathological changes in fetal brain.
Materials and methods
To participate in the study, a group of women was selected from 50 patients, who underwent US and MRI in the second and third trimester of pregnancy. Among them, 10 patients were excluded from the study due to the time difference more than 2 weeks between MRI and US implementation; 6 patients were excluded from the study because none of the imaging techniques confirmed the preliminary diagnosis of FGR; 7 patients were excluded due childbirth at other medical facilities and absence of verification of the diagnosis; 2 patients had multiple pregnancies; 1 patient had concomitant pathology – infectious and inflammatory disorder. The final analysis included 24 women.
The retrospective study included 24 patients. All women were examined and gave birth at the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of the Ministry of Health of Russia from 2021 to 2022. Time difference between US and MRI was no more than 2 weeks. Fetal ultrasound and MRI was performed in the second and in the third trimester of pregnancy to assess both fetal head measurements and differential diagnosis of pathological changes in the fetal brain. Ultrasound scanners of expert level were used. US was performed by widely experienced specialists in this diagnostic area. The percentile nomograms were used to calculate the parameters of fetal growth restriction. MRI examinations were performed using scanners GE Signa 1.5T, Toshiba Vantage Titan 1.5T with MRI sequences T1VI, T2WI, T2FS, FIESTA. Fetal brain MR images with 3 mm slice thickness were obtained in the saggital, frontal and axial planes. For fetal brain size estimation, the following measurements were made: brain fronto-occipital length, brain biparietal diameter, skull occipitofrontal diameter, skull biparietal diameter, transverse cerebellar diameter, head circumference, vermis height, vermis width, vermis area, with further entering of ultrasound measurements into Fetal Centiles Calculator (https://www.developingbrain.co.uk/fetalcentiles/) (Fig. 1).
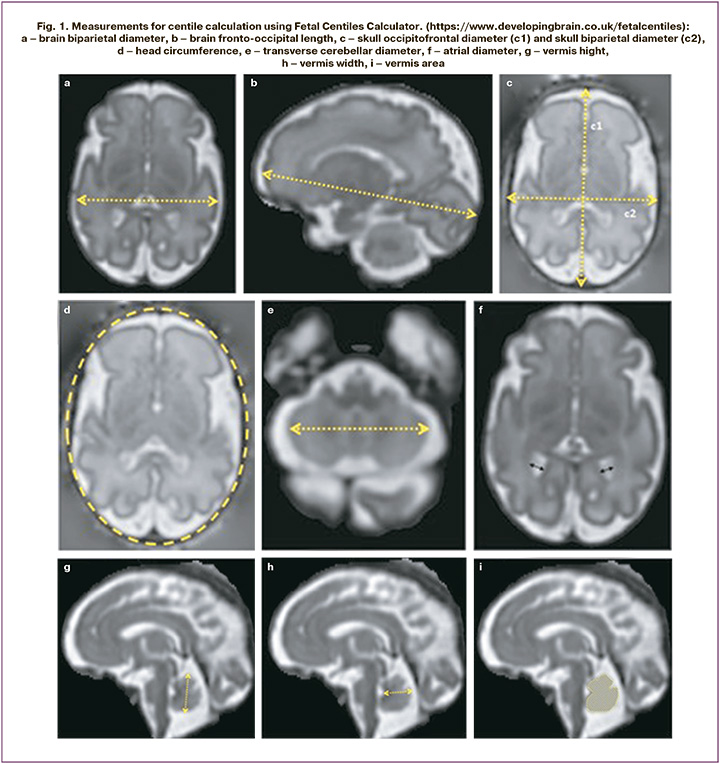
Inclusion criteria: singleton pregnancy ≥ 20 weeks with FGR, informed consent obtained from the patients to participate in the study, delivery at National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov, verification of the diagnosis after childbith.
Exclusion criteria: general contraindications to MRI, maternal severe somatic diseases and preeclampsia, multiple pregnancy, maternal infectious diseases, exacerbation of chronic diseases, not verified diagnosis during delivery at another hospital, performance of MRI and ultrasound at different terms of pregnancy (the interval between MTI and US is more than 2 weeks).
MRI-based and ultrasound-based measurements of the parameters was compared as follows:
- The conformity with fetal brain volume using the percentile nomograms.
- The conformity with the identified concomitant brain injury – the pathology of the corpus callosum, ventriculomegaly, brain malformations, cerebellar hypoplasia and cerebellar vermis hypoplasia, hypoxic-ischemic changes in the brain and their effects, pathological formation of the sulci and delayed sulcation.
The study was approved by the local Ethics Committee of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of the Ministry of Health of Russia.
Results
The mean age of patients (Me (Q1–Q3)) was 33 (25–41) years; the mean gestational age was 27.5 (20–35) weeks.
Comparative characteristics of the changes evaluated by US and MRI is represented in Table 1.
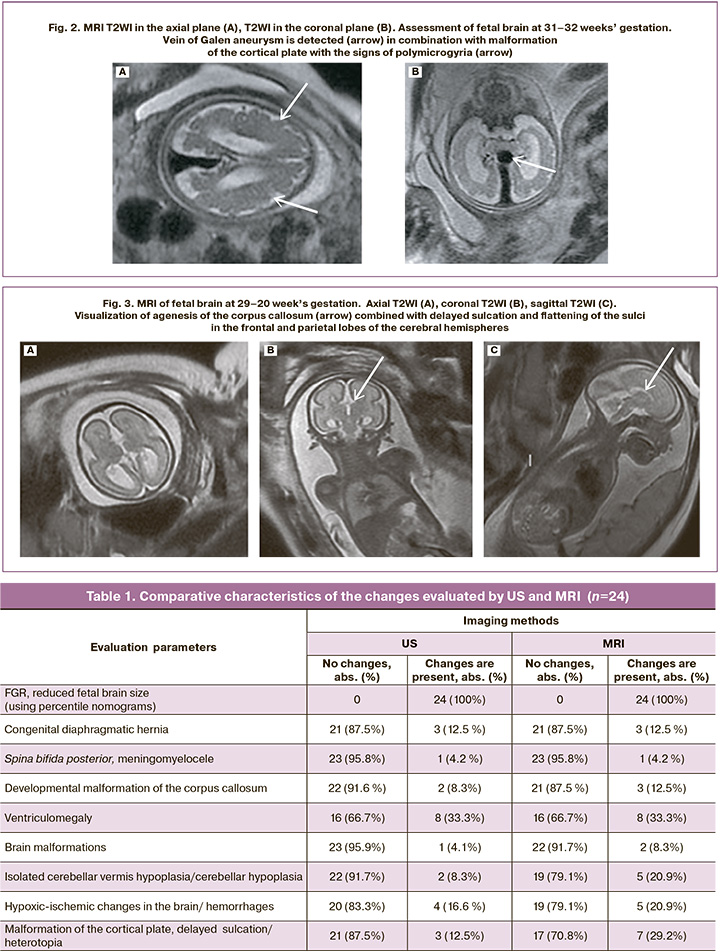
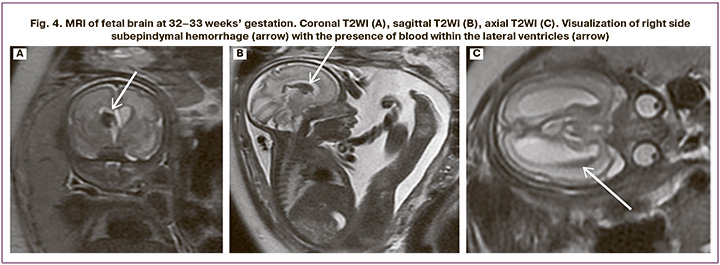
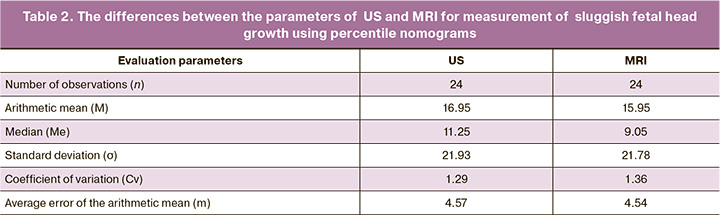
Analysis of the data obtained by US and MRI showed discrepancies in the interpretation of pathological changes. So, false positive results were not observed (the presence of changes on US images, and absence of changes on MRI). False-negative results of ultrasound were most often found in assessing malformation of the cortical plate, delayed sulcation and suspected gray matter heterotopia in 3 diagnostic cases (12.5%) versus 7 (29.2%) cases using MRI (Fig.2). There were fewer discrepancies between ultrasound diagnosis of isolated cerebellar vermis hypoplasia/cerebellar vermis in 2/24 patients (8.3%) and MRI in 5/24 (20.9%) patients. Also, there were minor discrepancies between US diagnosis of developmental malformation of the corpus callosum in 2/24 fetuses (8.3%) and MRI in 3/24 (12.5%) fetuses (Fig. 3); between US diagnosis of brain malformations in 1/24 fetus (4.1%) and MRI in 2/24 fetuses (8.3%), between US diagnosis of hypoxic-ischemic changes in the brain, hemorrhages in 4/24 fetuses (16.6 %) and MRI in 5/24 fetuses (20.9%) (Fig. 4). MRI and ultrasound data completely coincided in the diagnosis of fetal growth restriction in all 24 fetuses (100%), including the severity of FGR using percentile nomograms. The diagnosis of fetal growth restriction combined with congenital diaphragmatic hernia also coincided using both techniques in 3/24 fetuses (12.5%), spina bifida posterior with meningomyelocele in 1/24 fetus (4,2%), and ventriculomegaly in 8/24 fetuses (33.3%).
To find the differences between US and MRI data with regard to the measured parameters of sluggish fetal head growth using percentile nomograms, indicators of the variation series were calculated, that demonstrated the absence of significant differences between both methods (Table 2).
Discussion
Our study showed a high role of ultrasound in assessing pathological changes, such as reduced fetal brain volume in FGR and in evaluation of fetal brain pathology. Fetal ultrasound is a traditional ultrasound technique, which is most widely used in monitoring of high-risk pregnancies. However, the accuracy of US in predicting perinatal and neonatal outcomes continues to be debated [19–21]. For example, a scoring system was proposed for assessment of the neurological status of the fetus by 4D sonography [22]. Nevertheless, this method remains unconfirmed in large studies, that currently limits its widespread use. Our study demonstrates the necessity of using MRI as an additional diagnostic test for comprehensive evaluation of the fetal brain, since MRI is the most accurate, non-invasive imaging technique enabling to evaluate not only fetal brain volume, but also pathological changes in the fetal brain. MRI of the fetal brain is very important in early detection of central nervous system injuries in fetuses in high-risk pregnancies. Fetal MRI can be technically challenging test, since obtaining fetal brain MR images of high diagnostic quality depends on transabdominal intrauterine environment and fetal movements [23]. There are also a number of difficulties arising from insufficient experience of radiologists and laboratory technicians in examining the fetus. Due to this, it is best to perform MRI of the fetal brain at the medical center by well-trained radiologists, who are experts in this field. Currently, MRI of the fetal brain in fetal growth restriction is used preferentially to exclude significant brain abnormalities and assess hypoxic-ischemic brain injury associated with FGR. Fetal MRI provides detailed images for assessment of the developing brain at high-risk pathology, including fetal growth restriction, with the possibility to correlate fetal brain structural abnormalities with later neurodevelopmental outcomes [24]. MRI of the fetal brain is also most informative in fetuses with FGR for detecting malformations the cerebral cortex and sulci of the cerebral hemispheres [25], that is very important in predicting long-term neurological outcomes for the infants born with this pathology [26]. In addition, MRI data confirm that the size of the corpus callosum in fetuses with FGR is significantly smaller than the normal size, that also correlates with adverse neurological outcomes in newborns [27]. In our study, there were differences between final diagnoses in a number of cases according to ultrasound and MRI data for a number of brain pathologies. In particular, MRI-based assessement of the corpus callosum and cortical plasticity, as well as various hypoxic-ischemic brain injuries, was more accurate. This may be associated both with the technical characteristics of implementing ultrasound and the subjectivity in data interpretation, low feasibility of ultrasound examination of the fetal brain in conditions of unstable lie, transverse position and breech presentation. At the same time, no differences were found between the diagnostic value of MRI and ultrasound in confirmation of the diagnosis of FGR and assessment of the brain size using percentile nomograms.
Conclusion
Comparison of US and MRI data showed that both diagnostic techniques are comparable in assessment of the fetal brain size using percentile nomograms. It was found that MRI is a complimentary tool added to ultrasound and is very useful in the diagnosis of concomitant brain pathology, especially in detection of malformations of the cortical plate and sulci of the cerebral hemispheres, pathology of the corpus callosum, differential diagnosis of ischemic brain injury, that significantly changes patient-management strategies due to worsening of neonatal prognosis.
References
- Ananth C.V., Friedman A.M. Ischemic placental disease and risks of perinatal mortality and morbidity and neurodevelopmental outcomes. Semin. Perinatol. 2014; 38(3): 151-8. https://dx.doi.org/10.1053/j.semperi.2014.03.007.
- Большакова А.С., Барков И.Ю., Франкевич Н.А., Ярыгина Т.А., Шмаков Р.Г. Задержка роста плода при редком сочетании хромосомного и моногенного заболеваний. Акушерство и гинекология. 2024; 1: 74-81. [Bolshakova A.S., Barkov I.Yu., Frankevich N.A., Yarygina T.A., Shmakov R.G. Fetal growth restriction in the rare co-occurrence of chromosomal and monogenic diseases. Obstetrics and Gynecology. 2024; (1): 74-81. (in Russian)]. https://dx.doi.org/10.18565/aig.2023.288.
- Нефтерева А.А., Сакало В.А, Гладкова К.А., Костюков К.В., Ходжаева З.С. Возможные молекулярно-биологические механизмы формирования синдрома селективной задержки роста плода при монохориальной беременности. Акушерство и гинекология. 2021; 10: 5-12. [Neftereva A.A., Sakalo V.A., Gladkova K.A., Kоstyukov K.V., Khodzhaeva Z.S. Possible molecular and biological mechanisms for the development of selective fetal growth restriction in monochorionic twin pregnancy. Obstetrics and Gynecology. 2021; (10): 5-12. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.10.5-12.
- Resnik R. Intrauterine growth restriction. Obstet. Gynecol. 2002; 99(3): 490-6. https://dx.doi.org/10.1016/s0029-7844(01)01780-x.
- Cetin I., Alvino G. Intrauterine growth restriction: implications for placental metabolism and transport. A review. Placenta. 2009; 30(Suppl. A):S77-S82. https://dx.doi.org/10.1016/j.placenta.2008.12.006.
- Geva R., Eshel R., Leitner Y., Valevski A.F., Harel S. Neuropsychological outcome of children with intrauterine growth restriction: a 9-year prospective study. Pediatrics. 2006; 118(1): 91-100. https://dx.doi.org/10.1542/peds.2005-2343.
- Miller S.L., Huppi P.S., Mallard C. The consequences of fetal growth restriction on brain structure and neurodevelopmental outcome. J. Physiol. 2016; 594(4): 807-23. https://dx.doi.org/10.1113/JP271402.
- Dubois J., Benders M., Borradori-Tolsa C., Cachia A., Lazeyras F., Ha-Vinh Leuchter R. et al. Primary cortical folding in the human newborn: an early marker of later functional development. Brain. 2008; 131(Pt. 8): 2028-41. https://dx.doi.org/10.1093/brain/awn137.
- Tolcos M., Bateman E., O'Dowd R., Markwick R., Vrijsen K., Rehn A. et al. Intrauterine growth restriction affects the maturation of myelin. Exp. Neurol. 2011; 232(1): 53-65. https://dx.doi.org/10.1016/j.expneurol.2011.08.002.
- Nitsos I., Rees S. The effects of intrauterine growth retardation on the development of neuroglia in fetal guinea pigs. An immunohistochemical and an ultrastructural study. Int. J. Dev. Neurosci. 1990; 8(3): 233-44. https://dx.doi.org/10.1016/0736-5748(90)90029-2.
- Olivier P., Baud O., Bouslama M., Evrard P., Gressens P., Verney C. Moderate growth restriction: deleterious and protective effects on white matter damage. Neurobiol. Dis. 2007; 26(1): 253-63. https://dx.doi.org/10.1016/j.nbd.2007.01.001.
- Fischi-Gomez E., Muñoz-Moreno E., Vasung L., Griffa A., Borradori-Tolsa C., Monnier M. et al. Brain network characterization of high-risk preterm-born school-age children. Neuroimage Clin. 2016; 11: 195-209. https://dx.doi.org/10.1016/j.nicl.2016.02.001.
- Eixarch E., Muñoz-Moreno E., Bargallo N., Batalle D., Gratacos E. Motor and cortico-striatal-thalamic connectivity alterations in intrauterine growth restriction. Am. J. Obstet. Gynecol. 2016; 214(6): 725.e1-e9. https://dx.doi.org/10.1016/j.ajog.2015.12.028.
- Волочаева М.В., Кан Н.Е., Тютюнник В.Л., Гасымова Ш.Р., Борисова А.Г. Особенности течения беременности и состояния здоровья новорожденных при задержке роста плода. Медицинский Совет. 2023; 13: 200-5. [Volochaeva M.V., Kan N.E., Tyutyunnik V.L., Gasymova Sh.R., Borisova A.G. Features of the course of pregnancy and health of newborns with intrauterine growth restriction. Medical Council. 2023; (13): 200-5. (in Russian)]. https://dx.doi.org/10.21518/ms2023-173.
- Baschat A.A. Neurodevelopment after fetal growth restriction. Fetal Diagn. Ther. 2014; 36(2): 136-42. https://dx.doi.org/10.1159/000353631.
- Longo S., Bollani L., Decembrino L., Di Comite A., Angelini M., Stronati M. Short-term and long-term sequelae in intrauterine growth retardation (IUGR). J. Matern. Fetal Neonatal Med. 2013; 26(3): 222-5. https://dx.doi.org/10.3109/14767058.2012.715006.
- Malhotra A., Ditchfield M., Fahey M.C., Castillo-Melendez M., Allison B.J., Polglase G.R. et al. Detection and assessment of brain injury in the growth-restricted fetus and neonate. Pediatr. Res. 2017; 82(2): 184-93. https://dx.doi.org/10.1038/pr.2017.37.
- Ганичкина М.Б., Мантрова Д.А., Кан Н.Е., Тютюнник В.Л., Хачатурян А.А., Зиганшина М.М. Ведение беременности при задержке роста плода. Акушерство и гинекология. 2017; 10: 5-11. [Ganichkina M.B., Mantrova D.A., Kan N.E., Tyutyunnik V.L., Khachaturyan A.A., Ziganshina M.M. Pregnancy management complicated by intrauterine growth restriction. Obstetrics and Gynecology. 2017; (10): 5-11. (in Russian)]. https://dx.doi.org/10.18565/aig.2017.10.5-11.
- Figueras F., Gardosi J. Intrauterine growth restriction: new concepts in antenatal surveillance, diagnosis, and management. Am. J. Obstet. Gynecol. 2011; 204(4): 288-300. https://dx.doi.org/10.1016/j.ajog.2010.08.055.
- Ярыгина Т.А., Гус А.И. Задержка (замедление) роста плода: все, что необходимо знать практикующему врачу. Акушерство и гинекология. 2020; 12: 14-24. [Yarygina T.A., Gus A.I. Fetal growth restriction (retardation): everything the practitioner should know. Obstetrics and Gynecology. 2020; (12): 14-24. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.12.14-24.
- Гуменюк Е.Г., Ившин А.А., Болдина Ю.С. Поиск предикторов задержки роста плода: от сантиметровой ленты до искусcтвенного интеллекта. Акушерство и гинекология. 2022; 12: 18-24. [Gumeniuk Е.G., Ivshin A.A., Boldina Yu.S. Search for the predictors of fetal growth restriction: from a measuring tape to artificial intellect. Obstetrics and Gynecology. 2022; (12): 18-24. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.185.
- Kurjak A., Predojevic M., Stanojevic M., Kadic A.S., Miskovic B., Badreldeen A. et al. Intrauterine growth restriction and cerebral palsy. Acta Inform. Med. 2012; 18(2): 64-82. https://dx.doi.org/10.5455/aim.2010.18.64-82.
- Damodaram M.S., Story L., Eixarch E., Patkee P., Patel A, Kumar S., Rutherford M. Foetal volumetry using magnetic resonance imaging in intrauterine growth restriction. Early Hum. Dev. 2012; 88 Suppl 1: S35-40. https://dx.doi.org/10.1016/j.earlhumdev.2011.12.026.
- Banović V., Škrablin S., Banović M., Radoš M., Gverić-Ahmetašević S., Babić I. Fetal brain magnetic resonance imaging and long-term neurodevelopmental impairment. Int. J. Gynaecol. Obstet. 2014; 125(3): 237-40. https://dx.doi.org/10.1016/j.ijgo.2013.12.007.
- Egaña-Ugrinovic G., Sanz-Cortes M., Figueras F., Bargalló N., Gratacós E. Differences in cortical development assessed by fetal MRI in late-onset intrauterine growth restriction. Am. J. Obstet. Gynecol. 2013; 209(2): 126.e1-e8. https://dx.doi.org/10.1016/j.ajog.2013.04.008.
- Hüppi P.S. Cortical development in the fetus and the newborn: advanced MR techniques. Top. Magn. Reson. Imaging. 2011; 22(1): 33-8. https://dx.doi.org/10.1097/RMR.0b013e3182416f78.
- Egaña-Ugrinovic G., Sanz-Cortés M., Couve-Pérez C., Figueras F., Gratacós E. Corpus callosum differences assessed by fetal MRI in late-onset intrauterine growth restriction and its association with neurobehavior. Prenat. Diagn. 2014; 34(9): 843-9. https://dx.doi.org/10.1002/pd.4381.
Received 20.12.2023
Accepted 04.04.2024
About the Authors
Polina V. Kulabukhova, Radiologist, Radiology Department, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Oparina str., 4, +7(916)618-88-97, kulpola@mail.ru, https://orcid.org/0000-0002-0363-3669Vladimir G. Bychenko, PhD, Radiologist, Head of the Department of Radiation Diagnostics, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
117997, Russia, Moscow, Oparina str., 4, v_bychenko@oparina4.ru, https://orcid.org/0000-0002-1459-4124
Roman G. Shmakov, Dr. Med. Sci., Professor of the RAS, Non-staff Chief Specialist in Obstetrics at Ministry of Health of Russia; Director of GBUZ MO MONIIAG, 101000, Russia, Moscow, Pokrovka str., 22А, https://orcid.org/0000-0002-2206-1002



