Embryo tranfer at zygote stage in patients with failed embryonic development in previous in vitro fertilization programs
Objective: Assessment of the effectiveness of embyo transfer into uterine cavity at the zygote stage in patients with multiple IVF/ICSI failures in history and the absence of embryonic cleavage cultured in vitro.Nazarenko T.A., Martirosyan Ya.O., Krasnova V.G., Biryukova A.M., Sokolova Yu.V.
Materials and methods: The study included 37 patients. The mean age of patients was 38.3 (3.3) лет, and infertility duration was 6.1 (2.3) years. Idiopathic infertility was a major nosologic form of infertility. Independently of the chosen ovarian stimulation protocol and the used type of gonadotropins, the absence of embryos that could be offered for transfer and stoppage of embryonic development took place in all attempts. In 37 women in the study group, embryo transfer into the uterine cavity was performed on day 1 after transvaginal ovarian puncture, immediately after fertilization was confirmed.
Results: The average number of IVF/ICSI attempts in history of the patients included in the study was 4.2 (1.6). The retrospective analysis confirmed that the parameters of early embryo development was impaired in all patients in the study group. Pregnancy rate in the group of women below 40 years was 45.0% (9/20), and 5.8% (1/17) in the group of patients of late reproductive age.
Conclusion: It is supposed that in vitro embryo culture may be one of the cause of impaired embryo development and early embryonic developmental arrest. Due to this, embryo transfer strategy into the uterine cavity at zygote stage, can influence physiological preimplantation development and as a result lead to the onset of pregnancy in difficult clinical situations, when embryos stop developing. This method can be effective for the patients of young age, who mainly have euploid oocytes.
Keywords
For the recent decades, a continuous progress in the development of reproductive medicine leading to improvement of assisted reproductive techniques have been observed. Despite this, according to the world statistics, the pregnancy rate has reached a plateau and has not changed dramatically over the past decade [1, 2]. In this connection, the attention of specialists is currently focused on searching the causes of repeated in vitro fertilization (IVF) failures and the absence of implantation, when good quality embryos are transferred [3]. Another no less important and extremely interesting aspect is the phenomenon of early embryo developmental arrest that implies that embryo stops developing at early stages, or the production of good quality blastocysts is insufficient. It has been proven that the blastocyst formation frequency, which should be at least 60%, is a key indicator of the effectiveness of IVF programs [4].
It is known, that about 40% of embryos stop developing at various stages during preimplantation period [5]. An embryo that stops developing is characterized by the absence of signs of mitotic cell division for at least 24 hours, as well as degeneration and lysis.
Depending on the developmental stage, at which the embyo stops developing, the following is observed: no cleavage after the zygote stage, cleavage stops on day 2–3, when it is supposed that embryo is between 2–8 cell stage, failure of morula compaction or formation of blastocyst [6]. The researchers offered different theories trying to explain the mechanisms of impairment of preimplantation embryonic development [7–9]. One of the best-known theories considers the “arrest” as a protective mechanism to prevent further development of the poor quality embryos [7, 8]. Another hypothesis suggests that in addition to developmental arrest, which reflects a predetermined internal scenario of natural selection, when poor quality embryos are rejected, it is necessary to take into account a certain role of in vitro embryo culture under the conditions that are different from natural [9].
Based on the hypothesis that a possible cause of impairement of embryonic development can be embryo culture conditions, which undoubtedly differ from natural ones, this study attempted to eliminate the stage of embryo development outside the body and transfer the embryo into the uterine cavity at the zygote stage, i.e. immediately after fertilization was confirmed. This decision was taken empirically for those patients who had similar situation in multiple (3 or more) failed IVF attempts, namely, embryos stopped developing on day 3 of embryo culture. Generally, in these cases the doctor may offer the use of donor oocytes, and this is a hard decision for young patients. This article describes the experience of using the technique of zygote-stage embryo transfer in 37 patients with embryo developmental arrest in the previous IVF attempts.
The aim of the study was assessment of the effectiveness of embyo transfer into uterine cavity at the zygote stage in patients with impairment of embryonic development in multiple IVF attempts.
Materials and methods
The prospective study included 37 couples, who underwent fertility treatment using IVF/ICSI in the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia in the period from January to December, 2021.
The patients included in the study had at least 3 failed IVF/ICSI attempts in history, which were characterized by embryo developmental arrest at cleavage stage on days 2–3 of embryo culture.
The inclusion criteria were: normal caryotype of the partners/spouses; obtaining of at least 5 cumulus oocyte complexes (COC) after transvaginal follicular puncture; absence of severe form of pathozoospermia in the partners and high percentage of sperm DNA fragmentation; at least 3 failed IVF attempts in history.
Taking into account the negative outcomes in pevious attempts, the patients were offered embryo transfer into the uterine cavity at the zygote stage. All couples have signed informed concent to participate in the study. The patients underwent controlled ovarian stimulation according to the standard protocol with gonadotropin releasing hormone (GnRH) antagonists. Follitropin alfa and human menopausal gonadotropin were both used as follicle growth inducers. The starting dose of gonadotropins was calculated depending on the patient’s age, body mass index and ovarian reserve. During ovarian stimulation, ultrasound guided assessment of the number of antral, growing and preovulatory follicles was performed. Transvaginal puncture was performed 36 hours after human chorionic gonadotropin (hCG) injection with a doze of 10000 IU. Cumulus oocyte complexes were detected in aspirated follicular fluid.
Manipulations were carried out at stable temperature (+37.0°С). The COCs isolated from follicular fluid and plasma were washed and placed in sterile dishes (Nunc, Denmark) with culture media G-TL Vitrolife (Vitrolife, Sweden) for 2–3 hours for preliminary incubation at temperature +37.0°С and in atmosphere of 6% СО2. Further, the enzyme hyaluronidase (CooperSurgical, Denmark) was used for oocytes denudation from cumulus cells for 20 sec, and the oocytes were washed in G-TL medium (Vitrolife, Sweden) for subsequent fertilization procedure. After fertilization, the oocytes were placed in culture medium G-TL (Vitrolife, Sweden) for further culture. Fertilization assessment was performed 14–16 hours after fertilization. The result of assessment was visualization of two pronuclei (zygote formation). Fertilization was considered a failure in the absence of two pronuclei. All stages of cell culture in 25 µl drops were carried out in K-Systems multi gas incubators (Kivex Biotec LTD., Denmark) under a layer of oil (Vitrolife, Sweden).
Zygote quality and pronuclear morphology were assessed according to zygote-grading system of Scott et al. [10] taking into account the following parameters: alignment; the size of pronuclei; calculation of nucleolar spread in pronuclei; cytoplasm homogeneity.
The method included examination of zygoes 16–18 hours after insemination and 24–26 hours after insemination.
Embryo transfer, assessment criteria for the effectiveness of transfer (the endpoints of the study)
Embryo transfer into the uterine cavity was performed on day 1, when the presence of two pronuclei in the zygote were confirmed using Wallace soft catheter (Germany) or Cook catheter (Australia). One or two zygotes of the best morphological quality (Z1 according to zygote scoring system of Scott et al.) [10] were transferred. Vaginal administration of micronized progesterone at a dosage of 600 mg per day was used as luteal support following induced transfer cycle. The biochemical pregnancy rate was determined based on serum concentration of ß-hCG subunit (ß-hCG), when ß-hCG level was greater than 20 IU/L). The clinical pregnancy rate was based on visualization of gestational sack by transvaginal ultrasonography 21 days after embryo transfer.
Statistical analysis
The results were processed using Microsoft Excel 2010 and SPSS V22.0 statistical tools for data analysis. To determine the type of data distibution before the comparative analysis of quantitative data in the study groups, the Kholmogorov–Smirnov test or Shapiro–Wilk test was used depending on the sample size. The arithmetic mean (M) and standard deviation (SD) was used to describe the quantitative parameters with normal distribution. Distirbution of qualitative parameters that differed from normal distribution was measured as median (Me) and interquartile range Me (Q1; Q3). The qualitative parameters are shown in absolute and relative values (%). Parametric statistics (t-test for independent variables) was used to assess the differences between the groups for the normal distribution. For data distribution that differed from the normal distribution, non-parametric statisctics were used (Mann–Whitney U-test for independent variables). All differences were considered statistically significant at p<0.05.
Results
The clinical and laboratory data of the patients included in the study are shown in Table 1. The study included 37 patients. The mean age of women was 38.3 (3.3) years. Taking into account a negative impact of patients’ age on the outcomes of IVF programs, the data of women of late reproductive age – over 40 years – were considered separately. Thus, the mean age of 20 patients was 36.2 (2.4) years, and the mean age of 17 women was 41.8 (1.1) years. The vast majority of patients (35/37) were diagnosed with primary infertility with duration of 6.1 (2.3) years.
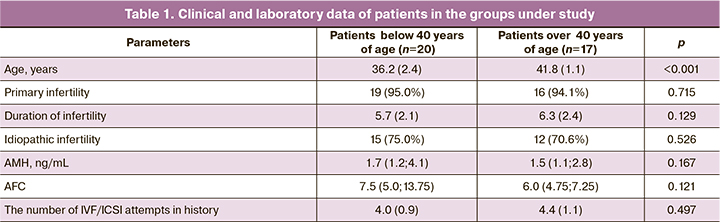
The average number of IVF/ICSI attempts in history was 4.2 (1.6). Ovarian reserve in most women (92%) was evaluated as normal: anti-Müllerian hormone (AMH) level was 1.7 (1.2; 4.1) ng/mL in women of younger age and 1,5 (1.1; 2.8) ng/mL in women of the older age, the average antral follicle counts (AFC) were 7.5 (5.0; 13.75) and 6.0 (4.75; 7.25), respectively. Analysis of reproductive function conditions and the causes of infertility showed that 73% (27/37) of women had idiopathic infertility.
The previous IVF attempts in the group of patients under study (n=37) were assessed. The average number of IVF/ICSI attempts per 1 patient in this group was 4.2 (1.0). All previous attempts were characterized by moderate ovarian response to stimulation with optimal number of obtained COC – 10.2 (3.2) per 1 puncture. It should be noted, that ovarian stimulation in the previous attampts was performed using different protocols. From the submitted documents, it was found that fertilization rate was 66.3% (615/928) and it was consistent with the normal values in IVF programs. Moreover, impairment of embryoninc development at early stages of cleavage was in all patients.
Given the fact that the notion of embryo developmental arrest is heterogenous, it was interesting to analyze the previous embryological data and detect at which stages embryos stopped developing. In the vast majority of patients (30/37) the development stopped on days 2–3 of embryo culture, in other women (7/37) on day 3 of embryo culture the presence of 6–8 embryonic cells was observed, which prognostically had a potential for further development and blastocyst formation. However, on day five of embryo cultrure there were no blastocysts. The results of the retrospective analysis confirmed that the parameters of early embryogenesis were impaired in all patients in the study group.
The characteristics of ovulation induction cycle and embryonic stage in IVF/ICSI programs in patients in the study groups is presented in Tables 2 and 3.
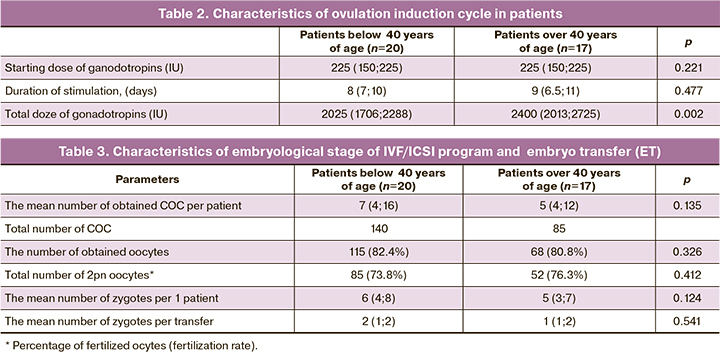
The data in Table 2 show that despite the seemingly increased gonadotropins dose during the course of treatment and the duration of stimulation, these parameters did not reach significant differences in older patients versus younger women. This suggested potential perspective of performing IFV programs in women of older reproductive age participating in the study.
The results of embryonic stage confirmed that the age of patients have an impact on the effectiveness of IVF programs. In women of older reproductive age, the number of COC and mature oocytes was significantly lower, fertilization rate was lower, and respectively, the number of obtained zygotes was lower verus younger patients.
In 20 patients in group 1, the total number of transferred zygotes was 36, on average, 1.8 (1; 2) per patient. The embryos from 14 patients underwent further culture. It is important, that among cultured embryos, developmental arrest was registered in 81.8% (11/14) of embryos on day 3 of culture, in neither case the blastocysts were obtained. This fact is consistent with the data in previous IVF attempts. 52 zygotes were obtained from 17 patients over 40 year of age, and each of them underwent embryo transfer on day 1 of culture. The remaining 18 embryos for further culture stopped developing.
14 days after embryo transfer, positive ß-hCG levels were in 14 patients in group 1 and in 4 patiens of late reproductive age. In patients in group 1, the biochemical pregnancy and anembryonic pregnancy rates were 27.3% (3/14) and 9.1% (2/14), respectively. Biochemical pregnancy was in one patient of late reproductive age, and anembryonic pregnancy was in 2 patients of 4. Progressive intrauterine pregnancy was detected in 10 patients, and reached 45.0% (9/20) in 9 women of young age, and reached 5.8% (1/17) in 1 woman in the group of late reproductive age; OR=7.65 (95% CI 1.08–54.43), p= 0.009.
Discussion
IVF/ICSI programs and embryo transfer were performed in accordance with recommendations by Edwards R.G. et al. (1980) to transfer embryos into the uterine cavity at the cleavage stage [11]. Zygote transfer was performed by some specialists, but the results were conflicting in the general population of patients. Some authors reported no difference between the implantation and pregnancy rates in embryo transfer on day 1 and 2/3 of culture [11–15]. According to other data, pregnancy rate was significantly lower after embryo transfer at the zygote stage versus cleavage-stage transfer [16–17]. Sermondade N. et al. (2012) reported that the strategy of embryo transfer into the uterine cavity at the zygote stage was used for the patients with multiple IVF/ICSI failures in history, and with a high degree of embryo fragmentation in previous attempts. Pregnancy rate in patients in the main group was 26.4% [18].
The obtained clinical results induced us to think over the characteristics that are inherent to embryonic cleavage. The quality of the obtained embryo is determined not only by euploidy, which is detected by molecular genetic testing, but also by the impact of epigenetic factors and embryo culture conditions.
The embryos at early stages of preimplantation development demonstrate flexibility and capability to adapt to environmental factors. Howerever, some authors noted that these adaptive mechanisms can lead to developmental arrest or significantly decreased quality of embryos [19, 20]. The absence of embryo cleavage, the so-called “arrest” of early embryogenesis, is a rare phenomenon in the clinical practice, but it is quite dramatic for the patients who have to use donor oocytes.
In this study we made an attempt to overcome this phenomenon by transferring embryos at the zygote stage. The first positive experience, i.e. achieving pregnancy in 10/37 women who underwent IVF treatment, forces to think over possible causes of embryo developmental arrest in IVF programs. It is interesting to note that pregnancy rate was high in women of younger age with embryo transfer at the zygote stage; and there was no significant increase in the rate of pregnancy among the patients of late reproductive age. The obtained results suggest that in young women mostly having euploid embryos, epigenetic factors can play a significant role in formation of early embryonic arrest, and it is not unlikely that these factors are associated with in vitro culture conditions. Despite the fact, that one of the major steps of IVF program is in vitro embryo culture during 5 days, possible effects of culture media and culture conditions on embryo development is still not fully explored. Moreover, multiple recent studies demonstrated that the process of embryo culture causes significant changes in gene expression, and epigenic changes occur during transfer from the zygote stage to blastocyst stage [21, 22]. At later stages of development, these changes may have effect on embryo implantation capability, fetal development and offspring health [19].
Most studies analyzing the influence of different culture conditions on embryo development focused on oxygen concentration, as this can disrupt the intracellular redox balance of embryo [23]. Undoubtedly, the choice of the appropriate culture medium is of crucial importance for optimizing embryo developmental capability, but the issue of “ideal” culture medium remains unsettled [24].
It should be noted that married couples of young age included in the study, had no tangible causes of infertility, i.e. natural pregnancy did not occur for some reasons, despite the presence of ovulatory cycle and patency of fallopian tubes in women and healthy sperm in spouses. Nevertheless, the success achieved due to zygote-stage transfer in IVF programs, forced to intensify research effors to study the factors affecting in vitro embryo development. Therefore, this will help to solve the problem of unexplained infertility. With regard to women of older age, the causes of impairment of embryoninc development at early stages are more obvious. It is known that in women after 40 years of age, up to 90% of oocytes are aneuploidy, and most likely, this causes impairment of embryonic development of obtained embryos.
Conclusion
The occurrence of pregnancy in rather complex clinical cases of repeated embryo developmental arrest during IVF programs in young women demonstrates the need for further reseach to study a number of factors that are of crucial importance in the process of early embryogenesis, and to accumulate the clinical experience confirming advisability of using this method.
References
- Khalife D., Nassar A., Khalil A., Awwad J., Abu Musa A., Hannoun A. et al. Cumulative live-birth rates by maternal age after one or multiple In Vitro fertilization cycles: An institutional experience. Int. J. Fertil. Steril. 2020; 14(1): 34-40. https://dx.doi.org/10.22074/ijfs.2020.5855.
- Leijdekkers J.A., Eijkemans M.J.C., van Tilborg T.C., Oudshoorn S.C., van Golde R.J.T., Hoek A. et al.; OPTIMIST Study Group. Cumulative live birth rates in low-prognosis women. Hum. Reprod. 2019; 34(6): 1030-41. https://dx.doi.org/10.1093/humrep/dez051.
- Busnelli A., Somigliana E., Cirillo F., Baggiani A., Levi-Setti P.E. Efficacy of therapies and interventions for repeated embryo implantation failure: a systematic review and meta-analysis. Sci. Rep. 2021; 11(1): 1747. https://dx.doi.org/10.1038/s41598-021-81439-6.
- ESHRE Special Interest Group of Embryology and Alpha Scientists in Reproductive Medicine. The Vienna consensus: report of an expert meeting on the development of ART laboratory performance indicators. Reprod. Biomed. Online. 2017; 35(5): 494-510. https://dx.doi.org/10.1016/j.rbmo.2017.06.015.
- Mohebi M., Ghafouri-Fard S. Embryo developmental arrest: review of genetic factors and pathways. Gene Rep. 2019; 17: 100479. https://dx.doi.org/10.1016/j.genrep.2019.100479.
- ESHRE Special Interest Group of Embryology; Alpha Scientists in Reproductive Medicine. The Vienna consensus: report of an expert meeting on the development of art laboratory performance indicators. Hum. Reprod. Open. 2017; 2017(2): hox011. https://dx.doi.org/10.1093/hropen/hox011.
- Betts D.H., Madan P. Permanent embryo arrest: molecular and cellular concepts. Mol. Hum. Reprod. 2008; 14(8): 445-53. https://dx.doi.org/10.1093/molehr/gan035.
- Xu Y., Qian Y., Liu Y., Wang Q., Wang R., Zhou Y. et al. A novel homozygous variant in NLRP5 is associate with human early embryonic arrest in a consanguineous Chinese family. Clin. Genet. 2020; 98: 69-73. https://dx.doi.org/10.1111/cge.13744.
- Cívico S., Agell N., Hernández L., Campo E., Bachs O., Balasch J. Increased messenger ribonucleic acid expression of the cyclin-dependent kinase inhibitor P27Kip1 in cleavage-stage human embryos exhibiting developmental arrest. Fertil. Steril. 2008; 89(5, Suppl.): 1557-62. https://dx.doi.org/10.1016/j.fertnstert.2007.06.003.
- Scott L., Alvero R., Leondires M., Miller B. The morphology of human pronuclear embryos is positively related to blastocyst development and implantation. Hum. Reprod. 2000; 15(11): 2394-403. https://dx.doi.org/10.1093/humrep/15.11.2394.
- Edwards R.G., Steptoe P.C., Purdy J.M. Establishing full-term human pregnancies using cleaving embryos grown in vitro. Br. J. Obstet. Gynaecol. 1980; 87(9): 737-56. https://dx.doi.org/10.1111/j.1471-0528.1980.tb04610.x.
- Dale B., Fiorentino A., de Simone M.L., di Matteo L., di Frega A.S., Wilding M. et al. Zygote versus embryo transfer: a prospective randomized multicenter trial. J. Assist. Reprod. Genet. 2002, 19(10): 456-61. https://dx.doi.org/10.1023/A:1020354318164.
- Quinn P., Stone B.A., Marrs R.P. Suboptimal laboratory conditions can affect pregnancy outcome after embryo transfer on day 1 or 2 after insemination in vitro. Fertil. Steril. 1990; 53(1): 168-70. https://dx.doi.org/10.1016/S0015-0282(16)53236-1.
- Scott L.A., Smith S. The successful use of pronuclear embryo transfers the day following oocyte retrieval. Hum. Reprod. 1998; 13(4): 1003-13. https://dx.doi.org/10.1093/humrep/13.4.1003.
- Ahuja K., Smith W., Tucker M., Craft I. Successful pregnancies from the transfer of pronucleate embryo in an outpatient in vitro fertilization program. Fertil. Steril. 1985; 44(2): 181-4. https://dx.doi.org/10.1016/S0015-0282(16)48732-7.
- Jaroudi K., Coskun S., Hollanders J., Al-Hassan S., Al-Sufayan H., Atared A., Merdad T. Advanced surgical sperm recovery is a viable option for intracytoplasmic sperm injection in patients with obstructive or nonobstructive azoospermia. Fertil. Steril. 1999; 72(3): 479-83. https://dx.doi.org/10.1016/S0015-0282(99)00298-8.
- Margreiter M., Weghofer A., Kogosowski A., Mahmoud K.Z., Feichtinger W.A. Prospective randomized multicenter study to evaluate the best day for embryo transfer: does the outcome justify prolonged embryo culture? J. Assist. Reprod. Genet. 2003; 20(2): 91-4. https://dx.doi.org/10.1023/A:1021744209193.
- Sermondade N., Delarouziere V., Ravel C., Berthaut I., Verstraete L., Mathieu E. et al. Characterization of a recurrent poor-quality embryo morphology phenotype and zygote transfer as a rescue strategy. Reprod. Biomed. Online. 2012; 24(4): 403-9. https://dx.doi.org/10.1016/j.rbmo.2012.01.004.
- Barker D.J. The origins of the developmental origins theory. J. Intern. Med. 2007; 261(5): 412-7. https://dx.doi.org/10.1111/j.1365-2796.2007.01809.x.
- Calle A., Miranda A., Fernandez-Gonzalez R., Pericuesta E., Laguna R., Gutierrez-Adan A. Male mice produced by in vitro culture have reduced fertility and transmit organomegaly and glucose intolerance to their male offspring. Biol. Reprod. 2012; 87(2): 34. https://dx.doi.org/10.1095/biolreprod.112.100743.
- Rizos D., Clemente M., Bermejo-Alvarez P., de La Fuente J., Lonergan P., Gutiérrez-Adán A. Consequences of in vitro culture conditions on embryo development and quality. Reprod. Domest. Anim. 2008; 43(Suppl. 4): 44-50. https://dx.doi.org/10.1111/j.1439-0531.2008.01230.x.
- Fauque P., Léandri R., Merlet F., Juillard J.C., Epelboin S., Guibert J. et al. Pregnancy outcome and live birth after IVF and ICSI according to embryo quality. J. Assist. Reprod. Genet. 2007; 24(5): 159-65. https://dx.doi.org/10.1007/s10815-007-9115-z.
- Bavister B. Oxygen concentration and preimplantation development. Reprod. Biomed. Online. 2004; 9(5): 484-6. https://dx.doi.org/10.1016/s1472-6483(10)61630-6.
- Biggers J.D., Summers M.C. Choosing a culture medium: making informed choices. Fertil. Steril. 2008; 90(3): 473-83. https://dx.doi.org/10.1016/j.fertnstert.2008.08.010.
Received 11.05.2022
Accepted 03.06.2022
About the Authors
Tatiana A. Nazarenko, Dr. Med. Sci., Professor, Head of the Scientific and Educational Center for ART with the Clinical Division named after F. Paulsen, Head of the Institute of Reproductive Technologies, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, t.nazarenko@mail.ru, 117997, Russia, Moscow, Ac. Oparin str., 4.Yana O. Martirosyan, Researcher at the Scientific and Educational Center for ART with the Clinical Division named after F. Paulsen, Academician V.I. Kulakov
NMRC for OG&P, Ministry of Health of Russia, +7(925)124-99-99, ya_martirosyan@oparina4.ru, 117997, Russia, Moscow, Ac. Oparin str., 4.
Valeria G. Krasnova, clinical resident, Scientific and Educational Center for ART with the Clinical Division named after F. Paulsen, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(909)915-05-82, lkrasnova27@gmail.com, 117997, Russia, Moscow, Ac. Oparin str., 4.
Almina M. Biryukova, PhD, Head on Clinical Work, Scientific and Educational Center for ART with the Clinical Division named after F. Paulsen,
Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, a_birukova@oparina4.ru, 117997, Russia, Moscow, Ac. Oparin str., 4.
Julia V. Sokolova, embryologist, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)438-13-41, julietsok@gmail.com,
117997, Russia, Moscow, Ac. Oparin str., 4.
Authors’ contributions: Nazarenko T.A., Martirosyan Ya.O., Krasnova V.G., Biryukova A.M., Sokolova Yu.V. – design of the study, obtaining the data for analysis, review and translation of publications on the issue under study, statistical data analysis, writing the text of the article; Nazarenko T.A., Martirosyan Ya.O. – design of the study, analysis of the obtained data, writing and editing the article.
Conflicts of interest: The authors declare that they have no conflicts of interest.
Funding: The study was carried out in the frames of the State Assingment No. 22-A21.
Ethical Approval: The study was approved by the local Ethics Committee of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Nazarenko T.A., Martirosyan Ya.O., Krasnova V.G., Biryukova A.M., Sokolova Yu.V. Embryo transfer at zygote stage in patients with failed embryonic development in previous in vitro fertilization programs.
Akusherstvo i Gynecologia/Obstetrics and Gynecology. 2022; 6: 83-89 (in Russian)
https://dx.doi.org/10.18565/aig.2022.6.83-89



