Features of the cervical canal microbiota in prenatal amniorrhea and full-term pregnancy
Objective. To investigate the composition of the microflora in full-term pregnancy and premature rupture of the membranes (PROM) in comparison with timely amniorrhea.Kaganova M.A., Spiridonova N.V., Kazakova A.V., Devyatova O.O., Galkina D.A., Golovina O.N.
Materials and methods. At the N.I. Pirogov City Clinical Hospital One, 143 pregnant women at 37–41 weeks’ gestation, including 85 women with PROM and 58 control group patients with timely amniorrhea during childbirth, underwent microscopic examination of vaginal smears, bacteriological cultures from the cervical canal for flora and antibiotic sensitivity, as well as real-time PCR of swabs taken from the cervical canal.
Results. In PROM, the scrapes from the cervical canal exhibited a marked predominance of the anaerobic microflora in 5 (22.7%) cases, a preponderance of Lactobacillus spp. and aerobes in one (4.5%) patient and absolute prevalence of Lactobacillus spp. only in 7 (31.8%) cases (p = 0.002). Lactobacillus spp. decreased significantly and Raoultella spp. was more frequently found in the PRPO group (14% versus 1.3% in the study group).
Conclusion. The cervical canal in PROM is characterized by a predominance of anaerobic bacteria, which was diagnosed by real-time PCR diagnosis.
Keywords
The problem of premature rupture of membranes (PROM) in full-term pregnancy does not lose its relevance, despite the large number of studies in this area and the invention of various methods for preparing the cervix and labor induction.
According to various authors, PROM at least an hour before the onset of the labor occurs in 2.7% to 17% of cases [1, 2]. PROM in full-term pregnancy is associated with the high percentage of the labor dystocia, fetal distress, birth injuries, septic complications for both mother and newborn. These PROM complications as well as patient’s refusal of the cervix preparing or its ineffectiveness, increase in labor duration lead to the growing number of cesarean sections among such patients. Usually these are primiparous women. Therefore, the percentage of cesarean sections and maternal and perinatal morbidity may be reduced due to the proper management of patients with PROM in full-term pregnancy.
The association of the infectious factor and PROM has been proved in a large number of scientific works [3-6]. Modern studies demonstrate that inflammatory changes of the membranes due to ascending infection are one of the PROM leading causes [7]. In the rupture of membranes, leukocyte infiltration over the internal os is detected in every third case [8]. Analysis of vaginal and cervical canal microflora in patients with PROM will help to explain the role of microbial factor in the implementation of PROM.
The aim of this research was to investigate the microbiota of the vagina and cervical canal in pregnant patients over 37 weeks gestation and to compare it with the patients’ microbiota in timely rupture of the membranes.
Materials and Methods
This was a prospective study of 143 patients at 37-42 weeks gestation who were investigated in the Maternity Department of the N.I. Pirogov City Hospital One. The main group included 85 women with PROM, and the control group included 58 patients with timely rupture of the membranes (they had a rupture of fetal membranes after the onset of labor). Swabs and bacteriological culture were taken at the moment of the membranes rupture in the main group of the patients, or at the active phase of the first stage of the labor in case of the intact fetal membranes. The investigation included the results of bacteriological analysis of the cervical and vaginal flora carried out by cultivating aerobic and anaerobic microorganisms on the special germ culture media, microscopical examination of the vaginal and cervical smears by Gram’s staining. It also included real-time PCR analysis taken from the cervical canal (Femoflor 16) using DT-96 detecting amplifier produced by DNA-Technology LLC (2009/04663, patent №2362808 dated 13.02.08). Originally the system Femoflor 16 was developed for estimating the condition of the vagina. But we used it for the assessment and comparison of the bacteriological culture of microbial landscape of the cervical canal. This technology provides the analysis of biota of different biotops, and cervical canal is among them [9,10]; the analysis includes the identification of the folloing microorganisms: Lactobacillus spp, Enterobacteriaceae, Streptococcus spp., Staphylococcus spp., Gardnerella vaginalis / Prevotella bivia / Porphyromonas spp., Eubacterium spp., Sneathia spp. / Leptotrihia spp. / Fusobacterium spp, Megasphaera spp. / Veillonella spp. / Dialister spp., Lachnobacterium spp. / Clostridium spp., Mobiluncus spp. / Corynebacterium spp., Peptostreptococcus spp., Atopobium vaginae, Mycoplasma hominis, Ureaplasma (urealyticum + parvum), Candida spp.
A large number of immunological and microbiological investigations were conducted at the Department of Obstetrics and Gynecology of Institute for Continuing Medical Education at Samara State Medical University together with a private medical company INVITRO. Special attention was given to the study of the vaginal biocenosis in different obstetric and gynecological pathologies [11, 12] and at different age periods using microflora screening test systems “Femoflor 8”, “Femoflor 16”, “Femoflor 17” [13, 14]. This technique has proved to be highly sensitive and specific in detecting certain groups of microorganisms.
Only samples with a sufficient number of the cells from the cervical canal in test-tube with the analysed sample and sufficient total bacterial mass (TBM) were used to obtain reliable results. The samples where the number of human DNA cells was greater than 104 genome-equivalent (GE) in the sample were considered, it was the collection material estimation. The TBM should contain at least 106 GE in the sample for conducting the reliable assessment of biocenosis. Total amount of microbiological mass, Lactobacillus spp., different types of opportunistic microorganisms were automatically defined after amplification. Quantitative estimation of cervical microflora was performed using both absolute and relative indices of TBM. Absolute index refers to the DNA number of the revealed microorganism in the sample which is expressed in GE and presented in the form of the decimal logarithm, lg. The relative quantitative index was calculated as a ratio of the quantity of the revealed microorganism and the quantity of TBM. It was presented in two formats: the difference in decimals lg of the amount of the corresponding microorganisms’ group and TBM, and in percentage as regards to TBM.
The amount of epithelial cells, white blood cells, presence of bacilli, cocci or mixed flora, including pathogenic flora such as gonococci, trichomonas and others were assessed in the swab microscopy. According to the ratio of the indices, the results of swab examination were divided to normobiosis, bacterial vaginosis, vaginitis. Bacterial vaginosis was diagnosed due to the increased number of epithelial cells in the smear in the presence of normal or low number of leukocytes, pH > 4.5. The diagnosis of vaginitis was evidenced by a large number of leukocytes, cocci, flora with cocci and bacilli, identification of pathogenic microorganisms.
Statistical analysis was carried out using program Statistica 10.0, SPSS 13. Median (Me) was used to describe the interval-scale variables, as for interval estimation there were upper (Q1) and low (Q3) quartiles, because these variables do not to follow the law of normal distribution (it was estimated using Shapiro-Wilk test). The quantitative variables included natural logarithm of different species of microorganisms in the analysis of real-time PCR. The remaining features referred to categorial binary variables (yes/no), frequencies were given in absolute numbers and percentages. The analysis of categorical variables was carried out using crosstabulation tables, using the χ2 test or Fisher’s exact test. Differences were considered statistically significant at p <0.05.
Results and Discussion
Swab microscopy is a standard examination of pregnant women and women in labor at admission to the department. According to the estimation criteria of the swab, the pattern of the vaginal microflora was distributed the following way: normobiosis was revealed in 38 patients (52.05%) of 73 in the main group, and in 35 patients (62.1%) of 58 in the control group. Biocenosis disturbances were observed as follows: bacterial vaginosis was in 12 women (16.4%) of main group and in 12 women (20.6%) in the control group, vaginitis was in 12 patients (20.6%) and 10 patients (17.2%) , respectively, but differences were statistically not significant (χ2 =3,5; d.f.=2, р=0.17).
The analysis of the bacteriological studies of the cervical secretions revealed a paradoxical result: microorganisms did not grow on the standard media in 40 cases (49.4%) of 81 patients in the main group and in 29 cases (50%) of 58 women in the control group, so there were no significant differences in the bacterial content of the cervix according to the results of culturing.
A variety of microbial associations in bacteriological culturing was represented by facultative anaerobes. Monocultures were identified in the main group in 24 pregnant women (29.6%), which were nearly twice as many as in the control group, 10 patients (17.2%). The remaining patients had associations of two, three, four microorganisms in almost equal ratio (χ2 = 6.64; d.f. = 4, p=0.16). The most frequent associations were Enterococcus spp. Staphylococcus spp. Candida spp., E. coli, Klebsiella spp.
Lactobacillus spp. were detected more often in the control group, in every fifth patient; they were also observed in the group with PROM but with a lower frequency (Table 1).
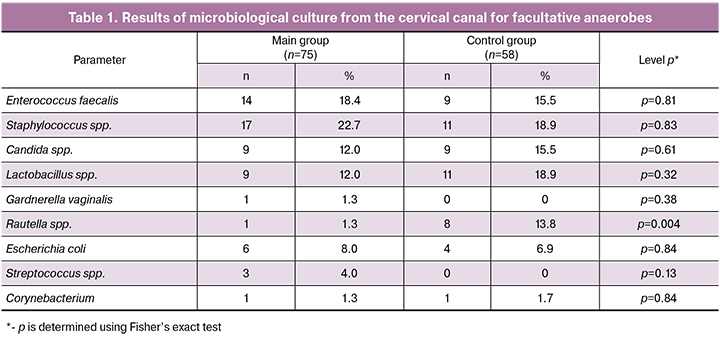
Different types of opportunistic staphylococci (Staphylococcus haemolyticus, Staphylococcus epidermidis, Staphylococcus hominis, Staphylococcus pasteuri) dominated in both groups, all of them were in large numbers, more than 10 6 CFU. Every fifth patient in the main and control groups had Staphylococcus spp. and there were no significant differences in the groups.
Enterococcus faecalis was the second bacterium in frequency: 14 patients (18.4%) in the main group and 9 women (15.5%) in the control group, differences were not statistically significant.
Streptococcus agalactiae from the large family of Streptococcus spp., was revealed only in the main group in 3 patients (4.0%), in the control group it was absent.
The frequency of Escherichia coli’s detection was comparable in both groups: in 6 patients (8.0%) with PROM and in 4 patients (6.9%) with timely rupture of fetal membranes, which corresponded to the data of other studies 2.5-7% [15, 16].
It should be noted that Raoultella ornithinolytica was detected much more frequently in the control group than in the main group, 8 cases (13.8%) versus 1 case (1.3%), respectively, (р=0.004).
We detected Сorynebacterium spp. in single cases in both groups.
The determination of the number of microorganisms from the cervical canal using the method of real-time PCR was performed in 22 pregnant women in the main group and 17patients in the control group. The results are shown in Table 2.

TBM was lower in women of the main group than in ones of the control group, and its median was 6.0 (5.3; 6.5) GE/sample, whereas in the control group it was 7.5 (7.0; 8.1) GE/sample (U=24.5; Z=-4.5; р=0.000), with reliable indicator of collection material estimation. So TBM was sufficient to perform the analysis. According to the literature [9], microbial content of the cervical canal is 10-100 times less than in the vagina (106-109).
The main representatives of the normal vaginal microflora in reproductive-aged healthy women are Lactobacillus spp. Normally, the share of Lactobacillus spp. producing hydrogen peroxide compose from 80% to 100% of all microorganisms (Nugent et al., 1991, Tikhomirov A.L., Oleynik Ch.G., 2004). The absolute amount of Lactobacillus spp. in healthy women does not significantly differ from TBM, so it is nearly 106–108 (Ankirskaya A.S., Muravyova V.V., 2006). The presence of Lactobacillus spp. in the cervical canal can be considered as normal, and in percentage it should prevail over other microorganisms (more than 80%). In our case the absolute amount of Lactobacillus spp. was lower in the main group (median 105.8), than in control - 107.6 (р=0.000). There were no Lactobacillus spp. in two cases (10%) in the main group.
TBM in the main group was 6.0 (5.3; 6.5) which was significantly lower than in the control group – 7.5 (7.0; 8.1). According to the results of our study, four patterns of the cervical canal microflora were obtained using real-time PCR: low TBM (less than 106 ) was in 9 patients (40.9%) in the main group, and in 1 patient (5.9%, р=0.02) in the control group. The significant predominance of anaerobic microflora was observed only in patients of the main group, in 5 cases.
Staphylococcus spp. were most frequently revealed in the control group, in 15 patients (88%), in the main group in 10 patients (45%), p=0.007 (Fisher’s exact test). Despite the fact that in the control group different species of Staphylococcus spp. were identified more often, but in lower absolute quantity and, therefore in the presence of predominance of Lactobacillus spp. these results were considered to be a normal pattern.
The bacteria of the Enterobacteriaceae family were detected twice more often in the control group than in the main group: 8 (47%) versus 4 (18%) pregnant women, respectively, p=0.09 (Fisher’s exact test). As for Streptococcus spp., differences in groups were observed neither in quantity, nor in the frequency of detection.

The patients with PROM did not have predominance of any types of obligate anaerobic microflora either in quantity, for example Gardnerella vaginalis + Prevotella bivia + Porphyromonas spp., or in the frequency of detection, namely 17 (77%) and 104.3 in the main group and 13 (76%) and 104.2 in the control group (р=0.9). Eubacterium spp. were revealed equally often in both groups, 15 (68%) and 11 (64%) cases, respectively (р=0.9), the percentage of remaining microflora was not different. Such anaerobes as Sneathia spp. + Leptotrichia spp.+Fusobacterium spp., Megasphaera spp. + Veillonella spp. + Dialister spp., Lachnobacterium spp. + Clostridium spp., Peptostreptococcus spp. were detected in the main group more often and in more absolute and relative concentration. This fact supports our assumption on the prevalence of anaerobic microflora in patients with PROM. The predominance of Candida spp. was also noted in the main group, 8 patients (36%) in comparison with 3 patients of the control group (17%, р=0.28), but the differences were not statistically significant.
Mycoplasma spp. were distributed in groups as follows: Mycoplasma hominis was detected only in one case in the main group, there was also a higher frequency of occurrence for Ureaplasma (urealyticum + parvum), but differences were not statistically significant (p=0.62). As for Mycoplasma genitalium, which belongs to the absolute pathogens, and the results of the analysis indicate only the presence of this microorganism or its absence without specifying the number of colonies, in our study it was identified only in one case in a patient from the group with PROM.
Discussion
The normal vaginal microflora of a healthy reproductive-aged woman contains gram-positive and gram-negative aerobic, facultative anerobic and obligate anaerobic microorganisms; 95-98% of all microorganisms are represented by Lactobacillus spp. [12]. Mycoplasma hominis at a titer less than 104 CFU/ml, Candida spp. at a titer less than 103 CFU/ml also refer to the normal microflora [17]. The application of the real-time PCR is promising because not only vaginal biotope can be studied, but also the one of the cervical canal. However, the normal indicators for this technique in different biotopes have not been developed yet, especially for the pregnant women. Our studies have shown that TBM in patients with PROM is significantly lower than in the patients with intact fetal membranes, while there is a decrease in the absolute and relative amount of Lactobacillus spp. and the predominance of obligate anaerobes in the cervical canal. Low TBM can be linked to the antimicrobial activity of the amniotic fluid, which consequently leads to the reduction of contamination of the cervical canal bacterial flora, in comparison with the group of patients without rupture of membranes.
According to our research, different types of Staphylococcus spp. in small quantities (103.3-103.9) are the most common agents of the cervical canal biotope. These microorganisms can be found in the normal microflora of the gastrointestinal tract and are present in almost all patients in the control group, unlike the main group. Our studies are confirmed by other scientific works [16, 17]. Changes in the characteristics of the local immunity leads to the intensive reproduction of Staphylococcus spp.
Enterococcus faecalis was the second most common bacterium in the bacteriological culture of the cervical canal. According to the authors Yegorova J.V., Nesterova A.C. [14], its frequency in the biotope of the cervical canal of a healthy woman is 13%; in the presence of inflammatory diseases it increases twice. In our study, Enterococcus faecalis was detected in 14 cases (18.4%) in the main group and 9 cases (15.5%) in the control group; we did not reveal any significant differences in the groups.
Streptococcus spp. were identified in our study in every fifth patient. It is an opportunistic microorganism which is a natural inhabitant of the human body and localized mainly in the intestine, nasopharynx, and vagina. According to the Centers for Disease Control and Prevention (CDC, 2018, Creti et al., 2013), in 30% of cases this type of Streptococcus spp. inhabits the vagina, and this can be considered as a normal condition. Streptococcus spp. result in the development of diseases only under certain negative conditions that weaken the immune system and reduce the overall resistance of the body.
The frequency of detecting Escherichia coli was the same in both groups, it was about 6 (8.0%) at the bacteriological study of the cervical discharge. Our results fully confirmed the data of other studies, namely 2.5-7% [16, 17]. These microorganisms are most frequently associated with the diseases of the urinary tract; there were cases of neonatal sepsis, especially in very early preterm birth. The real-time PCR analysis makes it possible to identify the family of Еnterobacteriaceae, the representatives of which are often observed in the control group, 8 cases (47.5%, р=0.08) versus 4 cases in the main group (18.2%) (p=0.08; Fisher’s exact test).
It should be noted that Raoultella ornithinolytica was observed quite often in the results of microbiological culture in the patients of the control group, 8 cases (13.8%) versus 1 case (1.3%) of the main group, p=0.01. Indirectly, its predominance in the control group can also be confirmed by real-time PCR analysis, microorganisms of the Еnterobacteriaceae family were more frequently identified. Raoultella ornithinolytica is a gram-negative encapsulated aerobic bacillus contained in aquatic environments, fish and insects; it belongs to the Enterobacteriaceae family and includes Raoultella electrica, Raoultella terrigena, Raoultella planticola and Raoultella ornithinolytica. The literature describes cases of bacteremia caused by Raoultella terrigena, Raoultella planticola and Raoultella ornithinolytica, which accounts for about 0.15% of all cases of bacteremia [18]. In 2018, the first case of early neonatal sepsis caused by Raoultella ornithinolytica multiresistant to antibacterial therapy [19] with a fatal outcome was described. As for obstetric complications, it has been proved that Raoultella ornithinolytica may cause septic postpartum complications [20]. A number of researchers tend to associate a low detection of Raoultella spp. in the group with PROM with metabolic features of these microorganisms, which could lead to an increased histamine production of this microorganism. In turn, it caused cervical ripening and the onset of labor until the rupture of the membranes with subsequent change in the quality characteristics of the fetal membranes [21]. For this reason, there is no association of PROM and the presence of Raoultella ornithinolytica in bacteriological culture.
In general, most of the above-mentioned opportunistic microorganisms are included in the list of bacteria associated with aerobic vaginitis (Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, Streptococcus Group B). Numerous studies have demonstrated their relationship with adverse pregnancy outcomes [22, 23], such as preterm labor, PROM, early neonatal sepsis, chorioamnionitis and postpartum metroendometritis.
Significant differences in the frequency of detection in both groups were not found. The second place in the frequency of occurrence is taken by Eubacterium spp., Megasphaera spp. + Veillonella spp. + Dialister spp.; the third most common microorganisms are Mobiluncus spp. + Corynebacterium spp.
The distribution of Atopobium vaginae in groups is of great interest as it is associated with the diagnosis of anaerobic dysbiosis. According to the research data, Atopobium vaginae in the third trimester of pregnancy may occur normally in small amounts, namely 103 [16]. According to other studies, Atopobium vaginae is a marker of the vaginal dysbiosis, especially if the absolute number exceeds 103 [24]. Our data were identical to the results of M.A. Vlasova and co-authors [17], who emphasized the dominant role of anaerobic microorganisms in the structure of vaginal dysbiosis in women with PROM in the period of 26-34 weeks of pregnancy. We have identified the predominant role of anaerobic microorganisms, but only in the cervical canal. According to our data, the frequency of detection of Atopobium vaginae in the cervical canal was almost the same in groups, however, the excess of GE in the sample was observed only in the main group. The detection of Mycoplasma genitalium and Mycoplasma hominins was also characteristic only of the main group, as for Ureaplasma (urealyticum + parvum), it was isolated in both groups in the amount of 103-4 almost in equal proportions.
Conclusion
Thus, according to the results of the studies, patients with PROM more frequently experience vaginal dysbiosis as bacterial vaginosis. TBM of the cervical canal in patients with PROM according to the real-time PCR test is lower than in patients of the control group. We tend to associate this paradox with the antimicrobial action of the amniotic fluid and the mechanical flushing of microorganisms. There was an absolute and relative decrease in the proportion of Lactobacillus spp. and an increase in the absolute and relative number of obligate anaerobic microorganisms, as well as Mycoplasma spp. and Ureaplasma spp.
According to the results of the research, Raoultella ornithinolytica can be identified as a protective factor for PROM, its frequency was significantly lower in the main group.
References
- Князева Т.П. Причины и факторы риска преждевременного разрыва плодных оболочек. Дальневосточный медицинский журнал. 2016; 2: 128-35. [Knyazeva T.P. Causes and risk factors for premature rupture of the membranes. Far Eastern Medical Journal. 2016; 2: 128-35. (in Russian)].
- Huang S., Xia W., Sheng X., Qiu L., Zhang B., Chen T. et al. Maternal lead exposure and premature rupture of membranes: a birth cohort study in China. BMJ Open. 2018; 8(7): e021565. https://dx.doi.org/10.1136/bmjopen-2018-021565.
- Soucy-Giguère L., Gasse C., Giguère Y., Demers S., Bujold E., Boutin A. Intra-amniotic inflammation and child neurodevelopment: a systematic review protocol. Syst. Rev. 2018; 7(1): 12. https://dx.doi.org/10.1186/s13643-018-0683-z.
- Ehsanipoor R.; Committee on Practice Bulletins-Obstetrics. Premature rupture of membranes. Practice Bulletin No.172. Obstet. Gynecol. 2016; 128(4): e165-77. https://dx.doi.org/10.1097/AOG.0000000000001712.
- Romero R., Chaemsaithong P., Docheva N., Korzeniewski S.J., Kusanovic J.P., Bo Hyun Yoon. et al. Clinical chorioamnionitis at term VI: acute chorioamnionitis and funisitis according to the presence or absence of microorganisms and inflammation in the amniotic cavity. J. Perinat. Med. 2016; 44(1): 33-51. https://dx.doi.org/10.1515/jpm-2015-0119.
- Островская О.В., Кожарская О.В., Супрун С.В., Мусатов Д.В., Обухова В.Г., Ивахнишина Н.М., Наговицына Е.Б., Власова М.А., Лебедько О.А. Морфометрическая характеристика терминальных ворсин при инфицировании плаценты возбудителями внутриутробных инфекций. Тихоокеанский медицинский журнал. 2018; 4: 29-33. [Ostrovskaya O.V., Kozharskaya O.V., Suprun S.V., Musatov D.V., Obukhova V.G., Ivakhnishina N.M., Nagovitsyna E.B., Vlasova M.A., Lebedko O.A. Morphometric characteristics of the terminal villi during infection of the placenta by pathogens of intrauterine infections. Pacific Medical Journal. 2018; 4: 29-33. (in Russian)].
- Каганова М.А., Спиридонова Н.В., Ларина Т.В., Бесков Д.С., Зотова Ю.В., Сыресина С.В., Мамедзаде Ф.Р. Плацента и плодные оболочки при дородовом излитии околоплодных вод и доношенной беременности. Аспирантский вестник Поволжья. 2018; 5-6: 57-64. [Kaganova M.A., Spiridonova N.V., Larina T.V., Beskov D.S., Zotova Yu.V., Syresina S.V., Mamedzade F.R. Placenta and fetal membranes in prenatal rupture of amniotic fluid and full-term pregnancy. Postgraduate Bulletin of the Volga region. 2018; 5-6: 57-64. (in Russian)].
- Кира Е.Ф. Бактериальный вагиноз. М.: МИА; 2012. 472 с. [Kira E.F. Bacterial vaginosis. M .: MIA; 2012. 472 p. (in Russian)].
- Болдырева М.Н., Липова Е.В., Алексеев Л.П., Витвицкая Ю.Г., Гуськова И.А. Характеристика биоты урогенитального тракта у женщин репродуктивного возраста методом ПЦР в реальном времени. Журнал Акушерства и женских болезней. 2009; LVIII(6): 36-42. [Boldyreva M.N., Lipova E.V., Alekseev L.P., Vitvitskaya Yu.G., Guskova I.A. Characteristics of the biota of the urogenital tract in women of reproductive age by real-time PCR. Journal of Obstetrics and Women’s Diseases. 2009; LVIII(6): 36-42. (in Russian)].
- Сухих Г.Т., Прилепская В.Н., Трофимов Д.Ю., Донников А.Е., Айламазян Э.К., Савичева А.М., Шипицына Е.В. Применение метода полимеразной цепной реакции в реальном времени для оценки микробиоценоза урогенитального тракта у женщин (тест фемофлор): медицинская технология. М.; 2011. 36 с. [Sukhikh G.T., Prilepskaya V.N., Trofimov D.Yu., Donnikov A.E., Aylamazyan E.K., Savicheva A.M., Shipitsyna E.V. The use of the method of real-time polymerase chain reaction to assess the microbiocenosis of the urogenital tract in women (femoflor test): medical technology. M .; 2011. 36 p. (in Russian)].
- Спиридонова Н.В., Басина Е.И., Мелкадзе Е.В. Неспецифический вагинит у беременных: возможно ли лечение с сохранением вагинальных лактобацилл? Акушерство, гинекология, репродукция. 2012; 6(1): 6-15. [Spiridonova N.V., Basina E.I., Melkadze E.V. Non-specific vaginitis in pregnant women: is treatment possible with preservation of vaginal lactobacilli? Obstetrics, gynecology, reproduction. 2012; 6 (1): 6-15. (in Russian)].
- Уварова Е.В., Артюх Ю.А., Казакова А.В. Соотношение аэробной и анаэробной микрофлоры влагалища в различные периоды полового созревания. Современные проблемы науки и образования. 2017; 1: 124. [Uvarova E.V., Artyukh Yu.A., Kazakova A.V. The ratio of aerobic and anaerobic microflora of the vagina in different periods of puberty. Modern problems of science and education. 2017; 1: 124. (in Russian)].
- Спиридонова Н.В., Казакова А.В. Микробный пейзаж влагалища в зависимости от степени слипчивого процесса гениталий девочек нейтрального периода. Фундаментальные исследования. 2015; 1-3: 603-8. [Spiridonova N.V., Kazakova A.V. Microbial landscape of the vagina, depending on the degree of the adherent process of the genitalia of girls in a neutral period. Basic research. 2015; 1-3: 603-8. (in Russian)].
- Егорова Ю.В., Нестеров А.С. Характеристика цервико-вагинальной микробиоты у женщин с урогенитальным хламидиозом. Современные проблемы науки и образования. 2014; 6: 1188. [Egorova Yu.V., Nesterov A.S. Characteristics of cervical vaginal microbiota in women with urogenital chlamydiosis. Modern problems of science and education. 2014; 6: 1188. (in Russian)].
- Ворошилина Е.С., Тумбинская Л.В., Донников А.Е., Плотко Е.Э., Хаютин Л.В. Биоценоз влагалища с точки зрения количественной полимеразной цепной реакции: что есть норма? Акушерство и гинекология. 2011; 1: 57-65. [Voroshilina E.S., Tumbinskaya L.V., Donnikov A.E., Plotko E.E., Khayutin L.V. Vaginal biocenosis in terms of quantitative polymerase chain reaction: what is the norm? Obstetrics and gynecology. 2011; 1: 57-65.(in Russian)].
- Гомберг М.А. Бактериальный вагиноз и новые инфекции, с ним ассоциированные. Российский вестник акушера-гинеколога. 2010; 10(2): 32-4. [Gomberg M.A. Bacterial vaginosis and new infections associated with it. Russian Bulletin of the obstetrician-gynecologist. 2010; 10 (2): 32-4. (in Russian)].
- Власова М.А., Островская О.В., Супрун С.В., Кондрашова Е.А., Ивахнишина Н.М., Наговицына Е.Б., Хорук В.В. Оценка состояния микробиоценоза генитального тракта у беременных женщин с преждевременным разрывом околоплодных оболочек с применением тест «Фемофлор». Бюллетень физиологии и патологии дыхания. 2014; 54: 92-6. [Vlasova M.A., Ostrovskaya O.V., Suprun S.V., Kondrashova E.A., Ivakhnishina N.M., Nagovitsyna E. B., Khoruk V.V. Assessment of the microbiocenosis of the genital tract in pregnant women with premature rupture of the membranes using the Femoflor test. Bulletin of physiology and pathology of respiration. 2014; 54: 92-6. (in Russian)].
- Chun S., Yun J.W., Huh H.J., Lee N.Y. Clinical characteristics of Raoultella ornithinolytica bacteremia. Infection. 2015; 43(1): 59-64. 10.1007/s15010-014-0696-z.
- Abbas A., Ahmad I. First report of neonatal early-onset sepsis caused by multi-drug-resistant Raoultellaornithinolytica. Infection. 2018; 46(2): 275-7. https://dx.doi.org/10.1007/s15010-014-0696-z.
- Шляпников М.Е., Кузнецова Л.В., Тарасова А.В. Эффективность применения деэскалационной ступенчатой комбинированной антибактериальной терапии в комплексном лечении пациенток с тяжелыми инфекционно-воспалительными заболеваниями матки и ее придатков. Вестник медицинского института «РЕАВИЗ»: реабилитация, врач и здоровье. 2018; 2: 82-6. [Shlyapnikov M.E., Kuznetsova L.V., Tarasova A.V. Effectiveness of using de-escalation stepwise combined antibacterial therapy in the complex treatment of patients with severe infectious and inflammatory diseases of the uterus and its appendages. Bulletin of the medical institute „REAVIZ”: rehabilitation, doctor and health. 2018; 2: 82-6. (in Russian)].
- Циркин В.И., Хлыбова С.В. Роль гистамина в репродукции. Вятский медицинский вестник. 2006; 3-4: 62-7. [Tsirkin V.I., Khlybova S.V. The role of histamine in reproduction. Vyatka medical messenger. 2006; 3-4: 62-7. (in Russian)].
- Kaambo E.Б., Africa C.W.J. The threat of aerobic aginitis to pregnancy and neonatal morbidity. Afr. J. Reprod. Health. 2017; 21(2): 108-18.
- Дятлова Л.И. Особенности микробиоценоза влагалища при преждевременном разрыве околоплодных мембран при сроках гестации 22-34 недели. Международный журнал экспериментального образования. 2015; 3: 502-6. [Dyatlova L.I. Features of the vaginal microbiocenosis with premature rupture of the membranes during gestational age 22-34 weeks. International Journal of Experimental Education. 2015; 3: 502-6.(in Russian)].
- Доброхотова Ю.Э., Бондаренко К.Р., Гущин А.Е., Румянцева Т.А., Долгова Т.В., Кузнецов П.А., Джохадзе Л.С. Результаты исследования цервико-вагинальной микробиоты методом ПЦР в реальном времени у беременных с угрожающими преждевременными родами. Акушерство и гинекология. 2018; 11: 50-59. [Dobrokhotova Yu.E., Bondarenko K.R., Gushchin A.E., Rumyantseva T.A., Dolgova T.V., Kuznetsov P.A., Dzhokhadze L.S. The results of the study of cervical-vaginal microbiota by real-time PCR in pregnant women with threatened preterm labor. Obstetrics and gynecology. 2018; 11: 50-59. (in Russian)].
Received 08.04.2019
Accepted 19.04.2019
About the Authors
Kaganova, Maria A., PhD, associate professor of the Department of Obstetrics and Gynecology, Institute for Improvement of Physicians, Samara State Medical University, Ministry of Health of Russia. 443100, Russia, Samara, Polevaya str. 80. Tel.: +78462071968. E-mail: mkaganova@yandex.ru ORCID: 0000-0001-5879-418xSpiridonova, Natalia V., MD, professor, head of the Department of Obstetrics and Gynecology, Institute for Improvement of Physicians, Samara State Medical University, Ministry of Health of Russia. 443100, Russia, Samara, Polevaya str. 80. Tel.: +78462071968. E-mail: nvspiridonova@mail.ru ORCID: 0000-0003-3928-3784
Kazakova, Anna V., MD, associate professor of the Department of Obstetrics and Gynecology, Institute for Improvement of Physicians, Samara State Medical University, Ministry of Health of Russia. 443100, Russia, Samara, Polevaya str. 80. Tel.: +78462071968. E-mail: amigo14021980@yandex.ru ORCID: 0000-0002-9483-8909
Devyatova, Olga O., obstetrician-gynecologist, N.I. Pirogov City Clinical Hospital One.
443100, Russia, Samara, Polevaya str. 80. Tel.: +78462071949. E-mail: dewyatowa.olya@yandex.ru
Galkina, Daria A., obstetrician-gynecologist, N.I. Pirogov N.I. Pirogov City Clinical Hospital One.
443100, Russia, Samara, Polevaya str. 80. Tel.: +78462071949. E-mail: darya.golubeva.1992@mail.ru
Golovina, Olga N., obstetrician-gynecologist, N.I. Pirogov City Clinical Hospital One. 443100, Russia, Samara, Polevaya str. 80. Tel.: +78462071949. E-mail: doktorola@yandex.ru
For citations: Kaganova M.A., Spiridonova N.V., Kazakova A.V., Devyatova O.O., Galkina D.A., Golovina O.N. Features of the cervical canal microbiota in prenatal amniorrhea and full-term pregnancy. Akusherstvo i Ginekologiya/ Obstetrics and Gynecology. 2019; (5): 77-84. (in Russian)
http://dx.doi.org/10.18565/aig.2019.5.77-84



