Optimizing pre-pregnancy management of patients with post-cesarean section uterine scar
Objective: To develop a strategy for pre-pregnancy management of patients with a post-cesarean section uterine scar.Kurtser M.A., Egikyan N.M., Savelyeva N.A., Vatagina M.A., Pisarskaya E.S., Platitsyn I.V., Kutakova Yu.Yu., Logunova A.O.
Materials and methods: A multicenter study including 209 patients planning pregnancy naturally or by assisted reproductive technologies. The patients met the inclusion/exclusion criteria and were managed at a pre-pregnancy stage in clinical hospitals of the Mother and Child Group of Companies from 2018 to 2021 and. Baseline clinical evaluation included medical history taking, contrast sonohysterography on days 5–8 of the menstrual cycle, and pelvic magnetic resonance imaging on days 5–8 of the menstrual cycle. The statistical analysis was performed using the IBM SPSS Statistics v22 software (IBM Corp., USA).
Results: We developed a scoring scale for grading scar niche severity. Based on this scoring scale, the patients were classified into three groups: in group I [n=107/209 (51.2%)] surgery at the pre-pregnancy stage is not recommended, in group II [n=36/209 (17.22%)] hysteroscopic uterine metroplasty is recommended, and group III [n=66/209 (31.58%)] laparoscopic uterine metroplasty is recommended. There were statistically significant differences between the groups of patients regarding the prevalence of clinical complaints, data on residual myometrium thickness, and niche volume. There was a high level of agreement between contrast sonohysterography and magnetic resonance imaging.
Conclusion: The proposed scoring scale for grading scar niche severity helps a clinician choose the optimal managing strategy for the patient at the pre-pregnancy stage. Further clinical studies are needed to determine optimal threshold values of the uterine scar defect to select between a hysteroscopic and laparoscopic approach and evaluate the effectiveness of the proposed management strategy based on the results of prospective data regarding the elimination of gynecological symptoms, restoration of fertility, and assessment of obstetric complications.
Keywords
Markedly increased use of cesarean section (CS) delivery worldwide over the last several decades is associated with long-term postoperative complications, including a uterine scar defect (with or without niche formation). According to an international research group, a niche is defined as an indentation at the site of CS with a depth of at least 2 mm [1]. The actual incidence of cesarean scar defect is unknown, but according to some estimates, it ranges from 19 to 84% of women with previous cesarean section [2].
Cesarean scar defect is diagnosed by transvaginal sonography, saline infusion sonohysterography (SIS), magnetic resonance imaging (MRI), hysterosalpingography, sometimes using diagnostic hysteroscopy; however, there is no consensus on the gold standard for diagnosis.
Scar tissue thinning along the outer contour is often asymptomatic while thinning along the inner contour – a niche – can be a diagnostic finding and may be associated with gynecological and obstetric complications.
The present study aimed to optimize pre-pregnancy management of patients with a post-cesarean section uterine scar to relieve gynecologic symptoms, restore fertility, and reduce the risk of obstetric complications.
Materials and methods
The study included 209 patients managed from 2018 to 2021 for post-cesarean section uterine scar defect. All patients met the eligibility requirements and provided signed informed consent to participate in the study. The Research Ethics Committee of the Pirogov RNRMU approved this study (extract from the minutes of meeting No. 190 dated November 18, 2019) and agreed on the informed consent form for the patient.
The study inclusion criteria were age 18–42; post-cesarean section uterine scar defect confirmed by diagnostic imaging; the interval from CS to enrollment at least two years; planning pregnancy.
Exclusion criteria were uterine scar after corporal, isthmic-corporal incision of the uterus; pregnancy; a history of metroplasty at the pre-pregnancy stage; genital malformations; intrauterine pathology; women with contraindications to assisted reproductive technologies (ART); contraindications for MRI.
Clinical evaluation included medical history taking and cesarean scar assessment using SIS and pelvic MRI.
The clinical characteristics of the patients are presented in Table 1.
At baseline, all patients underwent transvaginal ultrasound (Voluson E8 device, General Electric, USA) to exclude uterine pathology, followed by SIS on days 5–8 of the menstrual cycle at clinical hospitals of the Mother and Child Group of Companies by one diagnostic medical sonographer to exclude the variability of subjective estimation. After visualization of the scar, the image was enlarged so that the scar area occupied at least 75% of the image to ensure consistent and accurate measurements, then 20.0 ml of sterile 0.9% NaCl solution was instilled transcervically into the uterine cavity through a Cook cervical ripening catheter. In the sagittal plane, the following were studied: the localization of the scar relative to the internal os, the presence of local myometrial thinning along the internal contour of a niche, the size of the niche (length, depth); residual myometrium thickness (RMT) in the projection of the scar (endometrium and perimetrium were not taken into account), adjacent myometrial thickness (AMT) were measured perpendicular to the serous membrane; the width of the niche was measured in the axillary plane, the presence of branches was taken into account; the presence of hyper- and anechoic inclusions was also assessed. The niche shape was classified according to classifications published by Bij de Vaate et al. [3]. The assessment of the volume of the niche was carried out automatically by multiplying niche measurements: length, width, and depth (in cm3) by a factor of 0.523 [4].
Pelvic MRI was performed on a 1.5T clinical scanner MAGNETOM Aera, Siemens, Germany at the Mother and Child Group of Companies, assessed by one MRI specialist radiographer to exclude the variability of subjective measurements. Before the procedure, all patients were given standard recommendations to prepare for the study, performed on days 5–8 of the menstrual cycle. The block of sections was inserted along the bony landmarks of the femoral heads and the wings of the iliac bones. The scanning level was located from the L4 vertebral body to the bladder neck level. According to the images in the sagittal plane, subsequent T2-WIs were positioned in other planes, corresponding to the anatomical position of the uterine body. Images in the coronal plane were oriented along the body of the uterus, in the axial plane – perpendicular to the body of the uterus (slice thickness 2–3 mm). Positioning perpendicular to the body of the uterus provided a detailed analysis of the area of the scar, RMT in the projection of the scar, AMT; in the case of a niche, its localization relative to the internal os, as well as the depth, width, length, and volume of the defect were assessed. On T1-WI, the presence of congestive (hemorrhagic) contents in the niche projection was evaluated.
SIS and MRI findings were compared for each patient regarding the length, depth, and width of the niche, RMT in the scar projection, AMT, niche volume, and the presence/absence of congested contents.
Considering the clinical and instrumental data, a scoring scale for assessing the severity of the niche was developed to optimize pre-pregnancy management of patients with a post-cesarean section uterine scar. Concerning the clinical picture, the leading complaints associated with the niche, such as postmenstrual spotting and secondary infertility, were taken into account [5] (Table 2).
Interpretation of the results: 0–3 points – a clinically insignificant niche, 3–4 points – a moderately expressed niche, 5–6 points – a critical niche.
We recognize that the proposed thresholds in the niche scoring system are arbitrary and should only be considered until appropriate values are available based on data from extensive systematic studies.
Statistical analysis
The statistical analysis was performed using IBM SPSS Statistics v22 (IBM Corp., USA). The normality of the distribution was tested by the Shapiro–Wilk test. Variables not meeting normality assumptions were reported as the median (Me) and interquartile range (Q25%; Q75%). Categorical variables were summarized using frequency counts and percentages (%). The Kruskal–Wallis test was used to compare numerical data between three groups, followed by pairwise comparison using the Mann – Whitney U-test. When several groups were compared, Bonferroni correction was applied for multiple comparisons. For categorical variables, differences between independent groups were assessed using the Fisher’s Exact Test. When testing statistical hypotheses, the critical significance level was considered at p<0.05 and 0.017 for multiple comparisons. To assess the degree of agreement between the measurements performed by the SIS and MRI methods, Kendall’s concordance coefficient, the Pearson–Spearman correlation analysis, and the Bland–Altman method were used. The 95% CI for the sensitivity was calculated using the Wilson formula.
Results
The patients were divided into three groups based on niche severity scores. Comparative data are presented in table 3.
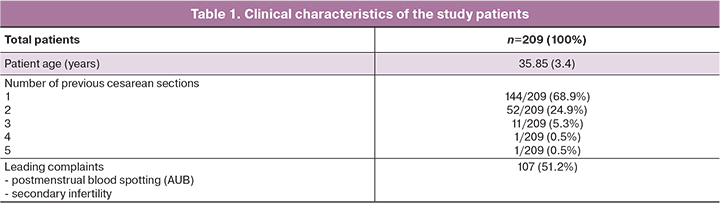
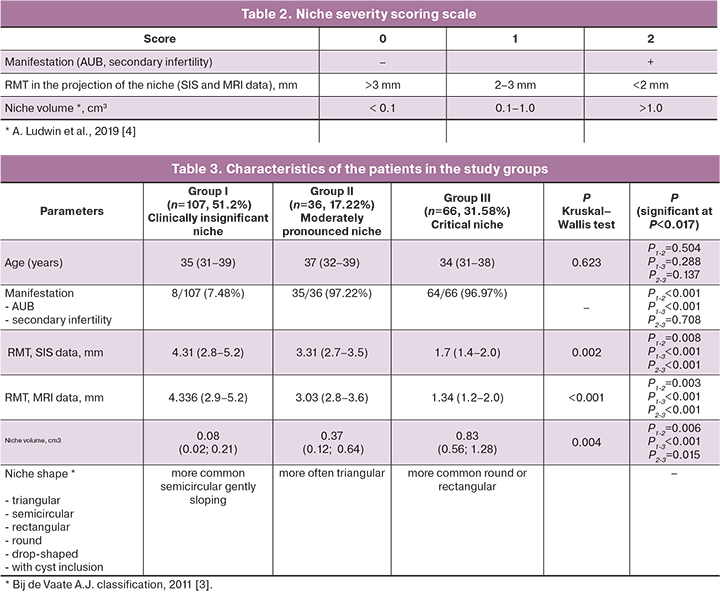
There were statistically significant differences between patient groups regarding the prevalence of clinical complaints, RMT and niche volume. There was a high degree of consistency between RMT findings in the SIS and MRI protocols: Kendall's concordance coefficient τ=0.9380, Z=20.17, p<0.001 (95% CI 0.919; 0.952). The data of assessing the consistency of the results of paired RMT measurements using MRI and SIS are also presented on the Bland–Altman graph (Fig. 1): mean absolute error (bias) 0.028 (95% CI 0.0067; 0.0501); upper bias limit +0.340 (95% CI 0.302; 0.378); lower bias -0.283 (95% CI -0.321; -0.246). The mean difference between measurements (bias) was 0.028, which indicates no systematic discrepancy; the standard deviation of the differences was 0.15, which is small compared to the values themselves. Also, there was no dependence of the measurement difference on the RMT value. Spearman's rank correlation coefficient between measurements made by both methods was R=0.995; 95% CI (0.993; 0.996), p<0.001. Pearson's correlation coefficient was also r=0.995, 95% CI (0.993; 0.996), p<0.001 (Fig. 2). A high value of the correlation coefficient indicates a close relationship between measurements.
Thus, the measurements obtained by SIS and MRI were in good agreement with each other. The sensitivity of the SIS compared to MRI was 83.25% (95% CI 77.60%; 87.71%).
There was no statistically significant difference in age between the groups of patients (p>0.05). The most common niche shape was determined for each group of patients. Patients of group I were offered conservative management, excluding surgery at the pre-pregnancy stage. It was recommended to plan pregnancy through the regular sexual activity without contraception for 12 months (for a group of patients under the age of 35), 6 months (for a group of patients over the age of 35), or undergo an ART program.
Patients in group II were recommended to undergo hysteroscopic metroplasty at the pre-pregnancy stage. Hysteroscopic surgery consists of remodeling the edges of the niche (more often – resection of the distal, protruding edge of the triangular niche), removing micropolyps, coagulation (ablation) with a ball electrode of pathological vessels, and endometrioid heterotopies in the bottom of the niche.
Group III patients were offered laparoscopic metroplasty at the pre-pregnancy stage. Surgery consists of sequential dissection of the bladder, hysterotomy, and excision of the niche (with mechanical scissors, monopolar needle, harmonic scalpel, or laser); the hysterotomy incision was closed by double row vicryl suture followed by peritonization. With pronounced retroflection of the uterus, an additional shortening of the round ligaments was performed according to the Baldy method, using long-term absorbable suture material to temporarily move the uterus to the anteversio position and thereby reduce the load on the postoperative wound area during the first weeks.
Before laparoscopic metroplasty, patients aged > 38 were offered superovulation in the ART program with oocyte /embryo cryopreservation to enable women to postpone pregnancy to a later date after surgery.
The present study did not include patients with a scar defect along the external contour. Their management required measuring RMT in the projection of the scar and laparoscopic metroplasty.
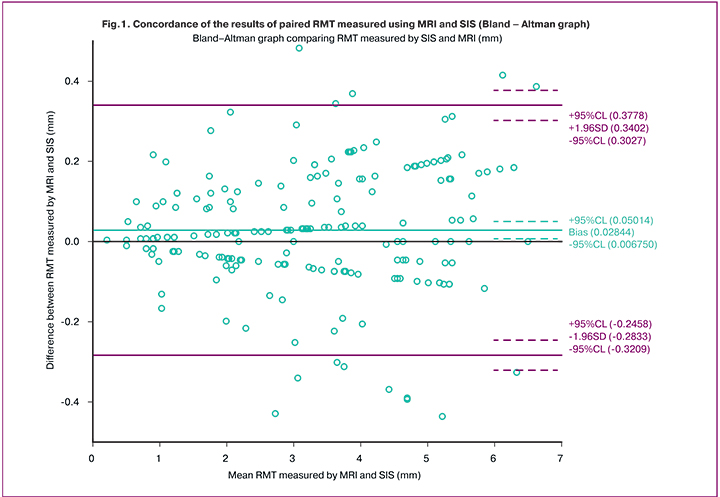
The MRI images (Fig. 3) show clinical examples.
Discussion
Currently, there is a certain terminological dissonance in the description of the post-cesarean section uterine scar [6, 7]. In clinical practice, we consider it appropriate to use the term "scar defect on the uterus" with its location: along the external/internal contour of the uterus (with or without the niche formation).
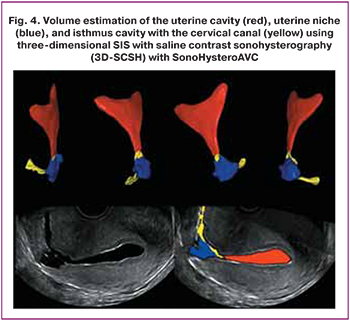
From the standpoint of diagnostics, ultrasound examination is of fundamental importance. However, there may be variability due to subjective measurements of different specialists. Gubbini G. et al. used ultrasound to evaluate the niche area since they believed that the shape of the niche corresponds to an isosceles triangle. The authors proposed to calculate using the formula: niche depth × niche length/2. The niche was classified as follows: grade 1 (area <15 mm2); grade 2 (area from 16 to 25 mm2), grade 3 (area> 25 mm2) [8]. Ludwin et al. evaluated the niche volume using 3D contrast SIS and automatic volume calculation software (SonoHysteroAVC) [4] (Fig. 4).
MRI facilitates clinical decision-making due to its higher resolution and larger field of view than ultrasound [9]. The disadvantages of the method include high cost and lack of widespread availability. In addition, due to the lack of knowledge by radiologists of the clinical significance of a post-cesarean uterine scar, this pathology is often not described.
According to Satpathy G. et al., the diagnostic accuracy of MRI in diagnosing the post-cesarean uterine scar was 90%, SIS – 96.7%. However, compared to ultrasound, where the reliability of the data obtained depends on the experience of a specialist, MRI is a more objective method, allowing for analyzing the images retrospectively and evaluating the surgery outcomes [10].
The decision on the treatment methods is made on a case-by-case basis, taking into account the severity of clinical complaints and the data of clinical investigations.
In a systematic review and meta-analysis, Vitale S.G. et al. the benefit of surgical treatment was confirmed only for symptomatic niches. A meta-analysis showed the resolution of clinical symptoms in 85% of cases after hysteroscopic correction and 92.77% after laparoscopic/robotic correction [11].
Research by Tsuji S. et al. demonstrated that hysteroscopic surgery effectively increases RMT and reduces niche volume [12], which is essential for subsequent gestation. Mashiach R. et al. emphasized that hysteroscopic metroplasty is the preferred intervention for patients with RMT in a niche projection> 2–3 mm to eliminate gynecological symptoms. On the contrary, the authors recommend laparoscopic metroplasty for patients with RMT <2–3 mm. The authors propose 2.5 mm as the threshold value for RMT based on common practice and expert opinion [13].
Conclusion
The proposed scoring scale for assessing the severity of the niche helps the clinician choose the optimal management strategy for pre-pregnancy management of patients with a post-cesarean section uterine scar at the pre-pregnancy stage to relieve gynecologic symptoms, restore fertility and reduce the risk of obstetric complications.
Our findings showed a high level of agreement between SIS and MRI in diagnosing post-cesarean section uterine scar. It seems rational to use SIS as the first line of diagnosis. In patients with moderately expressed and critical niches planning pre-pregnancy surgery, the diagnosis should be verified by MRI.
Further clinical studies are needed to determine optimal threshold values of the uterine scar defect to select between a hysteroscopic and laparoscopic approach and evaluate the effectiveness of the proposed management strategy based on the results of prospective data regarding the relief of gynecologic symptoms, restoration of fertility, and assessment of obstetric complications.
References
- Jordans I.P.M., de Leeuw R.A., Stegwee S.I. Amso N.N., Barri-Soldevila P.N., van den Bosch T. et al. Sonographic examination of uterine niche in non-pregnant women: a modified Delphi procedure. Ultrasound Obstet. Gynecol. 2019; 53(1): 107-15. https://dx.doi.org/10.1002/uog.19049.
- Setubal A., Alves J., Osório F., Guerra A., Fernandes R., Albornoz J. et al. Treatment for uterine isthmocele, a pouchlike defect at the site of a cesarean section scar. J. Minim. Invasive Gynecol. 2018; 25(1): 38-46. https://dx.doi.org/10.1016/j.jmig.2017.09.022.
- Bij de Vaate A.J., Brölmann H.A., van der Voet L.F., van der Slikke J.W., Veersema S., Huirne J.A. Ultrasound evaluation of the Cesarean scar: relation between a niche and postmenstrual spotting. Ultrasound Obstet. Gynecol. 2011; 37(1): 93-9. https://dx.doi.org/10.1002/uog.8864.
- Ludwin A., Martins W.P., Ludwin I. Evaluation of uterine niche by three-dimensional sonohysterography and volumetric quantification: techniques and scoring classification system. Ultrasound Obstet. Gynecol. 2019; 53(1): 139-43. https://dx.doi.org/10.1002/uog.19181.
- Курцер М.А., Егикян Н.М., Савельева Н.А., Ватагина М.А., Писарская Е.С., Логунова А.О., Кутакова Ю.Ю. Вторичное бесплодие, ассоциированное с нишей рубца на матке после кесарева сечения. Вопросы гинекологии, акушерства и перинатологии. 2020; 19(5): 95-101. [Kurtser M.A., Egikyan N.M., Savelyeva N.A., Vatagina M.A., Pisarskaya E.S., Logunova A.O. et al. Secondary infertility associated with a uterine niche after cesarean section. Gynecology, Obstetrics and Perinatology. 2020; 19(5): 95-101. (in Russian)]. https://dx.doi.org/10.20953/1726-1678-2020-5-95-101.
- Мартынов С.А., Адамян Л.В. Рубец на матке после кесарева сечения: терминологические аспекты. Гинекология. 2020; 22(5): 70-5. [Martynov S.A., Adamyan L.V. Cesarean scar defect: terminological aspects. Gynecology. 2020; 22(5): 70-5. (in Russian)]. https://dx.doi.org/10.26442/20795696.2020.5.200415.
- Лисицына О.И., Шмаков Р.Г. «Ниши» рубца на матке после кесарева сечения: диагностика, лечение и исходы. Акушерство и гинекология. 2019; 9: 24-31. [Lisitsyna O.I., Shmakov R.G. Niches of the uterine scar after cesarean section: diagnosis, treatment, and outcomes. Obstetrics and gynecology. 2019; 9: 24-31. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.9.24-31.
- Gubbini G., Centini G., Nascetti D., Marra E., Moncini I., Bruni L. et al. Surgical hysteroscopic treatment of cesarean-induced isthmocele in restoring fertility: prospective study. J. Minim. Invasive Gynecol. 2011; 18(2): 234-7. https://dx.doi.org/10.1016/j.jmig.2010.10.011.
- Bekiesinska-Figatowska M. Magnetic resonance imaging of the female pelvis after cesarean section: a pictorial review. Insights Imaging. 2020; 11(1): 75. https://dx.doi.org/10.1186/s13244-020-00876-5.
- Satpathy G., Kumar I., Matah M., Verma A. Comparative accuracy of magnetic resonance morphometry and sonography in assessment of post-cesarean uterine scar. Indian J. Radiol. Imaging. 2018; 28(2): 169-74. https://dx.doi.org/10.4103/ijri.IJRI_325_17.
- Vitale S.G., Ludwin A., Vilos G.A., Török P., Tesarik J., Vitagliano A. et al. From hysteroscopy to laparoendoscopic surgery: what is the best surgical approach for symptomatic isthmocele? A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2020; 301(1): 33-52. https://dx.doi.org/10.1007/s00404-020-05438-0.
- Tsuji S., Kimura F., Yamanaka A., Hanada T., Hirata K., Takebayashi A. et al. Impact of hysteroscopic surgery for isthmocele associated with cesarean scar syndrome. J Obstet Gynaecol Res. 2018; 44(1): 43-8. https://dx.doi.org/10.1111/jog.13464.
- Mashiach R., Burke Y.Z. Optimal isthmocele management: hysteroscopic, laparoscopic, or combination. J. Minim. Invasive Gynecol. 2021; 28(3): 565-74. https://dx.doi.org/10.1016/j.jmig.2020.10.026.
Received 14.07.2021
Accepted 05.10.2021
About the Authors
Mark A. Kurtser, Academician of the RAS, Dr. Med. Sci., Professor, Head of the Department of Obstetrics and Gynecology, Pediatric Faculty,N.I. Pirogov Russian National Research Medical University, Ministry of Health of Russia; Director General of Mother and Child Group of Companies,
1 Ostrovityanov str., Moscow, 117997, Russian Federation.
Natalya M. Egikyan, MD, PhD, Gynecologist, Head of the Department of Gynecology, Lapino Clinical Hospital, Mother and Child Group of Companies,
111 1st Uspenskoe highway, Lapino, Odintsovskii district, Moscow region, 143081, Russian Federation.
Natalya A. Savelyeva, PhD Student at the Department of Obstetrics and Gynecology, Pediatric Faculty, N.I. Pirogov Russian National Research Medical University,
Ministry of Health of Russia; Gynecologist, Lapino Clinical Hospital, Mother and Child Group of Companies, Nats4644@mail.ru,
https://orcid.org/0000-0001-9719-9447, 1 Ostrovityanov str., Moscow, 117997, Russian Federation.
Maria A. Vatagina, Gynecologist, Lapino Clinical Hospital, Mother and Child Group of Companies,
111 1st Uspenskoe highway, Lapino, Odintsovskii district, Moscow region, 143081, Russian Federation.
Ekaterina S. Pisarskaya, Diagnostic Medical Sonographer, Lapino Clinical Hospital, Mother and Child Group of Companies,
111 1st Uspenskoe highway, Lapino, Odintsovskii district, Moscow region, 143081, Russian Federation.
Igor V. Platitsyn, MD, PhD, Head of the Department of Diagnostic Imaging, Lapino Clinical Hospital, Mother and Child Group of Companies,
111 1st Uspenskoe highway, Lapino, Odintsovskii district, Moscow region, 143081, Russian Federation.
Yuliya Yu. Kutakova, MD, PhD, Medical Director for Organizational, Research and Educational Activity, Mother and Child Group of Companies,
111 1st Uspenskoe highway, Lapino, Odintsovskii district, Moscow region, 143081, Russian Federation.
Anna O. Logunova, Gynecologist, Lapino Clinical Hospital, Mother and Child Group of Companies,
111 1st Uspenskoe highway, Lapino, Odintsovskii district, Moscow region, 143081, Russian Federation.
Corresponding author: Natalya A. Savelyeva, Nats4644@mail.ru
Authors' contributions: Kurtser M.A., Egikyan N.M. – conception and design of the study, manuscript editing; Savelyeva N.A., Vatagina M.A., Kutakova Yu.Yu., Logunova A.O. – data collection and analysis; Savelyeva N.A., Pisarskaya E.S. – ultrasound examination; Platitsyn I.V. – MRI examination; Savelyeva N.A. – statistical analysis; Savelyeva N.A., Egikyan N.M. – manuscript drafting.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Kurtser M.A., Egikyan N.M., Savelyeva N.A., Vatagina M.A., Pisarskaya E.S., Platitsyn I.V., Kutakova Yu.Yu., Logunova A.O. Optimizing pre-pregnancy management
of patients with post-cesarean section uterine scar.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 12: 68-75 (in Russian)
https://dx.doi.org/10.18565/aig.2021.12.68-75



