Evaluation of the pharmacological activity of a somatostatin-containing protein-based preparation in a model of cyclophosphamide-induced ovarian failure in female mice
According to statistical studies, the number of infertile couples in the world is constantly growing and accounts for approximately 15%. Female infertility makes up 20–35%. The new drug being developed on the basis of somatostatin-containing protein is supposed to be used in female and male infertility, to increase the reproductive capacity of the body, to enhance the ovarian reserve and their entry into the growth phase, to accelerate the beginning of growth of resting follicles, to expand the volume of ejaculate and improve the quality characteristics of sperm.Matichin A.A., Kryshen K.L., Faustova N.M, Gushchin Ya.A., Makarova M.N., Reshetnik V.V., Sukhikh G.T., Yudin S.M., Lunin V.G.
Objective: Evaluation of the pharmacological activity of a somatostatin-containing protein-based preparation in a model of cyclophosphamide-induced ovarian failure in female mice.
Materials and methods: The drug "Endoxan" was used as an inducer of pathology once intraperitoneally at a dose of 70 mg/kg. Six groups were formed for the study: 1st – intact (n=15); 2nd – negative control (pathology+sodium chloride solution 0.9%, n=10); 3rd – positive control (pathology+recombinant murine somatotropic hormone at a dose of 800 µg/kg, n=10); 4th – pathology+test subject at a dose of 10 µg/kg (n=15); 5th – pathology+test subject at a dose of 50 µg/kg (n=15), 6th – pathology+test subject at a dose of 250 µg/kg (n=15).
The study included evaluation of microscopic changes in ovarian tissue, ovarian mass ratios, morphometric analysis of follicles, and plasma PGE2 and somatotropin levels.
Results: It was found that against the background of induced pathology the tested drug in the dose range of 10–250 µg/kg leads to a dose-dependent increase in the level of somatotropin and PGE2 and normalisation of folliculogenesis. It was revealed that a significant increase in the proportion of primordial and tertiary follicles and a decrease in the proportion of atretic follicles occurred under the effect of the tested preparation. This suggests a possible mechanism of action of the tested object, namely the synthesis of specific autoantibodies to somatostatin and a decrease in its concentration. This in turn leads to an increase in the content of endogenous somatotropic and sex hormones in the body.
Conclusion: The results obtained are consistent with the data of earlier studies on the increase in the size and number of follicles upon administration of exogenous somatotropic hormone to animals. We believe that clinical studies of the possibility of using a preparation based on somatostatin-containing protein for the treatment of female infertility associated with disorders of folliculogenesis are necessary.
Authors’ contributions: Matichin A.A. – text composition and editing, data processing; Kryshen K.L. – study concept and dezign; Faustova N.M., Gushchin Ya.A. – data collecting and processing; Makarova M.N. – article manuscript editing, critical analysis; Reshetnik V.V., Sukhikh G.T., Yudin S.M., Lunin V.G. – text editing, article manuscript approval.
Conflicts of interest: V.V. Reshetnik is the co-author of the publication. Mr. Reshetnik is the director of the company Yursfarm LLC, which financed this study under a partnership agreement.
Funding: The work was performed within the framework of contractual obligations between RMC “HOME OF PHARMACY” and Yurspharm LLC.
Ethical Approval: The study was approved at the meeting of the Bioethics Commission of RMC “HOME OF PHARMACY”
(No. of document 2.29/19 dated May 15, 2019).
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Matichin A.A., Kryshen K.L., Faustova N.M., Gushchin Ya.A., Makarova M.N., Reshetnik V.V., Sukhikh G.T., Yudin S.M., Lunin V.G. Evaluation of the pharmacological activity of a somatostatin-containing protein-based preparation in a model of cyclophosphamide-induced ovarian failure in female mice.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (7): 119-125 (in Russian)
https://dx.doi.org/10.18565/aig.2023.150
Keywords
Statistical studies show that approximately 15% of married couples face infertility. Infertility is usually defined as the inability to conceive after one year of regular sexual activity. Female infertility accounts for 20–35%. One of the most common causes of female infertility is ovulatory disorders [1–3].
In vitro fertilization (IVF) is one of the possible treatments for infertile couples [1–3]. IVF uses hormones to stimulate ovarian function to increase follicular growth and thus develop more than one oocyte. However, IVF in patients with poor ovarian response is often ineffective. Most patients with poor response to ovarian stimulation in IVF cycles have reduced ovarian reserve. Some literature reports that the use of growth hormone (growth hormone (GH), somatotropin) in patients with poor ovarian response to ovarian stimulation can increase the success of the IVF program [4].
It has also been shown that the administration of growth hormone stimulates follicle growth and development [6–9], and also increases the ovarian response to ovarian stimulation by gonadotropins [8, 9]. Thus, the use of somatotropin can significantly improve IVF outcomes, especially in women with poor response to standard hormonal therapy.
Prostaglandin E2 (PGE2) plays an important role in folliculogenesis. PGE2 is one of the mediators that promote the growth and maturation of the follicular epithelium that nourishes the oocyte. Some data show that inhibition of PGE2 production in follicles or genetic knockout of production of PGE2 and its receptors can block ovulation. Increased luteinizing hormone levels have been shown to increase PGE2 levels in the dominant follicle, leading to an increase of follicular epithelial cells and expression of proteases associated with follicular rupture. In an experiment on mice with PGE2 receptor deficiency, abnormal division of follicular epithelial cells and absence of ovulation were observed, which led to infertility [10].
The mechanism of action of the new medication under development is induction of synthesis of specific autoantibodies to somatostatin (anti-COM-IgG), reduction of its concentration and, increase in the content of endogenous somatotropin [11].
The aim of this work was to evaluate the pharmacological activity of a medication based on somatostatin-containing protein on the model of cyclophosphamide-induced ovarian failure in female mice.
Materials and methods
The tested object (TO) is a new medication made of somatostatin-containing protein (LLC “Urspharm”, Russia). The active substance is a recombinant protein containing somatostatin (SOM), consisting of a carrier protein - glucan binding domain (GBD), a spacer sequence (-) and two copies of somatostatin antigenic determinant (GBD-SOM-SOM) with a molecular mass of 39 kDa [11].
Outbred female ICR mice (CD-1) aged 8–10 weeks, weighing 17–24 g, obtained from RMC “HOME OF PHARMACY” (Leningrad region, Russia) were used in the experiment. The animals were kept under standard conditions in accordance with Directive 2010/63/EU of September 22, 2010 on the protection of animals used for scientific purposes. The feed used was “Feed for keeping laboratory animals” PK-120-1, prepared according to National State Standard 34566-2019. The study was approved by bioethical commission of RMC “HOME OF PHARMACY” (No. BEC 2.29/19 dated May 15, 2019). The study complied with the principles of the “three Rs” (Reduction, Refinement, Replacement) [12].
Endoxan medication (INN: cyclophosphamide, produced by Baxter Oncology GmbH, Germany, series 8l264D) was used as a pathology inducer in a single intraperitoneal dose of 70 mg/kg [13]. The data indicate that this model pathology shows a decrease in ovarian weight, a significant decrease in the number of normal follicles and an increase in the number of atretic follicles, a decrease in the level of PGE2, and an increase in the level of follicle-stimulating hormone (FSH) [13].
6 groups were formed for the study: Group 1 – intact (n=15); Group 2 – negative control (pathology+sodium chloride solution 0.9% (LLC “Solopharm”, Russia, series 320319), n=10); Group 3 – positive control (pathology+recombinant mouse growth hormone (rmGH) at a dose of 800 µg/kg, n=10); Group 4 – pathology+test medication at a dose of 10 µg/kg (n=15); Group 5 – pathology+test medication at a dose of 50 µg/kg (n=15), Group 6 – pathology+test medication at a dose of 250 µg/kg (n=15). The test medication and physiologic solution were injected into animals subcutaneously twice with an interval of 14 days (on the 1st and 14th days of the experiment). The positive control substance was injected in a single intraperitoneal dose on the 28th day of the experiment. The pathology inducer was injected in a single dose on day 21 of the experiment. The total duration of the experiment was 43 days (Fig. 1). Euthanasia was performed using carbon dioxide (CO2) followed by exsanguination from the heart cavities.
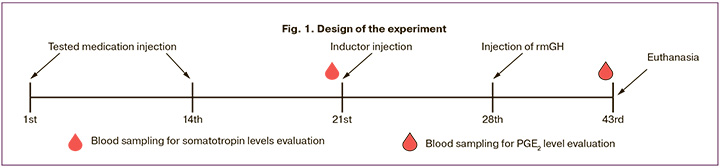
The animals were monitored daily. Body weight was measured once before injection of the test medicine and then weekly. To evaluate the level of somatotropin in 5 animals of the intact group and 5 animals from the groups receiving the test medicine in the studied doses, blood samples were taken from the heart cavities on the 21st day of the experiment during planned euthanasia. To determine the level of PGE2, blood samples were taken from the remaining animals of all groups on the 43rd day of the experiment, according to a similar protocol. Blood was sampled into tubes with sodium heparin. On the 43rd day of the experiment, during planned euthanasia, ovarian weight was measured in 10 animals from each group and then endometrium and ovaries were sampled for further histologic analysis. The design of the experiment is presented in Figure 1.
To evaluate the concentration of somatotropin and PGE2, commercially available kits were used: Elisa kit for Growth Hormone (GH), No. SEA044Mu, lot L190619110, (Cloud-Clone Corp., USA) and Elisa kit for Prostaglandin (PGE2), No. CEA538Ge, lot L190619122, (Cloud-Clone Corp., USA). Measurements were performed on a multifunctional microplate analyzer “CLARIOstar” (BMG Labtech, Germany) at two wavelengths 450 nm and 650 nm according to the manufacturer's instructions.
In the process of euthanasia, the uterus with ovaries was removed from the animals. The extracted ovaries were weighed in pairs with preliminary fixation in 10% neutral formalin solution for further calculation of mass coefficients. The uterus and ovaries were fixed in 10% neutral formalin solution for 24 h and then embedded in paraffin for further histologic analysis [14]. Then 5–7 μm thick sections were made and stained with hematoxylin and eosin. Histological objects were analyzed using a light-optical microscope Accu-Scope 3000 SERIES (“Accu Scope Inc.”, USA) at magnifications of 40, 100, and 400. To assess ovarian function, primary, secondary, early antral, antral follicles, corpus luteum, and pathological follicles in the atresia stage in one ovary were counted. Then, from the total sum of the follicles, the percentage of a certain type of follicles in the ovary was counted.
Statistical analysis
Data were analysed and plotted using Statistica 10.0 (StatSoft, USA) and Prism 9.0 (GraphPad Software, USA) software. Data were tested for conformity to the law of normal distribution using the Shapiro–Wilk test and presented as mean (M) and standard deviation (SD) (Shapiro–Wilk criterion, p>0.05). Differences were determined at 0.05 level of significance.
One-factor analysis of variance (ANOVA) was used for unrelated data. Subsequent intergroup comparison (post hoc) was performed using Tukey's HSD test (post hoc Tukey's).
Results and discussion
The animals remained in a satisfactory condition throughout the experiment. We observed a uniform increase in body weight of the experimental animals in all groups.
The level of PGE2 on the 43rd day of experiment in animals of intact group was 2,33 (1,81) pg/ml. In the negative control group, animals with cyclophosphamide-induced ovarian pathology had significantly lower PGE2 levels of 1.21 (0.87) pg/ml, which was on average 48% lower compared to those of the intact group.
The level of PGE2 in the group of animals that received rmGH treatment after pathology modelling was 2.56 (2.57) pg/ml. On average, the PGE2 level in this group was 112 % higher compared to the PGE2 level in the control group of animals that did not receive therapy. In female animal #3.8 the level of PGE2 was 8.30 pg/ml, which was significantly higher than the average PGE2 content in the group.
A dose-dependent increase in PGE2 levels was observed in animals treated with the study drug at doses of 10–250 µg/kg. On average, PGE2 levels were higher by 55% (10 µg/kg), 124% (50 µg/kg) and 181% (250 µg/kg) compared to the same levels in the negative control group (Fig. 2).

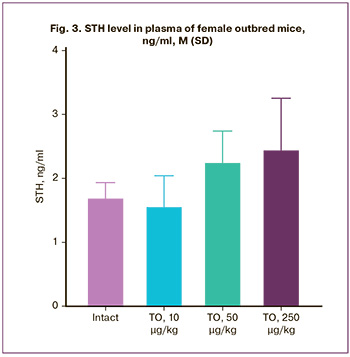
The range of somatotropin levels in blood plasma of animals of the intact group on the 21st day of the experiment was 1.72 (0.22) ng/ml. No statistically significant effect of the study drug in the studied doses on the level of somatotropin was found. The level of STH in animals that received the investigated drug in the dose of 10 µg/kg was within the range of STH values in animals of the intact group and amounted to 1.58 (0.46) ng/ml (Fig. 3). At the same time, an increase in the STH level was observed in the groups receiving the study drug in doses of 50 µg/kg (2.27 (0.47) ng/ml) and 250 µg/kg (2.47 (0.79) ng/ml) (Fig. 3). On average, the STH values in animals of these groups exceeded the STH levels in animals of the intact group by 32 and 44%, respectively. Despite the lack of statistical significance, the obtained data allow us to speak about the pharmacological activity of the drug at doses of 50 µg/kg and 250 µg/kg, manifested in the increase of the STH level.
A single administration of cyclophosphamide had a marked effect on the organs of the reproductive system of mice. We observed a significant decrease in weight (2.3-fold) and mass ratios of ovaries in animals of the negative control group compared to the same parameters in animals of the intact group (p<0.05, Table 1). A statistically significant increase in ovarian weight was observed in the animals of the positive control group compared to the same parameters in the animals of the group without treatment. A statistically significant increase in ovarian weight compared to the negative control group without treatment was observed only in the group of animals receiving the tested drug at a dose of 250 µg/kg. At the same time, the weight of ovaries in animals receiving the investigated drug at a dose of 250 µg/kg and rmGH at a dose of 800 µg/kg was similar to the values of ovarian weight in animals of the intact group.
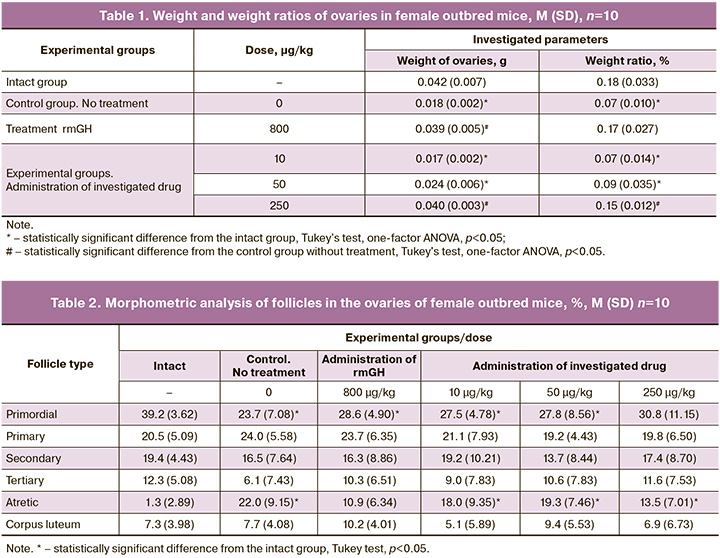
No pathologic changes in uterine and ovarian tissues were observed in animals of all experimental groups. Table 2 represents the data of morphometric analysis of follicles in the ovaries of female mice. In the animals of the intact group, numerous follicles at different stages of maturity, as well as separate corpus luteum were observed, which is normal. Primordial follicles (39.2%) formed the major part of the total number of follicles counted in the ovary. Primary follicles accounted for 20.5%, tertiary follicles for 12.3%, and atretic follicles for 1.3%. In the control group, the animals with model pathology had follicles with signs of cell atrophy and apoptosis. Against the pathology background, we observed a decrease in the proportion of primordial (23.7%) and tertiary (6.1%) follicles and an increase in the proportion of primary (24%) and atretic (22%) follicles. In the group of animals receiving rmGH at a dose of 800 µg/kg, a significant increase in the proportion of primordial and tertiary follicles and a decrease in the proportion of atretic follicles were observed compared to the control group. A dose-dependent tendency to increase the proportion of primordial and tertiary follicles with a parallel decrease in the proportion of atretic follicles was observed in animals receiving the study drug in doses of 10–250 µg/kg, which is consistent with literature data [15].
Conclusion
The pathology induced by cyclophosphamide administration was successfully generated in animals. The pathologic pattern was expressed in an increase in the number of follicles with signs of cell atrophy and apoptosis. There was an increase in the number of atretic follicles along with a decrease in the number of primordial follicles. The weight and weight ratio of ovaries decreased more than 2 times in comparison with similar data in animals of the intact group. There was a significant decrease in PGE2 concentration in comparison with PGE2 values in animals of the intact group, which corresponds to the literature data.
A dose-dependent increase in the weight and weight ratios of ovaries was observed during administration of the study drug. When the drug was administered to animals at a dose of 250 µg/kg, the weight of ovaries did not differ from the corresponding data in the intact group of animals. A dose-dependent tendency to increase the proportion of primordial and tertiary follicles along with a decrease in the proportion of atretic follicles was revealed, which correlated with the data on the level of PGE2 and may indicate normalization of the process of folliculogenesis against the background of pathology induced by cyclophosphamide administration. The obtained results of experimental studies correspond with the data of earlier studies, which demonstrated that administration of exogenous STH to animals leads to an increase in the size and number of follicles.
We believe that there is a need for clinical studies of the potential use of a drug based on somatostatin-containing protein for the treatment of female infertility associated with folliculogenesis disorders.
References
- Hart R.J., Rombauts L., Norman R.J. Growth hormone in IVF cycles: any hope? Curr. Opin. Obstet. Gynecol. 2017; 29(3): 119-25. https://dx.doi.org/10.1097/GCO.0000000000000360.
- Ho Y.K. Effects of growth hormone plus gonadotropins on controlled ovarian stimulation in infertile women of advanced age, poor responders, and previous in vitro fertilization failure patients. Taiwan. J. Obstet. Gynecol. 2017; 56(6): 806-10. https://dx.doi.org/10.1016/j.tjog.2017.10.018.
- Murtaza M., Hadi J., Iiizam E., Sani A. Male and female infertility: causes, and management. IOSR J. Dent. Med. Sci. 2019; 18(9): 27-32.https://dx.doi.org/10.9790/0853-1809132732.
- Cozzolino M., Cecchino G.N., Troiano G., Romanelli C. Growth hormone cotreatment for poor responders undergoing in vitro fertilization cycles: a systematic review and meta-analysis. Fertil. Steril. 2020; 114(1): 97-109.https://dx.doi.org/10.1016/j.fertnstert.2020.03.007.
- Ниаури Д.А., Гзгзян А.М., Коган И.Ю., Джемлиханова Л.Х., Крихели И.О., Объедкова К.В., Александрова Л.А. Эффективность применения соматотропного гормона в программах ЭКО/ЭКО+ИКСИ у женщин со «слабым» ответом яичников на стимуляцию гонадотропинами. Журнал акушерства и женских болезней. 2015; 64(6): 43-50. [Niauri D.A., Gzgzyan A.M.,Kogan I.Yu., Dzhemlikhanova L.Kh., Krikheli I.O., Obedkova K.V., Alexandrova L.A. Effectiveness of growth hormone use in women with "poor" ovarian response to the stimulation of gonadotropins in IVF/IVF+ICSI programs. Journal of Obstetrics and Women's Diseases. 2015; 64(6): 43-50.(in Russian)].
- Zhang Y., Zhang C., Shu J., Guo J., Chang H.-M., Leung P.C.K. et al. Adjuvant treatment strategies in ovarian stimulation for poor responders undergoing IVF: a systematic review and network meta-analysis. Hum. Reprod. Update. 2020; 26(2): 247-63. https://dx.doi.org/10.1093/humupd/dmz046.
- Cai M.H., Gao L.Z., Liang X.Y., Fang C., Wu Y.Q., Yang X. The effect of growth hormone on the clinical outcomes of poor ovarian reserve patients undergoing in vitro fertilization/intracytoplasmic sperm injection treatment: A retrospective study based on POSEIDON criteria. Front. Endocrinol. (Lausanne). 2019; 10: 775. https://dx.doi.org/10.3389/fendo.2019.00775.
- Yang P., Wu R., Zhang H. The effect of growth hormone supplementation in poor ovarian responders undergoing IVF or ICSI: a meta-analysis of randomized controlled trials. Reprod. Biol. Endocrinol. 2020; 18(1): 76.https://dx.doi.org/10.1186/s12958-020-00632-w.
- Liu F.T., Hu K.L., Li R. Effects of growth hormone supplementation on poor ovarian responders in assisted reproductive technology: a systematic review and meta-analysis. Reprod. Sci. 2021; 28(4): 936-48. https://dx.doi.org/10.1007/s43032-020-00298-0.
- Niringiyumukiza J.D., Cai H., Xiang W. Prostaglandin E2 involvement in mammalian female fertility: ovulation, fertilization, embryo development and early implantation. Reprod. Biol. Endocrinol. 2018; 16(1): 43.https://dx.doi.org/10.1186/s12958-018-0359-5.
- Патент RU 2 614 115 C1. Россия. Лунин В.Г., Юдин С.М. Рекомбинантный соматостатинсодержащий белок, способ его получения, инъекционный препарат для повышения мясной и молочной продуктивности сельскохозяйственных животных, а также способ использования препарата. Заявка: 2016.08.01. Публикация: 2017.03.22. [Patent RU 2 614 115 Russia. Lunin V.G., Yudin S.M. Recombinant somatostatin-containing protein, a method for its preparation, an injectable preparation for increasing meat and dairy productivity of farm animals, as well as a method for using the preparation. Application: 2016.08. Publication: 2017.03.22.(in Russian)].
- Решение Совета ЕЭК № 81 от 03.11.16 «Об утверждении Правил надлежащей лабораторной практики Евразийского экономического союза в сфере обращения лекарственных средств». [EEC Council Decision No. 81 of 03.11.16 "On Approval of the Rules of Good Laboratory Practice of the Eurasian Economic Union in the Field of Circulation of Medicines".(in Russian)].
- Liu T.E., Wang S., Zhang L., Guo L., Yu Z., Chen C., Zheng J. Growth hormone treatment of premature ovarian failure in a mouse model via stimulation of the Notch-1 signaling pathway. Exp. Ther. Med. 2016; 12(1): 215-21.https://dx.doi.org/10.3892/etm.2016.3326.
- Коптяева К.Е., Мужикян А.А., Гущин Я.А., Беляева Е.В., Макарова М.Н., Макаров В.Г. Некоторые особенности фиксации органов и тканей лабораторных животных для повышения качества гистологического анализа. Лабораторные животные для научных исследований. 2018; 2: 60-70. [Koptyaeva K., Muzhikyan A., Gushchin Ya., Belyaeva E., Makarova M., Makarov V. Some features of fixation of organs and tissues of laboratory animals for quality of gistological analysis. Laboratory animals for scientific research. 2018; (2): 60-70. (in Russian)]. https://dx.doi.org/10.29296/2618723X-2018-02-07.
- Hull K.L., Harvey S. Growth hormone and reproduction: a review of endocrine and autocrine/paracrine interactions. Int. J. Endocrinol. 2014; 2014: 234014. https://dx.doi.org/10.1155/2014/234014.
Received 13.06.2023
Accepted 10.07.2023
About the Authors
Aleksandr A. Matichin, Deputy Head of the Department of Specific Toxicology and Pharmacodynamics, RMC «HOME OF PHARMACY», matichin.aa@doclinika.ru,188663, Russia, Leningrad region, Vsevolozhsk district, p. Kuzmolovsky, Zavodskay str. 3, 245, https://orcid.org/0000-0001-7478-4942
Kirill L. Kryshen, PhD (Bio), Head of the Department of Specific Toxicology and Microbiology, RMC «HOME OF PHARMACY»,
188663, Russia, Leningrad region, Vsevolozhsk district, p. Kuzmolovsky, Zavodskay str. 3, 245, https://orcid.org/0000-0003-1451-7716
Natalya M. Faustova, PhD (Chem), Head of the Laboratory of Enzyme Immunoassay, RMC «HOME OF PHARMACY»,
188663, Russia, Leningrad region, Vsevolozhsk district, p. Kuzmolovsky, Zavodskay str. 3, 245, https://orcid.org/0000-0002-5557-1287
Yaroslav A. Gushchin, Head of the Laboratory Diagnostics Department, RMC «HOME OF PHARMACY»,
188663, Russia, Leningrad region, Vsevolozhsk district, p. Kuzmolovsky, Zavodskay str. 3, 245, https://orcid.org/0000-0002-7656-991Х
Marina N. Makarova, Dr. Med. Sci., Director, RMC «HOME OF PHARMACY»,
188663, Russia, Leningrad region, Vsevolozhsk district, p. Kuzmolovsky, Zavodskay str. 3, 245, https://orcid.org/0000-0003-3176-6386
Vyacheslav V. Reshetnik, Director, LLC "Urspharm", 117041, Russia, Moscow, Admiral Rudnev str., 4, office 6/31/1.
Gennady T. Sukhikh, Academician of the Russian Academy of Sciences, Dr. Med. Sci., Professor, Honoured Scientist of the Russian Federation, Director, Academician
V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation,
117997, Russia, Moscow, Oparina str., 4.
Sergei M. Yudin, Dr. Med. Sci., Professor, Director General, Centre for Strategic Planning of FMBA of Russia, 119121, Russia, Moscow, Pogodinskaya str., 10 bld. 1.
Vladimir G. Lunin, Dr. Bio. Sci., Leading Researcher, National Research Center for Epidemiology and Microbiology named after Honorary Academician N.F. Gamaleya,
Ministry of Health of the Russian Federation, 123098, Russia, Moscow, Gamaleya str., 18.
Corresponding author: Aleksandr A. Matichin, matichin.aa@doclinika.ru



