Rationale for preoperative treatment of advanced-stage endometrial ovarian cysts in infertile women
Lipatov I.S., Tezikov Yu.V., Tyutyunnik V.L., Kan N.E., Martynyuk D.A., Belousov I.S.
Objective: This study aimed to optimize the preoperative treatment of advanced-stage endometrial ovarian cysts (EOC) in infertile women to ensure ovarian protection, improve endometrial receptivity, and normalize the pro-inflammatory status.
Materials and methods: A comprehensive examination and treatment were performed on 114 patients with stage III–IV EOC. Group I consisted of 65 infertile women who received differentiated treatment with GnRH antagonists or dienogest to overcome infertility preoperatively. The aim was to reduce the size of the EOC, decrease the severity of pain, preserve the ovarian reserve, normalize endometrial receptivity, and correct the inflammatory status. Group II consisted of 49 women who underwent surgical treatment for infertility, similar to the patients in Group I. The examination was conducted before the start of treatment/surgery, in the postoperative period, and after six months of waiting for spontaneous pregnancy. To obtain reference values for the studied parameters, control group III was formed consisting of 35 healthy women with intact ovaries and male factor infertility. These women underwent infertility treatment with ART.
Results: Changes in EOC size, ovarian reserve (antral follicle count, AMH, FSH, and E2 levels), sex steroid receptors, molecular markers of cellular transformation and apoptosis, levels of pro-inflammatory cytokines in the eutopic endometrium, and EOC capsules in the study groups demonstrated the protective effect of preoperative hormonal preparation against surgical trauma. These changes resulted in an increase in the spontaneous pregnancy rate by 9.3 times, pregnancy rate with the use of ART by 2.4 times, and live birth rate by 3.5 times.
Conclusion: This study demonstrated the advantage of the preoperative hormonal stage of treatment in infertile patients with advanced-stage EOC. This advantage was observed in relation to the structural and functional state of the EOC, preservation of the ovarian reserve, normalization of endometrial receptivity and pro-inflammatory state, and increased effectiveness in achieving pregnancy with favorable perinatal outcomes.
Authors' contributions: Lipatov I.S., Tezikov Yu.V., Martynyuk D.A., Belousov I.S. – material collection and processing, conception and design of the study, drafting of the manuscript, analysis of relevant literature; Tyutyunnik V.L., Kan N.E. – data interpretation, editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the 1Samara State Medical University.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Lipatov I.S., Tezikov Yu.V., Tyutyunnik V.L., Kan N.E., Martynyuk D.A., Belousov I.S.
Rationale for preoperative treatment of advanced-stage endometrial ovarian cysts in infertile women.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (4): 75-86 (in Russian)
https://dx.doi.org/10.18565/aig.2023.300
Keywords
Currently, there is controversy surrounding the management strategies for infertile patients with various stages of endometrial ovarian cysts (EOC) [1]. This ambiguity stems from the multifactorial nature of endometriosis and infertility, leading to different approaches among obstetrician-gynecologists, surgeons, and reproductive specialists. Key factors influencing fertility include preservation of ovarian reserve, oocyte quality, eutopic endometrium receptivity (EER), and the ability to form a functional maternal-fetal-placental unit [2].
A widely accepted approach for addressing infertility in advanced-stage EOC and severe pain involves therapeutic and diagnostic laparoscopy. Ideally, this procedure should be performed only once in patients with infertility. Subsequently, patients wait for a spontaneous pregnancy. If pregnancy does not occur, assisted reproductive technology (ART) can be introduced into treatment plans [3]. However, there is ongoing debate regarding the negative impact of surgical intervention on germ cells and the sensitivity of ovaries to superovulation stimulation [4].
Numerous studies have shown that surgical trauma during EOC treatment leads to a decrease in ovarian reserve due to increased local inflammation, impaired iron metabolism and enzyme activity in the follicles, mitochondrial dysfunction, and intracellular oxidative stress [5, 6]. The size of the cysts removed also affects egg quality, which should be considered before gonadal surgery [7].
In recent years, meta-analyses have indicated that surgical treatment of EOC impairs ovarian reactivity to controlled hyperstimulation in ART cycles, resulting in a significant decrease in the ovarian reserve [8]. Assessing the outcomes of oocytes after the removal of large EOCs remains challenging [9, 10]. It is important to consider the high likelihood of developing premature ovarian failure syndrome when removing advanced-stage bilateral EOCs [11]. Despite the incomplete understanding of the mechanisms of infertility in EOC, careful removal of EOC has been shown to enhance the effectiveness of ART [12].
Dysregulation of progesterone receptor (PR) and progesterone resistance play significant roles in early reproductive loss. In EOC, both ectopic and eutopic endometria demonstrate impaired sensitivity to progesterone, leading to hyperstimulation by estrogen and negatively impacting endometrial readiness for complete decidualization and implantation [13]. Adjuvant hormone therapy after surgery has shown positive effects on oocyte quality and EER by inhibiting the progression of the endometriotic process and preventing recurrence. However, hormonal therapy after surgery cannot reverse the negative consequences of surgical trauma on oocyte preservation [14, 15].
To minimize the loss of ovarian reserve, enhance EER, and correct the proinflammatory state, optimal conditions for surgical intervention can be established preoperatively. This provides an additional method to implement ovarian protection [14, 15]. Despite the reduced ability to conceive with a large EOC, a sufficient ovarian reserve maintains the likelihood of pregnancy and live birth [16].
Functional ovarian cysts, which are connective tissue capsules with liquid contents, can change in size and even disappear based on the effectiveness of anti-inflammatory/hormonal treatment, cyclic processes of the reproductive axis, and elimination of the underlying cause [17]. EOCs are hormonally sensitive, and progesterone has an inhibitory effect on them during pregnancy, resulting in disease remission, regression, and reduced size and functional activity of EOC [18, 19]. Some studies have demonstrated that long-term therapy with the gonadotropin-releasing hormone (GnRH) agonist dienogest improves fertility rates in progressive EOC. Preoperative use of GnRH agonists and progestogens aims to reduce disease severity, enhance fertility restoration by increasing implantation frequency, and decrease early reproductive losses [20–22].
An analysis of several information databases, including PubMed, Scopus, eLibrary.Ru, MedLine, Cochrane, and Hinari, revealed a lack of specific recommendations regarding medical tactics for infertility caused by the severe stages of EOC. Furthermore, there is a dearth of evidence-based methods for preserving ovarian reserve and EER during pregnancy preparation that are currently available for practical use.
This study aimed to optimize the preoperative treatment of advanced-stage endometrial ovarian cysts in infertile women to ensure ovarian protection, improve endometrial receptivity, and normalize the pro-inflammatory status.
Materials and methods
We evaluated and treated 114 infertile patients with EOC stages III–IV, who formed two study groups. The treatment of patients in group I (n=65) consisted of four stages: preoperative, surgical, postoperative, waiting for spontaneous pregnancy for 6 months, and ART. The preoperative preparation aimed at reducing the severity of pain and the size of the EOC, preserving ovarian reserve, normalizing EER, and correcting the inflammatory status included preoperative hormonal treatment with GnRH agonist prescribed for 3 months (buserelin, starting in the first 5 days of the cycle at 3.75 mg, intramuscularly, once, every 4 weeks) or synthetic progestogen (dienogest for at least 4–5 months, 2 mg, daily, orally). GnRH agonist therapy was used in 46.2% (30/65) of the women with severe pain syndrome, while 53.8% (35/65) of the patients with moderate and mild pain syndrome received dienogest. Pain syndrome was assessed using a visual analog scale (VAS), where 0 points corresponded to no pain, 1–2 points corresponded to mild pain, 3–5 points corresponded to moderate pain, and ≥ 6 points corresponded to severe pain [23]. During the surgery, EOC desquamation, excision of endometriosis foci, and surgical dissection of adhesions were performed using laparoscopic access. After surgical treatment, 3.1% (2/65) of women with a critically diminished ovarian reserve were identified from group I (the level of anti-Mullerian hormone (AMH) was less than 0.5 ng/ml, follicle-stimulating hormone (FSH) was > 10 IU/ml). Both women were referred for infertility treatment by ART with oocyte donation. The remaining 63 patients in the postoperative period were prescribed dydrogesterone in a cyclical regimen (30 mg/day from days 16 to 25 of the cycle) to support phase II of the cycle. If spontaneous pregnancy did not occur within six months, the woman was referred for ART treatment. Group II consisted of 49 women who underwent surgical treatment to overcome infertility, similar to the patients in group I; no preoperative hormonal treatment was used. After surgical treatment, 16.3% (8/49) of women with critical ovarian reserve (AMH level less than 0.5 ng/ml) were also identified from patients in group II and sent for treatment with ART methods with oocyte donation. The remaining 41 women were managed expectantly for 6 months according to clinical recommendations: in the absence of spontaneous pregnancy, patients were referred for infertility treatment with ART [3, 24]. Control group III consisted of 35 apparently healthy women with intact ovaries and male factor infertility who were treated with ART. In ART programs, ovulation stimulation was performed according to a standard method with a long GnRH agonist stimulation protocol. EOC stage was determined according to the classification of current clinical guidelines [3]. The inclusion criteria for groups I and II were the presence of newly diagnosed EOC and histologically confirmed severe stages of EOC (III, IV), reproductive age less than 35 years, and first surgery for EOC. Non-inclusion criteria were hormonal therapy within 6 months before inclusion in the study, severe somatic and autoimmune pathology, endomyometritis, and male factor infertility (azoospermia). The exclusion criterion was failure to comply with the individual examination and treatment protocols.
The follow-up examinations of women included the stage of diagnosis with clarification of the cause of infertility before the start of preoperative hormonal treatment for ovarian protection (group I) or before surgery (group II), the 3rd day after surgery, and 6 months after surgery, before in vitro fertilization cycles (IVF). The examination of patients included collection of complaints and characteristics of menstrual function, assessment of medical history, and physical and bimanual vaginal examinations. Pain intensity was assessed using the VAS [23]. Based on intraoperative data, the fertility index was calculated [3, 25]. During the examination, all women underwent ultrasound examination, Doppler study (Voluson E6 GE Healthcare, GE USA); when calculating the antral follicle count (AFC), the recommendations of Khachkuzov S.G. were taken into account [26]. In patients of the control groups, the following parameters were determined by enzyme immunoassay, chemiluminescent immunoassay (Architect (Abbot, England)) before the 7th day of the cycle: AMH, estradiol (E2), luteinizing hormone (LH), FSH, prolactin (PRL), and thyroid-stimulating hormone (TSH), to exclude malignancy, and markers of neoplastic processes (CA125, HE4, ROMA index) were taken into account [27]. In the 2nd phase of the cycle (days 19–23), progesterone, pro-inflammatory cytokine interleukin (IL)-1β, and IL-6 levels were determined. To assess the protein synthesis function of the eutopic endometrium of menstrual blood, the concentration of glycodelin was determined, and a pipette biopsy was used for immunohistochemical (IHC) analysis and morphological characteristics of the endometrium. The EOC capsule was sent for morphological confirmation of diagnosis. Fragments of the EOC capsule were prepared for IHC analysis and determination of molecular marker expression (Biocare Test System, Germany). To assess the expression of sex steroid receptors (estrogen receptors (ER) and prolactin receptors (PR)), markers of cell proliferation Ki-67, and apoptosis p53, monoclonal antibodies were used (clone SP1, IgG isotype; clone PGR 16, IgG1 isotype; clone MM1, IgG1 isotype; clone 100 /D5, IgG1 isotype, respectively) [28].
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 25 PS. The distribution of continuous variables was tested for normality using the Shapiro-Wilk test. Continuous variables showing normal distribution were expressed as means (M) and standard deviation (SD); ANOVA tests were used to compare data. The equality of variance was assessed using Levene's test. Post hoc comparisons were performed using Tukey's test. Indicators with a different distribution than normal are presented in Me format [Q1 – 25%; Q3 – 75%]; For non-normally distributed parameters, the nonparametric Kruskal–Wallis was used followed by intergroup comparisons by the Mann–Whitney U test with Bonferroni correction (p<0.017). Within-group changes in the parameters during treatment were assessed using the paired Wilcoxon test. Frequencies were calculated for categorical indicators, and Pearson's χ² test with Yates correction was used to determine statistical differences. Correlation analysis was conducted by calculating the Spearman's rank correlation coefficients. The critical level of significance (p) when testing the statistical hypotheses was set at 0.05 [29, 30].
Results and discussion
For the purpose of objectifying the results of the impact of preoperative hormonal therapy in women with advanced EOC on ovarian reserve, EER, proinflammatory status, pain severity, and reproductive function, a comparative analysis of the baseline clinical and demographic characteristics of patients in the study groups was conducted, as shown in Table 1.
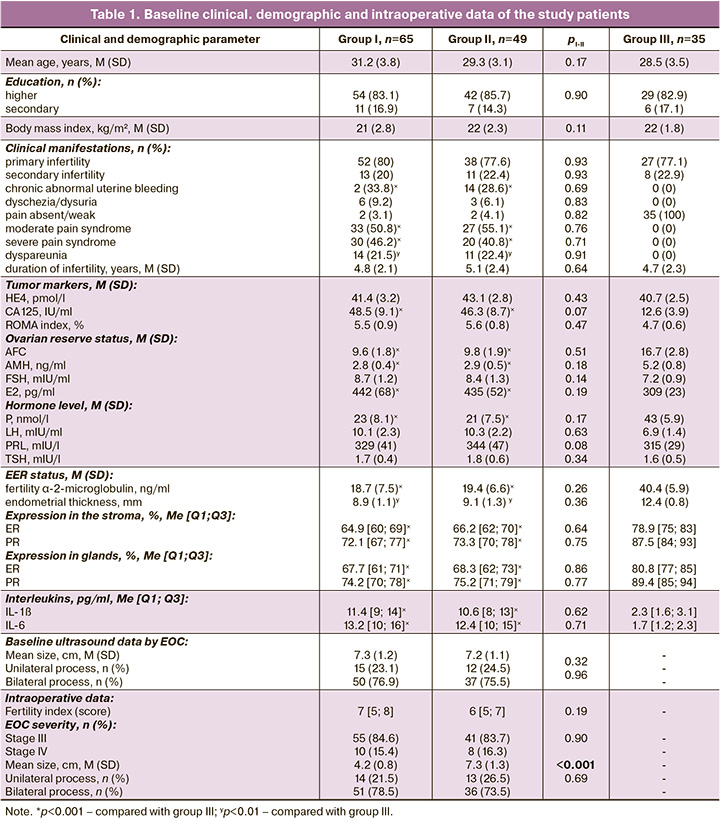
The groups were comparable in terms of age, social status, body mass index, duration of infertility and parity, PRL level, and thyroid function. Initially, attention was paid to women with ovarian tumor-like masses to exclude a malignant process through instrumental and laboratory examination for tumor markers (ultrasound, Doppler, and MRI data, cysto- and colonoscopy findings according to indications, CA-125, HE-4, and ROMA index). According to the results of the histological examination of the intraoperative material, a true neoplastic process was absent in all patients included in the study. Features of the clinical manifestations of advance-stage EOC include pain of varying severity, dysuria, dyschezia, and chronic abnormal uterine bleeding, the frequency of which was not statistically different between groups I and II. According to the data of the baseline examination, in patients with ovarian endometriosis of the study groups there were no significant differences in EOC size, thickness and protein synthetic function of the eutopic endometrium (α-2-microglobulin of fertility), and ovarian reserve parameters (AFC, AMH, E2, FSH), progesterone and pro-inflammatory IL). At the same time, the indicators differed from the results of the examination of women in group III (control): pI-III<0.001, pII-III<0.001. IHC analysis of the expression of ER and PR in biopsies of the eutopic endometrium of patients with advance-stage EOC and infertility in the study groups did not show statistically significant differences; however, differences were revealed when compared with the data from group III. An increase in the expression of ER and PR was noted in both stroma and glands (p<0.001). The obtained baseline data made it possible to compare groups I and II in terms of medical, demographic, and clinical indicators; baseline characteristics of the ovarian reserve; structural and functional state of the endometrium; and pro-inflammatory status.
Assessment of pain syndrome according to VAS in group I women after preoperative hormonal treatment showed a significant decrease in its severity compared with the baseline data: 89.2% (58/65) of patients had mild or no pain (χ²=93.63, p<0.001); 10.8% (7/65) had moderate pain syndrome (χ²=36.44, p<0.001), and there was no significant pain syndrome (χ²=22.57, p<0.001), which confirmed the antinociceptive effect of preoperative hormonal treatment.
The results obtained during diagnostic and treatment laparoscopy confirmed in group I a statistically significant decrease in the mean EOC size to 4.2 (0.8) cm, compared with the results of the baseline ultrasound before preoperative hormonal treatment – 7.3 (1.2) cm (p<0.001). Moreover, the intraoperative size of EOC in women in group I was statistically different from the intraoperative size of cysts in group II – 4.2 (0.8) versus 7.3 (1.3) cm, p<0.001). Assessment of the severity of EOC and their location did not refute the baseline intergroup similarity in these parameters (p>0.05) (Table 1).
The correlation analysis of ultrasound results of patients in groups I and II with intraoperative data showed the presence of a strong correlation between the size of the EOC (k 0.81–0.89, with p<0.05) and lateralization of the location of cysts (k 0.83–0, 94, p<0.05). Additionally, differences were noted in the presence of visible foci of endometriosis in the peritoneum. During preoperative hormonal treatment with GnRH agonist/progestagen, single lesions were detected in five (7.7%, 5/65) patients and in the absence of preoperative preparation in 20 (40.8%, 2/49) women (χ²I-II=16.02, pI-II <0.001). Calculation of the fertility index, taking into account data from anamnesis and intraoperative revision, showed no statistical difference between groups I and II (7 and 6 points, respectively; p=0.19), indicating the possibility of pregnancy with a probability of 40% [25].
The dynamics of the indicators of ovarian reserve, EER, IHC analysis of EOC capsules, IL, depending on the presence/absence of preoperative hormonal treatment, after surgical treatment are presented in Table 2, and before IVF in Table 3.
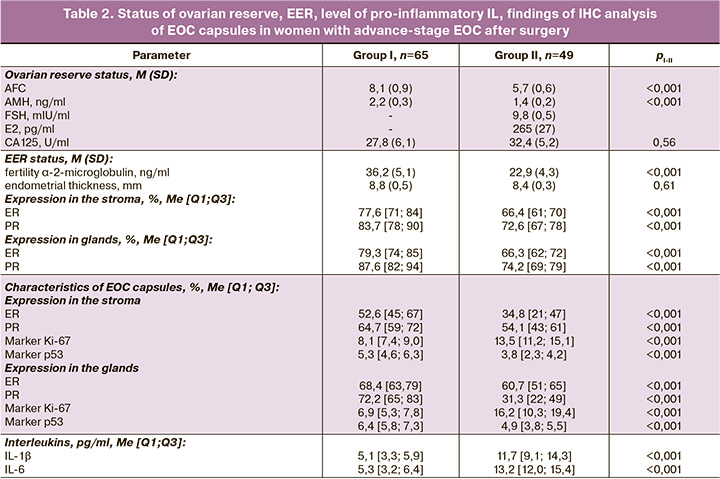
Postoperative examination for EOC stages III-IV showed a significant increase in FSH (p<0.01) in parallel with a decrease in E2 concentration by 39% (p<0.001) in group II compared to preoperative values, which can only be explained by the aggressive influence of surgical trauma on the ovaries and ovarian reserve. This conclusion is confirmed by the analysis of changes in the AFC during treatment: there was a decrease in preoperative AFC from 9.8 (1.9) to 5.7 (0.6) in the postoperative period (p<0.001), as well as in the AMH content from 2. 9 (0.5) to 1.4 (0.2) ng/ml (p<0.001), respectively. In addition, after surgical treatment in group II, eight patients (16.6%, 8/49) were identified with a sharply reduced ovarian reserve to critical values, for whom urgent ART with oocyte donation was indicated, and in group I with preoperative hormonal treatment, there were two such women (3.1%, 2/65) – χ²=4.59, p=0.03. Intergroup comparisons and intragroup dynamics of the AFC and AMH are shown in Figures 1 and 2, respectively.
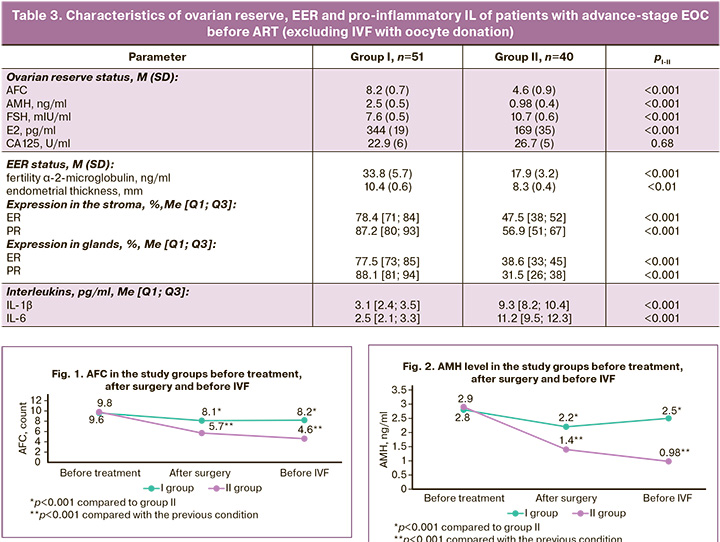
Consequently, after ovarian surgery, in the absence of preoperative hormonal treatment, a statistically significant decrease in all ovarian reserve parameters (AFC, AMH, FSH, and E2) was noted. At the same time, in group I with preoperative ovarian protection in the postoperative period, there was an insignificant decrease in ovarian reserve (AFC, AMH). This pattern was observed 6 months after surgical treatment in 51 patients in group I (12 women had spontaneous pregnancy, 2 had urgent IVF with oocyte donation) and 40 patients in group II (one had spontaneous pregnancy, eight had ART with donation of oocytes). The ovarian reserve parameters in group I were slightly changed without statistical differences from the baseline data (AFC 8.2 (0.7) versus 9.6 (1.8); AMH 2.5 (0.5) versus 2.8 ( 0.4) ng/ml; E2 344 (19) versus 442 (68) ng/ml; FSH 7.6 (0.5) versus 8.7 (1.2) mIU/ml), which is explained by the ovarian-protective effect of the prescribed preoperative hormonal treatment with GnRH agonist/dienogest. Attention should also be paid to a stable AFC level (8.2 (0.7) versus 8.1 (0.9) with a statistically significant increase in the number of obtained oocytes and M2 oocytes in IVF protocols – 6.5 (0.6) versus 3.3 (0.4) in group II, p<0.001 and 6.3 (0.4) versus 2.7 (0.3) in group II, p<0.001, according to the indicators (Table 4). even a slight increase in AMH content before IVF (2.5 (0.5) versus 2.2 (0.3) ng/ml), which is undoubtedly associated with the quantitative and qualitative preservation of the ovarian reserve, increased sensitivity to progesterone, local and systemic anti-inflammatory and antinociceptive effect, normalization of the conduction of nerve impulses, microcirculation and lymphatic drainage, inhibition of disease progression due to the use of GnRH agonist/dienogest at the preoperative stage, dydrogesterone after surgery. Characterizing the ovarian reserve in group II, progressive deterioration should be noted not only in the postoperative period but also within 6 months after surgery. Thus, there was a negative dynamics of AFC (from 5.7 (0.6) to 4.6 (0.9)), AMH (from 1.4 (0.2) to 0.98 (0.4) ng/ ml)), FSH (from 9.8 (0.5) to 10.7 (0.6) mIU/ml), E2 (from 265 (27) to 169 (35) ng/ml), which confirms oocyte alteration due to surgical trauma with subsequent progression. Undoubtedly, important evidence of the impact of preoperative hormonal treatment on EOC is the characterization of the IHC profile of the cyst capsule with an assessment of the representation of molecular markers of cell proliferation, programmed cell death, and sex steroid receptors. Heterogeneous data were obtained from the IHC profiles of EOC capsules in groups I and II (Table 2).
In the tissue of EOC capsules of women in group II, the representation of RP and ER was significantly reduced, both in comparison with the data on the eutopic endometrium and with the results of IHC analysis of EOC capsules in group I (p<0.001). Impaired ER expression is associated with changes in the synthesis of both forms of ER [31]. Increasing the receptivity of the heterotopic endometrium to progesterone increases the effectiveness of blocking the activity and progression of the process and the local anti-inflammatory action [2]. An increase in RP in group I was noted in both the stromal and glandular components with a range of indicators from 59 to 83%, approaching the data on EER of the control group, where the range of RP was from 84 to 93%, with variability of the RP indicator in group II EOC capsules, from 43 to 61%.
Cellular transformation processes play a key role in the structural and functional states of EOC [32]. Violation of the synthesis of proteins that regulate programmed cell death and proliferation contributes to the formation of pathological processes and their progression, recurrence, and lack of the expected effects of treatment [33]. The expression of the nuclear marker of cell proliferation and ribosomal RNA transcription (Ki-67), according to the results of the IHC profile of EOC capsules, differed significantly in group I, being in an intermediate position between the expression of the marker in control samples of eutopic endometrium and in EOC capsules of group II (in the stroma: 8.1% [7.4; 9.0] versus 6.6% [3.1; 7.5] in the control and 13.5% [11.2; 15.1] in group II EOC capsules, at pI-III<0.001 and pI-II<0.001; in the glands: 6.9% [5.3;7.8] versus 4.0% [3.0;4.8] in the control and 16.2% [10,3;19,4] in EOC capsules of group II, with pI-III<0.001 and pI-II<0.001).
It should be noted that there are clinical and laboratory parallels, namely that severe constant pain syndrome is characteristic of women with the greatest increase in Ki-67 expression. This is also explained by peritoneal infiltration with endometriosis foci in these women. The opposite pattern was observed for the apoptosis marker p53, where the variability of changes depended on the presence/absence of preoperative hormonal treatment. There was a significant increase in p53 in group I by 1.4 times and 1.3 times in the stroma and glands of the EOC capsules, compared with group II (pI-II<0.001), with a statistically insignificant decrease in p53 by 1.2 times, relative to the endometrial stroma of the control group (6.2% [4.8;6.5], p=0.08) and an increase of 2.9 times, relative to the glandular component of the control group endometrial samples (2.2% [1.5;3.5], pI-III<0.001). In general, a change in the ratio of cellular transformation markers in favor of apoptosis against the background of normalization of the representation of receptors for sex steroids due to the preoperative stage of hormonal preparation can convincingly explain the statistically significant decrease in the size of EOC by 42.5% (pI-II<0.001). Among the mechanisms that help reduce the size of EOC, one should highlight a decrease in local inflammation and swelling of the ovarian tissue and EOC, a change in the regulation of cellular transformation processes in the heterotopia focus, a decrease in the influence of hyperestrogenemia and an increase in sensitivity to progesterone, and a decrease in local immunopathological processes against the background of normalization of the connection between the hormonal and immunological circuit regulation of the state of the reproductive system, and the predominance of resorptive processes over secretory processes [9, 33]. As a result of reducing the volume of EOC, a clearer distinction between healthy and pathological tissues in the ovary and optimal conditions are created for more careful enucleation of the cyst, preservation of the ovarian reserve, reduction of local and systemic inflammation, which initiates the possible progression of oocyte alteration, and disturbance of EER in the long term.
The characteristics of EER markers, endometrial thickness, and protein-synthetic function in the postoperative period and 6 months after surgery in women in group I with pre- and postoperative hormonal therapy indicated a statistically significant increase in the representation of PR and ER (p<0.001), the concentration of glycodelin in menstrual blood (p<0.001), and a slight increase in endometrial thickness (10.4 (0.6) versus 9.6 (1.8) mm), which collectively indicate the normalization of EER and its ability to fully decidualize, implant, and participate in invasion and formation of the early placenta. At the same time, the initially impaired EER in group II women with advanced EOC persisted in the postoperative period, with an increase in changes during the 6-month wait for spontaneous pregnancy.
The negative influence of pro-inflammatory cytokines through epigenetic mechanisms on the reproductive axis, the synthesis of both forms of PR and sensitivity to progesterone, and the effectiveness of therapeutic measures, both in terms of the progression of endometriosis and overcoming infertility, has been demonstrated [34]. Systemic levels of IL-1β and IL-6 are shown in Tables 1, 2, and 3 and indicate normalization of inflammatory status in women in group I and preservation of inflammatory status in patients in group II, whose treatment was limited to only ovarian surgery.
The protocol parameters and IVF outcomes in groups I, II, and III are presented in Table 4.
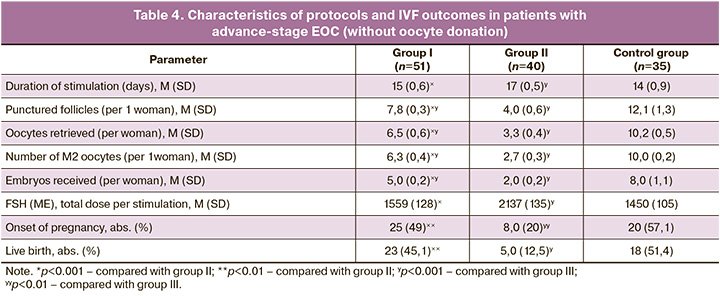
The primary outcomes of the effectiveness of management strategies for infertile women with EOC stages III–IV were the onset of pregnancy and delivery with a favorable perinatal outcome for a live fetus. Thus, in group II, after the surgical stage of treatment of EOC stages III–IV, spontaneous pregnancy rate within 6 months was 2% (1/49); according to the results of postoperative assessment of the ovarian reserve in 8 patients, a critical decrease was revealed, requiring IVF with oocyte donation; all observations resulted in live births. After 6 months of waiting for spontaneous pregnancy, ART was used in 40 patients, of whom 17.5% (7/40) became pregnant, 43% (3/7) had spontaneous abortions, and 57% (4/7) had live births. In total, in group II, pregnancy occurred in 32.7% (16/49) of cases, with the exception of ART with oocyte donation in 16.3% (8/49), and live births occurred in 26.5% (13/49) of patients without ART with oocyte donation in 10.2% (5/49) of observations. In group I, with the preoperative stage of hormonal ovarian protection and the postoperative administration of dydrogesterone in a cyclic period of 6 months, spontaneous pregnancy occurred in 18.5% (12/65) of the observations. Moreover, in the postoperative period, a critical ovarian reserve was detected in only two patients who successfully entered the IVF program with oocyte donation with a favorable perinatal outcome. All 51 patients without spontaneous pregnancies underwent ART). Pregnancy occurred in 25.5% (13/51) of cases, of which, in two cases, it ended in spontaneous miscarriage and in 21.6% (11/51) of live births. In general, in group I the pregnancy rate was 41.5% (27/65), without IVF with oocyte donation – 38.5% (25/65), the rate of live birth was 38.5% (25/65), without ART with oocyte donation – 35.4% (23/65).
In control group III, all 35 patients were prescribed ART because of male factor infertility. At the same time, pregnancy occurred in 57.1% (20/35) and live births in 51.4% (18/35) of the observations.
The difference in the management strategy of women with severe EOC stages I and II in the comparison groups was preoperative hormonal ovarian protection with GnRH agonist/dienogest, as well as cyclic administration of dydrogesterone in phase II of the cycle. Statistically significant reductions in pregnancy and live birth rates in group II, compared with group I, excluding the use of ART with donor oocytes (χ²I-II=5.6, pI-II=0.02 (OR 3.2 [1, 29;7.94]) and χ²I-II=8.3, pI-II<0.01 (OR 4.8 [1.68;13.85]), respectively, can only be explained by the aggressive effect of surgical trauma on the ovarian reserve, impaired EER, and pro-inflammatory state, which were successfully corrected by the use of hormonal ovarian protection in group I and made it possible, thanks to more careful surgical intervention, preserved ovarian reserve, and normalization of EER and inflammatory status, to optimize overcoming infertility at subsequent stages. This fact is also confirmed by an increase in the spontaneous pregnancy rate in group I by 9.3 times (18.5% versus 2%) – χ²I-II=5.92, pI-II=0.02 (OR 10.9 [1, 36;86,74]), which is undoubtedly due to the beneficial effects of preoperative hormonal treatment. Consequently, the preoperative stage of differentiated hormonal therapy with GnRH agonist/progestogen has shown effectiveness in overcoming infertility in women with EOC stages III–IV, which resulted in an increase in the pregnancy rate (without the use of donor oocytes) by 2.4 times and live births (without the use of donor oocytes) by 3.5 times.
Conclusion
Advanced-stage endometrial ovarian cysts are a complex interdisciplinary problem. They are associated with the versatility of the disease, individual risk factors, and the optimal use of surgical and reproductive approaches. In most cases, treating infertility in advanced-stage EOC requires less aggressive surgical interventions. It involves preserving the ovarian reserve and ensuring eutopic endometrial receptivity. Therefore, the proposed method of preoperative ovarian protection addresses the important issue of realizing reproductive potential in complex gynecological pathologies.
Utilizing a comprehensive examination, this study demonstrated the advantages of preoperative differentiated hormonal treatment for patients with EOC stages III–IV. It improves the structural and functional state of EOC, preserves the ovarian reserve, normalizes EER, corrects the proinflammatory state, and increases the likelihood of achieving pregnancy with a favorable perinatal outcome. These results support the need for preoperative preparation in women with severe forms of ovarian endometriosis and infertility. The method is based on pathogenetic evidence and is widely available for practical use, economically acceptable and safe.
References
- Адамян Л.В., Андреева Е.Н. Эндометриоз и его глобальное влияние на организм женщины. Проблемы репродукции. 2022; 28(1): 54-64. [Adamyan L.V., Andreeva E.N. Endometriosis and its global impact on the woman's body. Problems of Reproduction. 2022; 28(1): 54-64. (in Russian)]. https://dx.doi.org/10.17116/repro20222801154.
- Беженарь В.Ф., Кузьмина Н.С., Калугина А.С. Влияние хирургического лечения эндометриом яичников на состояние овариального резерва у пациенток с бесплодием. Эффективная фармакотерапия. 2022; 18(24): 6-11. [Bezhenar V.F., Kuzmina N.S., Kalugina A.S. Effect of surgical treatment of ovarian endometriomas on the state of ovarian reserve in patients with infertility. Efficient Pharmacotherapy. 2022; 18(24): 6-11. (in Russian)]. https://dx.doi.org/10.33978/2307-3586-2022-18-24-6-11.
- Министерство здравоохранения Российской Федерации. Эндометриоз. Клинические рекомендации. М.; 2020. Доступно по: https://roag-portal.ru/recommendations_obstetrics [Ministry of Health of Russian Federation. Endometriosis. Clinical Guidelines. Moscow; 2020. (in Russian). Available at: https://roag-portal.ru/recommendations_obstetrics].
- Федоров А.А., Попов А.А., Краснопольская К.В., Хабибуллах Т., Овсянникова М.Р., Ершова И.Ю., Коваль А.А., Тюрина С.С. Влияние хирургического лечения на фертильность у пациенток с эндометриоидными кистами. Российский вестник акушера-гинеколога. 2022; 22(5): 56-61. [Fedorov A.A., Popov A.A., Krasnopol’skaya K.V., Khabibullah T., Ovsyannikova M.R., Ershova I.Yu., Koval’ A.A., Tyurina S.S. Effect of surgical treatment on fertility in patients with endometrioid cysts. Effect of surgical treatment on fertility inpatients with endometrioid cysts. Russian Bulletin of Obstetrician-Gynecologist. 2022; 22(5): 56-61. (in Russian)]. https://dx.doi.org/10.17116/rosakush20222205156.
- Coccia M.E., Rizzello F., Capezzuoli T., Evangelisti P., Cozzi C., Petraglia F. Bilateral endometrioma excision: surgery-related damage to ovarian reserve. Reprod. Sci. 2019; 26(4): 543-50. https://dx.doi.org/10.1177/1933719118777640.
- Hong S.B., Lee N.R., Kim S.K., Kim H., Jee B.C., Suh C.S. et al. In vitro fertilization outcomes in women with surgery induced diminished ovarian reserve after endometrioma operation: comparison with diminished ovarian reserve without ovarian surgery. Obstet. Gynecol. Sci. 2017; 60(1): 63-8. https://dx.doi.org/10.5468/ogs.2017.60.1.63.
- Safdarian L., Ghalandarpoor Attar S.N., Aleyasin A., Aghahosseini M., Sarfjoo F.S., Hosseinimousa S. Investigation of anti-mullerian hormone (AMH) level and ovarian response in infertile women with endometriosis in IVF cycles. Int. J. Reprod. Biomed. 2018; 16(11): 719-22.
- Nagase Y., Matsuzaki S., Ueda Y., Kakuda M., Kakuda S., Sakaguchi H. et al. Association between endometriosis and delivery outcomes: a systematic review and meta-analysis. Biomedicines. 2022; 10(2): 478. https://dx.doi.org/10.3390/biomedicines10020478.
- Tanbo T., Fedorcsak P. Endometriosis-associated infertility: aspects of pathophysiological mechanisms and treatment options. Acta Obstet. Gynecol. Scand. 2017; 96(6): 659-67. https://dx.doi.org/10.1111/aogs.13082.
- De Ziegler D., Pirtea P., Carbonnel M., Poulain M., Cicinelli E., Bulletti C. et al. Assisted reproduction in endometriosis. Best Pract. Res. Clin. Endocrinol. Metab. 2019; 33(1): 47-59. https://dx.doi.org/10.1016/j.beem.2018.10.001.
- Patel B.G., Lenk E.E., Lebovic D.I., Shu Y., Yu J., Taylor R.N. Pathogenesis of endometriosis: interaction between endocrine and inflammatory pathways. Best Pract. Res. Clin. Obstet. Gynaecol. 2018; 50: 50-60. https://dx.doi.org/10.1016/j.bpobgyn.2018.01.006.
- Corachán A., Pellicer N., Pellicer A., Ferrero H. Novel therapeutic targets to improve IVF outcomes in endometriosis patients: a review and future prospects. Hum. Reprod. Update. 2021; 27(5): 923-72. https://dx.doi.org/10.1093/humupd/dmab014.
- Massarotti C., Mirabelli Badenier I., Paudice M., Scaglione G., Remorgida V., Vellone V.G. Steroids receptors immunohistochemical expression in different sites of endometriosis. J. Gynecol. Obstet. Hum. Reprod. 2021; 50(3): 101861. https://dx.doi.org/10.1016/j.jogoh.2020.101861.
- Murji A., Biberoğlu K., Leng J., Mueller M.D., Römer T., Vignali M. et al. Use of dienogest in endometriosis: a narrative literature review and expert commentary. Curr. Med. Res. Opin. 2020; 36(5): 895-907. https://dx.doi.org/10.1080/03007995.2020.1744120.
- Давыдов А.И., Белоцерковцева Л.Д., Таирова М.Б. Эндометриоидные кисты яичников: обоснование послеоперационной гормональной терапии. Вопросы гинекологии, акушерства и перинатологии. 2019; 18(2): 122-8. [Davydov A.I., Belotserkovtseva L.D., Tairova M.B. Endometrioid ovarian cysts: rationale for postoperative hormonal therapy. Gynecology, Obstetrics and Perinatology. 2019; 18(2): 122-8. (in Russian)]. https://dx.doi.org/10.20953/1726-1678-2019-2-122-128.
- Чернуха Г.Е., Марченко Л.А., Гусев Д.В. Поиск оптимальных решений и пересмотр тактики ведения пациенток с эндометриозом. Акушерство и гинекология. 2020; 8: 12-20. [Chernukha G.E., Marchenko L.A., Gusev D.V. Searching for optimal decisions and revising management tactics for patients with endometriosis. Obstetrics and Gynecology. 2020; (8): 12-20. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.8.12-20.
- Тимофеева О.С., Петров И.А., Гайфулина Ж.Ф., Самойлова Ю.Г., Тихоновская О.А., Кудлай Д.А., Петрова М.С., Логвинов С.В., Михеенко Г.А., Оккель Ю.В. Тактика ведения пациенток с функциональными кистами яичников в программах вспомогательных репродуктивных технологий. Вопросы гинекологии, акушерства и перинатологии. 2023; 22(2): 92-7. [Timofeeva O.S., Petrov I.A., Gaifulina Zh.F., Samoilova Iu.G., Tikhonovskaya O.A., Kudlay D.A., Petrova M.S., Logvinov S.V., Mikheenko G.A., Okkel U.V. Management strategies for patients with functional ovarian cysts in assisted reproductive technology programs. Gynecology, Obstetrics and Perinatology. 2023; 22(2): 92-7. (in Russian)]. https://dx.doi.org/10.20953/1726-1678-2023-2-92-97.
- Koninckx P.R, Zupi E., Martin D.C. Endometriosis and pregnancy outcome. Fertil. Steril. 2018; 110(3): 406-7. https://dx.doi.org/10.1016/j.fertnstert. 2018.06.029.
- Дубровина С.О., Берлим Ю.Д., Гимбут В.С., Вовкочина М.А., Воронова О.В., Александрина А.Д. Положительное влияние беременности на эндометриоз яичников – реальность или вымысел? Акушерство и гинекология. 2020; 5: 174-80. [Dubrovina S.O., Berlim Yu.D., Gimbut V.S., Vovkochina M.A., Voronova O.V., Aleksandrina A.D. The positive effect of pregnancy on ovarian endometriosis: reality or fiction? Obstetrics and Gynecology. 2020; (5): 174-80. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.5.174-180.
- Ozaki R., Kumakiri J., Jinushi M., Ikuma S., Murakami K., Kawasaki Y. et al. Comparison of effect of preoperative dienogest and gonadotropin-releasing hormone agonist administration on laparoscopic cystectomy for ovarian endometriomas. Arch. Gynecol. Obstet. 2020; 302(4): 969-76. https://dx.doi.org/10.1007/s00404-020-05691-3.
- Sahin G., Acet F., Biler A., Meseri R., Tavmergen Goker E.N., Tavmergen E. Assisted reproductive treatment outcomes of women with endometriomas: Either with or without previous ovarian surgery. Int. J. Clin. Pract. 2021; 75(12): e14991. https://dx.doi.org/10.1111/ijcp.14991.
- Muzii L., Galati G., Di Tucci C., Di Feliciantonio M., Perniola G., Di Donato V. et al. Medical treatment of ovarian endometriomas: a prospective evaluation of the effect of dienogest on ovarian reserve, cyst diameter, and associated pain. Gynecol. Endocrinol. 2020; 36(1): 81-3. https://dx.doi.org/10.1080/09513590.2019.1640199.
- Джеломанова О.А. Психоэмоциональные нарушения и их связь с интенсивностью боли у женщин репродуктивного возраста с синдромом хронической тазовой боли. Медико-социальные проблемы семьи. 2022; 27(2): 28-35. [Dzhelomanova O.A. Psycho-emotional disorders and their assotiation with intensity of pain in women of reproductive age with chronic pelvic pain syndrome. Medical and social problems of family. 2022; 27(2): 28-35. (in Russian)].
- Хамошина М.Б., Оразов М.Р., Абитова М.З., Волкова С.В., Арютин Д.Г., Алеев И.А., Байрамова А.А. Бесплодие, ассоциированное с эндометриозом яичников: современный взгляд на проблему. Вопросы гинекологии, акушерства и перинатологии. 2021; 20(1): 98-104. [Khamoshina M.B., Orazov M.R., Abitova M.Z., Volkova S.V., Aryutin D.G., Aleev I.A., Bayramova A.A. Infertility associated with ovarian endometriosis: a modern view to the problem. Gynecology, Obstetrics and Perinatology. 2021; 20(1): 98-104. (in Russian)]. https://dx.doi.org/10.20953/1726-1678-2021-1-98-104.
- Cook A.S., Adamson G.D. The role of the endometriosis fertility index (EFI) and endometriosis scoring systems in predicting infertility outcomes. Curr. Obstet. Gynecol. Rep. 2013; 2: 186-94. https://dx.doi.org/10.1007/s13669-013-0051-x.
- Хачкузов С.Г. УЗИ в гинекологии. Симптоматика. Диагностические трудности. Руководство для врачей. Санкт-Петербург: ЭЛБИ-СПб; 2016. 672 c. [Khachkuzov S.G. Ultrasound in gynecology. Symptomatology. Diagnostic difficulties. Manual for doctors. Saint-Petersburg: ELBI-SPb; 2016. 672p. (in Russian)].
- Чибисова Г.М., Хабаров С.В. Комплексное определение онкомаркеров СА125, HE4 и индекса ROMA как фактор прогноза развития рака яичников. Вестник новых медицинских технологий. 2018; 25(3): 15-20. [Chibisova G.M., Khabarov S.V. Complex determination of oncoprotein CA125, HE4 and ROMA indexas a prognosis of ovarian cancer. Journal of New Medical Technologies. 2018; 25(3): 15-20. (in Russian)]. https://dx.doi.org/10.24411/1609-2163-2018-16158.
- Kumar G.L., Rudbeck L., eds. Immunohistochemical staining methods. Education guide. Dako North America, Carpinteria, California. 2009. 172p. [Иммуногистохимические методы. Пер. с англ. Франк Г.А., Мальков П.Г., ред. Мoscow; 2011. 223с. (in Russian)].
- Котельников Г.П., Шпигель А.С. Доказательная медицина. Научно обоснованная медицинская практика. 2-е изд. М.: ГЭОТАР-Медиа; 2012. 242 с. [Kotel'nikov G.P., Shpigel' A.S. Evidence-based medicine. Evidence-based medical practice. 2nd ed. Moscow: GEOTAR-Media; 2012. 242p. (in Russian)].
- Ланг Т., Альтман Д. Основы описания статистического анализа в статьях, публикуемых в биомедицинских журналах. Руководство «Статистический анализ и методы в публикуемой литературе (САМПЛ)». Медицинские технологии. Оценка и выбор. 2014; 1: 11-6. [Lang T., Altman D. Basic description of statistical analysis in articles published in biomedical journals. The leadership of the «Statistical analyses and methods in the published literature (SAMPL)». Medical technologies. Evaluation and selection. 2014; (1): 11-6. (in Russian)].
- Пшеничнюк Е.Ю., Асатурова А.В., Адамян Л.В., Зайцев Н.В. Иммуногистохимические особенности эутопического и эктопического эндометрия у пациенток с рецидивирующим течением эндометриоидных кист яичников. Акушерство и гинекология. 2018; 3: 84-95. [Pshenichnyuk E.Yu., Asaturova A.V., Adamyan L.V., Zaitsev N.V. Immunohistochemical features of eutopic and ectopic endometrium in patients with recurrent ovarian endometrioid cysts. Obstetrics and Gynecology. 2018; (3): 84-95. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.3.84-95.
- Тезиков Ю.В., Липатов И.С., Печкуров Д.В., Тютюнник В.Л., Кан Н.Е., Протасов А.Д., Ковязина И.О. Прогнозирование нарушений предлактационной перестройки и профилактика патологического лактогенеза при метаболическом синдроме. Акушерство и гинекология. 2018; 11: 60-8. [Tezikov Yu.V., Lipatov I.S., Pechkurov D.V.,Tyutyunnik V.L., Kan N.E., Protasov A.D., Kovyazina I.O. Predicting impairment of pre-lactation transformation and preventing lactogenesis failure in metabolic syndrome. Obstetrics and Gynecology. 2018; (11): 60-8. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.11.60-68.
- Тезиков Ю.В., Стрижаков А.Н., Липатов И.С., Калинкина О.Б., Аравина О.Р., Мартынова Н.В. Клиническая значимость иммуногистохимического профиля эндометриоидных кист яичников. Акушерство и гинекология. 2020; 2: 116-24. [Tezikov Yu.V., Strizhakov A.N., Lipatov I.S., Kalinkina O.B., Aravina O.R., Martynova N.V. Clinical significance of the immunohistochemical profile of ovarian endometrioid cysts. Obstetrics and Gynecology. 2020; (2): 116-24. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.2.116-124.
- Сухих Г.Т., Серов В.Н., Адамян Л.В., Баранов И.И., Беженарь В.Ф., Габидуллина Р.И., Дубровина С.О., Козаченко А.В., Подзолкова Н.М., Сметник А.А., Тапильская Н.И., Уварова Е.В., Ших Е.В., Ярмолинская М.И. Алгоритмы ведения пациенток с эндометриозом: согласованная позиция экспертов Российского общества акушеров-гинекологов. Акушерство и гинекология. 2023; 5: 159-176. [Sukhikh G.T., Serov V.N., Adamyan L.V., Baranov I.I., Bezhenar V.F., Gabidullina R.I., Dubrovina S.O., Kozachenko A.V., Podzolkova N.M., Smetnik A.A., Tapilskaya N.I., Uvarova E.V., Shikh E.V., Yarmolinskaya M.I. Algorithms for the management of patients with endometriosis: an agreed position of experts from the Russian Society of Obstetricians and Gynecologists. Obstetrics and Gynecology. 2023; (5): 159-76 (in Russian)]. https://dx.doi.org/10.18565/aig.2023.132.
Received 22.12.2023
Accepted 29.03.2024
About the Authors
Igor S. Lipatov, Professor, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology of the Institute of Clinical Medicine, Samara State Medical University, Ministry of Health of Russia, 443099, Russia, Samara, Chapaevskaya str., 89, +7(846)958-24-18, i.lipatoff2012@yandex.ru, https://orcid.org/0000-0001-7277-7431,Researcher ID: С-5060-2018, SPIN-code: 9625-2947, Author ID: 161371, Scopus Author ID: 6603787595.
Yurii V. Tezikov, Professor, Dr. Med. Sci., Head of the Department of Obstetrics and Gynecology of the Institute of Clinical Medicine, Samara State Medical University,
Ministry of Health of Russia, 443099, Russia, Samara, Chapaevskaya str., 89, +7(846)958-24-18, yra.75@inbox.ru, https://orcid.org/0000-0002-8946-501X,
Researcher ID: С-6187-2018, SPIN-code: 2896-6986, Author ID: 161372, Scopus Author ID: 6603787595.
Victor L. Tyutyunnik, Professor, Dr. Med. Sci., Leading Researcher of Research and Development Service, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(903)969-50-41, tioutiounnik@mail.ru,
https://orcid.org/0000-0002-5830-5099, Researcher ID: B-2364-2015, SPIN-code: 1963-1359, Authors ID: 213217, Scopus Author ID: 56190621500,
Natalia E. Kan, Professor, Dr. Med. Sci., Deputy Director of Science, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(926)220-86-55, kan-med@mail.ru, https://orcid.org/0000-0001-5087-5946, Researcher ID: B-2370-2015, SPIN-code: 5378-8437, Authors ID: 624900, Scopus Author ID: 57008835600.
Darya A. Martynyuk, 6th year student of the Institute of Clinical Medicine, Samara State Medical University, Ministry of Health of Russia, 443099, Russia, Samara, Chapaevskaya str., 89, +7(846)958-24-18, martynuk.darya@yandex.ru, https://orcid.org/0009-0002-3253-7382
Ivan S. Belousov, 6th year student of the Institute of Clinical Medicine, Samara State Medical University, Ministry of Health of Russia, 443099, Russia, Samara,
Chapaevskaya str., 89, +7(846)958-24-18, beloucov_ivan@icloud.com, https://orcid.org/0009-0001-0889-0218



