New possibilities to use cardiotocography to assess the fetal functional state in the second trimester of pregnancy
Objective. To investigate the feasibility of using cardiotocography (CTG) in the 2nd trimester of pregnancy to assess the fetal functional state and identify criteria for characterizing fetal hypoxia in this period of gestation.Zamaleeva R.S., Maltseva L.I., Cherepanova N.A., Frizina A.V., Zefirova T.P.
Material and methods. This study analyzed the features of CTG in the 2nd trimester in 100 pregnant women with a physiological course of pregnancy and childbirth and 40 pregnant women with adverse perinatal outcomes.
Results. The following CTG parameters were characteristic of pregnant women with physiological pregnancy in the second trimester: STV more than 3.2 ms, LTV more than 37 ms, EHV more than 5, ELV less than 4 minutes, and fewer than three decelerations. The patients with gestational complications had the following CTG findings: an STV decrease below 3.2 ms; LTV below 37 ms; the combination of a decrease in EHV of less than 5 minutes with an increase in ELV of more than 5 minutes, and more than three decelerations.
Conclusion. The criteria for the pathological type of CTG in the 2nd trimester of pregnancy cannot be used to diagnose fetal hypoxia but may help predict the development of gestational complications.
Keywords
Despite the advances of modern medicine, perinatal morbidity and mortality rates in Russia remain high. Progress in reducing the stillbirth rate has been slow, which has declined only 2.3% over the last six years. Many authors attribute this fact to the failure to diagnose or delayed diagnosis of fetal hypoxia [1–5]. Reproductive losses and considerable health care costs associated with perinatal care for infants, who have suffered intrauterine hypoxia result in significant social and economic losses to society in general [6, 7]. One of the important diagnostic modalities based on registration of the heart rate for investigating fetal hypoxia is antenatal cardiotocography (CTG), the most common, painless, safe and widely available method for fetal assessment. However, CTG is underused, since its interpretation and computer analysis is possible at gestational age less than 24 weeks, but practically, according to the order of the Ministry of Health of the Russian Federation 572n of November 1, 2012, it is used only from 33 weeks’ gestation. Therefore, this diagnostic modality remains underutilized in the 2nd trimester of pregnancy. Fetal assessment is monitored by ultrasound and perinatal biochemical screening at 11–13 and 20–22 weeks of gestation. It is obvious that intermittent auscultation of fetal heart rate does not permit a diagnosis of fetal hypoxia [1–5]. However, there is evidence that the development of fetal hypoxia before 30 weeks of pregnancy results in a 15-fold increase in the risk of adverse pregnancy outcomes [8]. Chronic fetal hypoxia in the second trimester or long-term fetal hypoxia from early gestational age, especially in high-risk pregnancy cause such irreversible conditions like fetal growth restriction (FGR) and decompensated placental insufficiency. Currently, they are viewed as almost incurable pregnancy complications leading to intrauterine fetal demise or preterm labor, which does not always guarantee the birth of a live and healthy baby. Early detection of fetal hypoxia and its timely correction can improve the functional state of the fetal-placental unit, reduce negative outcomes, and also avoid the medication overload of pregnant women and the need for additional examinations of fetuses without hypoxia. Hence, there is doubtless feasibility of using fetal CTG in the second trimester of pregnancy to improve perinatal outcomes.
This study aimed to investigate the feasibility of using CTG in the second trimester of pregnancy to assess the fetal functional state and identify criteria for characterizing hypoxia in this period of gestation.
Materials and methods
This was a prospective observational study of 300 pregnant women, who were registered for antenatal care at 8–12 weeks’ gestation and underwent CTG at gestational ages ranging between 17 and 27 weeks. Of them, 150 women were, and 150 were not at risk for fetal hypoxia and placental insufficiency. All pregnant women at risk had a complicated obstetrical history, including spontaneous miscarriage, early fetal demise, three or more induced terminations of pregnancy, preterm birth, preeclampsia, antenatal fetal death, grade II-III FGR, infertility, chronic hypertension, pyelonephritis, recurrent inflammatory urogenital diseases, threatened miscarriage and frequent respiratory diseases in the first trimester of pregnancy and, often, early moderate and severe toxemia of pregnancy. Their scores measured on the scale of anamnestic (socio-biological, somatic and obstetric) prenatal risk factors were more than 15 points [9]. After the standardization of groups, 140 pregnant women were selected for analysis. The study group comprised 40 women with adverse gestational outcomes for this pregnancy, and the comparison group included 100 women with physiological pregnancy and childbirth.
The criteria for inclusion in the study group were singleton pregnancy, completed planned examination, informed consent to participate in the study, body mass index (BMI) less than 34 kg/m2, the presence of perinatal complications such as FGR, placental insufficiency, pre-eclampsia, antenatal fetal hypoxia , asphyxia of the newborn, antenatal fetal death. The exclusion criteria were multiple pregnancy, physiological pregnancy and childbirth, acute inflammatory diseases at the time of the examination, incomplete examination, refusal to participate, grade III obesity, neonatal asphyxia associated with iatrogenic or intranatal factors (anomalies of labor activity, shoulder dystocia, clinical mismatch between the fetal head and the mother’s pelvis). The comparison group included women with singleton pregnancies, completed planned examination, informed consent to participate in the study, a BMI of less than 34 kg/m2, and a physiological course of pregnancy and labor.
Clinical evaluation and observation of pregnant women were carried out by the order 572n. The fetal examination was performed every two weeks between 17 and 27 weeks’ gestation using a General Meditech CTG fetal monitor. During the observation in the second trimester, each woman underwent an average of 5 studies of fetal cardiac activity, a total of 700 CTG was analyzed. The significance of the obtained data was evaluated retrospectively after completion of the pregnancy and outcome analysis.
There turned out to be important details related to CTG technique. Before 24 weeks’ gestation, CTG was performed with the women in the supine position, since there was no risk of inferior vena cava compression syndrome due to the relatively small uterus size. After 24 weeks’ gestation, CTG was undertaken with the women lying on her side. Before 20 weeks’ gestation, the ultrasound cardiac sensor was placed at the level of the pubic symphysis; after 20 weeks it was placed at the point of the best detection of the fetal heartbeat. The sensor was fixed with an elastic belt. CTG recording was performed for 20 minutes. External ultrasonic sensors with an ultrasound frequency of 1.0 MHz, 1.5 MHz, and 2.0 MHz were used, which have no contraindications for use during pregnancy. According to the Clinical Safety Statement for Diagnostic Ultrasound of European Committee of Medical Ultrasound Safety (ECMUS), the main parameters of ultrasound are ultrasound frequency, thermal index (TI), mechanical index (MI), and ultrasound intensity (ISPTA). The Russian Association of Specialist in Ultrasound Diagnostics (RASUDM), referring to the International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) [10], determined the following sensor parameters while conducting a Doppler scan: thermal index (TI) values should not exceed 1 with the maximum exposure time of 60 minutes; MI should be not more than 0.7; ISPTA should be less than 0.94 mW/cm; the acoustic working frequency (FAWF) should be from 1 to 4 MHz. The CTG device used in this study had the following parameters: TI 0.028, MI no more than 004, ISPTA less than 0.2 mW/cm, the acoustic working frequency (FAWF) 1–1.5–2 MHz, which is significantly lower than the permissible ranges, i.e. the parameters of the CTG device fully complied with the fetal safety criteria.
Results and discussion
All pregnant women of the study group had complications of pregnancy (Table 1).
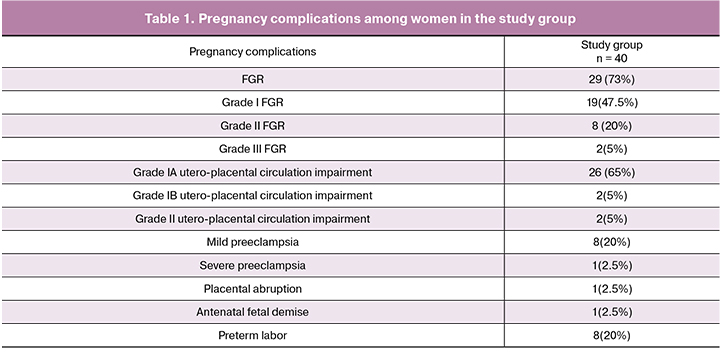
A majority (56%) of newborns of women in the study group had Apgar scores of 6 ± two as opposed to 8 ± 1 in the control group (p < 0.005). The mean birth weight of newborns in the study group was 2670 ± 243 g. In the control group, all 100 women had a physiological pregnancy and a full-term delivery; of them, 91 (91%) women had a vaginal delivery, and 9 (9%) underwent a cesarean section. Indications for cesarean section were abnormal labor patterns (n = 4, 4%), abnormal fetal presentations (n = 2, 2%), and uterine scar after cesarean section (n=3, 3%). The mean birth weight of newborns was 3470 ± 243 g and was higher than in the study group (p = 0.03).
The CTG analysis (Table 2) was performed using automatic evaluation of following parameters: baseline fetal heart rate (BHR), frequency of oscillations, the amplitude of oscillations, short-term variability (STV), long-term variability (LTV), episodes of high variability (EHV), episodes of low variability (ELV), the presence of accelerations and decelerations.

Comparison of STV and LTV between the study and control groups showed that in women with physiological pregnancy in 2nd trimester, the mean STV (4.1 ± 0.8 ms) and LTV (37 ± 8 ms) values were significantly (p = 0, 03) higher than in patients with adverse gestational outcomes (3.2 ± 0.7 and 31 ± 6 ms, respectively). The ranges of STV and LTV values in the study group were from 2 to 4.5 ms and from 24 to 46 ms and were lower than in the control group (3.2–7.1 ms and 32–78 ms, respectively). Thirty-four (85%) pregnant women in the study group showed a decrease in STV of less than 3.2 to 2 ms: in 5 (12.5%) in one of the examinations, in 22 (55%) in 2 examinations and in 7 (42.5) in 3 examinations.
In the control group, 9 (9%) pregnant women had a one-time decrease in STV to 2.8 ms, in 2 (2%) women a similar decrease was recorded twice, while in the vast majority of patients (89%) its values were above 3.2 ms. It is noteworthy that 31 (78%) women in the study group had at least a single decrease in LTV of less than 37 ms - to 24 ms, in 25 of them (62.5%) there was a multiple (3-4 times) decreases in this indicator. In contrast, 91 (91%) pregnant women in the control group had an LTV of more than 37 ms in all examinations, 9 (9%) had a one-time decrease taking into account parameters below 37 ms, and only 3 (3%) had a decrease of LTV to 31 ms twice. The sensitivity of a single reduction in LTV of less than 3.2 ms for predicting unfavorable perinatal outcomes was 64% and the specificity 70%; sensitivity and specificity of two instances of the decrease less than 3.2 ms were 81% and 84%. The sensitivity of a single decrease in LTV to less than 37 ms for predicting gestational complications was 58%, specificity - 66%, with two instances of the decrease - 84% and 88%, respectively. With a single simultaneous reduction of STV and LTV, the sensitivity was 81% and specificity 82%. With two or more similar observations, the sensitivity and specificity increased to 89%.
The duration of EHV at 17–27 weeks’ gestation in the study group was 4 ± 1.5 minutes and was significantly shorter (p = 0.02) than in the control group (7 ± 2.5 minutes). A single EHV decrease of fewer than 5 minutes was recorded in 32 (80%) pregnant women; two and three decreases were observed in 22 (55%) and 12 (30%) women, respectively (Table 2). ELVs were noted in both groups, but their duration among women with adverse outcomes (5 ± 1.3) was significantly longer than in healthy pregnant women (2 ± 1.4 min) (p = 0.04). The sensitivity of the predominance of ELV over EHV for predicting adverse perinatal outcomes was 79%, specificity - 81%. It is noteworthy that of 40 pregnant women, 28 (70%) had a predominance of ELV over EHV, which was observed repeatedly in 54% (n = 21). In 92 (92%) patients with physiological pregnancy and labor, on the contrary, the predominance of EHV over ELV was noted in most examinations.
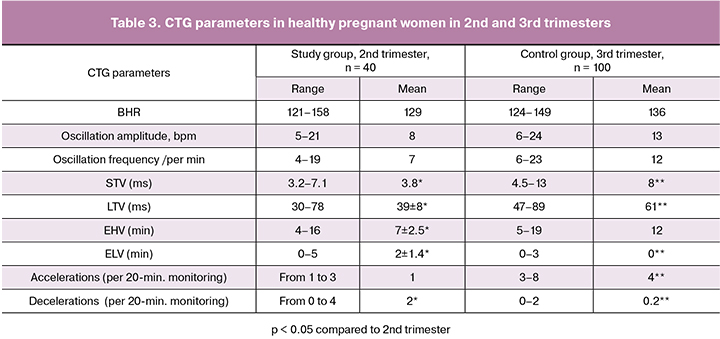
After 24 weeks of gestation, accelerations, as an indicator of the formation of the myocardial reflex, were observed in only 33 (24%) of all the examined patients and had no significant differences between the groups (p = 0.1); their height was within 10 beats with the duration from 2 to 12 seconds. Decelerations in the second trimester occurred in 50 (35%) women and also had a small depth (up to 10 beats) with their duration less than 15 seconds (mean 10 seconds). It should be emphasized that patients in the study group were more likely to have decelerations (from 2 to 6 per examination, mean 5 ± 2) than the patients in the control group (from 0 to 4, mean 2 ± 1), (p = 0.04). The sensitivity of more than three decelerations during one examination for predicting adverse perinatal outcomes was 61%, the specificity was 56%. The increase in the number of decelerations more than three per two or more CTGs was associated with an increase in sensitivity and specificity to 78%.
Assessment of CTG results in women with physiological pregnancy showed that there were no differences in BHR, amplitude, and frequency of oscillations measured during the 2nd and 3rd trimester (p > 0.05).
The modified STV and LTV dependencies in the 2nd trimester were lower than in the 3rd trimester. STV and LTV in the 3rd trimester are considered normal above 4 ms and 50 ms, respectively [11, 12], but in this study, most healthy pregnant women in the 2nd trimester had STV and LTV below 5 ms and 50 ms, respectively. EHV in II and III trimesters had comparable frequencies (p = 0.06), ELV indicating the risk of fetal metabolic acidosis in the 3rd trimester [12] were observed in the 2nd trimester even during normal pregnancy with a duration of up to 4 minutes (p = 0.02). With increasing gestational age, the number of ELVs in healthy patients decreased.
Accelerations and decelerations in the 2nd trimester of pregnancy did not meet the criteria for characteristics of the 3rd trimester. Normal CTG in the 3rd trimester suggests the absence of decelerations or the presence of spiky, shallow, short decelerations [11]. According to our data, in the 2nd trimester of pregnancy decelerations were present in 35% of women, from 1–3 spiky decelerations during physiological pregnancy, to more than three in women with a complicated pregnancy and signs of fetal hypoxia. Their diagnostic value was evaluated in combination with the other parameters.
Conclusion
Summarizing the results of 140 pregnant women, who were followed from early gestation to delivery with a retrospective assessment of CTG recorded in the 2nd trimester of pregnancy, we can conclude that the functional status of the fetus during the 2nd trimester can be effectively assessed using the CTG, while neither the Dawes-Redman criteria nor the Fisher Scale and FIGO guidelines in the generally accepted version cannot be used. To interpret CTG taken in the 2nd trimester of pregnancy, it is advisable to use only modified dependencies between the parameters of STV, LTV, EHV, ELV, and decelerations.
Given the significant differences in CTG parameters during the physiological and complicated pregnancy, the sensitivity and specificity of the analyzed parameters in patients with adverse perinatal outcomes, we can identify criteria that characterize the normal and the pathological functional state of the fetus in the 2nd trimester.
In 80% of pregnant women with a normal course of pregnancy, typical CTG parameters in the second trimester are as follows: STV - more than 3.2 ms, LTV - more than 37 ms, EHV - more than 5 minutes, ELV - less than 4 minutes, no more than three decelerations of 10 bpm below the baseline and lasting up to 10 sec.
In 82% of pregnant women with subsequent perinatal complications, typical CTG parameters include a decrease in STV from 2 to 3.2 ms; LTV decrease below 37 ms; a simultaneous decrease in EHV duration of less than 5 minutes in combination with an increase in ELV duration of more than 5 minutes, the presence of more than three decelerations.
The proposed criteria for the pathological type of CTG in the 2nd trimester of pregnancy cannot be used to make a diagnosis of fetal hypoxia at the time of CTG examination but may help predict the development of gestational complications, especially in patients with previous complicated pregnancies.
The presence of pathological CTG variants in the second trimester implies an increased risk for pregnancy complications such as FGR, placental insufficiency, and preeclampsia, which represent different types of incomplete trophoblast invasion. CTG does not allow determination of which of these complications will subsequently develop in a particular patient. However, given the generality of the consequences of these conditions, it is important to identify the overall risk of gestational complications and timely initiate preventive measures.
The findings of this study demonstrate the need for further research in this direction, the accumulation of evidence and the development of management strategies for pregnant women, who have pathological CTG variants to prevent adverse perinatal outcomes.
The proposed method for the evaluation of fetal condition was granted a patent for invention No. 2628240 on August 15, 2017.
References
1. Гус А.И., Баев О.Р., Александрова Н.В., Еремина О.В. Ультразвуковые методы в оценке состояния фетоплацентарного комплекса при беременности высокого риска. Ультразвуковая функциональная диагностика. 2011; 4: 62. [Gus A.I., Baev O.R., Aleksandrova N.V., Eremina O.V. Ultrasound methods in assessing the state of the placental complex in high-risk pregnancy. Ultrasound functional diagnostics. 2011; (4): 22.(in Russian]
2. Сафонова И.Н. Антенатальные допплерографические мониторинги при беременности высокого перинатального риска: обзор современной литературы. Медицинские аспекты здоровья женщины. 2014; 8: 5-16. [Safonova I.N. Antenatal Doppler monitoring in pregnancy of high perinatal risk: a review of current literature. Medical aspects of women’s health. 2014; (8): 5-16. (in Russian)]
3. Petraglia F., Boni C., Severi F.M., Norman J. Doppler examination of fetal and placental circulation. In: Buonocore G., Bracci R., Weindling M., eds. Neonatology. A practical approach to neonatal diseases. Springer; 2011: 60-3.
4. Сафонова И.Н. Фетальные аритмии: антенатальная ультразвуковая дифференциальная диагностика, прогнозирование постнатальных результатов и перинатальная тактика. SonoAceUltrasound. 2014; 26: 17-29. [Safonova I.N. Fetal arrhythmias: antenatal ultrasound differential diagnosis, prediction of postnatal results and perinatal tactics. SonoAceUltrasound. 2014; (26): 17-29. (in Russian)]
5. Hovsepyan G.A. Diagnostic and prognostic capabilities of cardiotocography in the III trimester of pregnancy. Медицинский вестник Эребуни. 2011; 2: 96-103.
6. Levine T.A., Grunau R.E., Mcauliffe F.M. Early childhood neurodevelopment after intrauterine growth restriction a systematic review. Pediatrics. 2015; 135(1): 126-41.
7. Ярыгина Т.В. Формирование репродуктивного потенциала у девочек с задержкой внутриутробного развития при рождении. Вопросы гинекологии, акушерства и перинатологии. 2014; 13(4): 63-9. [Yarygina T.V. Formation of reproductive potential in girls with intrauterine growth retardation at birth. Gynecology, obstetrics and perinatology issues. 2014; 13 (4): 63-9. (in Russian)]
8. Bailit J.L., Gregory K.D., Reddy U.M., Gonzalez-Quintero V.H., Hibbard J.U., Ramirez M.M. Maternal and neonatal outcomes by labor onset type and gestational age. Am. J. Obstet. Gynecol. 2010; 202(3): 245. e1–245. e12.
9. Радзинский В.Е., Князев С.А., Костин И.Н. Акушерский риск. Максимум информации - минимум опасности для матери и младенца. М.: ЭКСМО; 2009: 75-186. [Radzinsky V.E., Knyazev S.A., Kostin I.N. Obstetric risk. Maximum information - the minimum danger for the mother and baby. M.: Eksmo; 2009: 75-186. (in Russian)]
10. Salomon L.J., Alfirevic Z., Bilardo C.M., Chalouhi G.E., Ghi T., Kagan K.O. et al. ISUOG Practice guidelines: performance of first trimester fetal ultrasound scan. Ultrasound Obstet. Gynecol. 2013; 41(1): 102-13.
11. Воскресенский С.Л., Зеленко Е.Н. Кардиотокография в антенатальном периоде. Учебно-методическое пособие. Минск; 2011. 60с. [Resurrection S.L., Zelenko E.N. Cardiotocography in the antenatal period. Teaching manual. Minsk; 2011: 60p. (in Russia)]
12. Рыбалка А.Н. Методы исследования в акушерстве и гинекологии. Учебное пособие. Симферополь; 2016. 649с. [Fishing A.N. Research methods in obstetrics and gynecology. Tutorial. Simferopol; 2016: 649p. (in Russia)]
Received 16.03.2018
Accepted 20.04.2018
About the Authors
Zamaleeva, Rozaliya S., MD, professor of the Department of Obstetrics and Gynecology №1, Kazan State Medical Academy.420012, Russia, Kazan, Butlerov str. 36. Tel.: +78432364641. E-mail: zamaleewa@rambler.ru
Maltseva, Larisa I., MD, professor, head of the Department of Obstetrics and Gynecology №1, Kazan State Medical Academy.
420012, Russia, Kazan, Butlerov str. 36. Tel.: +78432364641. E-mail: laramalc@mail.ru
Cherepanova, Nataliya A., PhD, head of the Maternity Department,Volzhsk Central City Hospital.
425000, Republic of Mari El, Volzhsk, Sovetskaya str. 46. Tel.: 8(836)316-37-73. E-mail: nat26@list.ru
Frizina, Anastasia V., obstetrician-gynecologist of Volzhsk Central City Hospital.
425000, Republic of Mari El, Volzhsk, Sovetskaya str. 46. Tel.: +78363163773. E-mail: anastasia.frizina.84@mail.ru
Zefirova, Tatyana P., MD, professor of the Department of Obstetrics and Gynecology №1, Kazan State Medical Academy.
420012, Russia, Kazan, Butlerov str. 36. Tel.: +78432364641. E-mail: tzefirova@gmail.com.
For citation: Zamaleeva R.S., Maltseva L.I., Cherepanova N.A., Frizina A.V., Zefirova T.P. New possibilities to use cardiotocography to assess the fetal functional state in the second trimester of pregnancy. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2018; (12): 29-34. (in Russian)
https://dx.doi.org/10.18565/aig.2018.12.29-34



