Воспалительные заболевания органов малого таза (ВЗОМТ) – группа заболеваний, включающая эндометрит, сальпингоофорит, параметрит, оофорит. Наиболее тяжелыми формами ВЗОМТ являются пиосальпинкс, тубоовариальный абсцесс. ВЗОМТ занимает ведущее место среди всех причин госпитализаций в гинекологические стационары. Медико-социальная и экономическая значимость проблемы обусловлена тяжестью осложнений ВЗОМТ: бесплодие, эктопическая беременность, спаечный процесс в органах брюшной полости, синдром хронической тазовой боли, рак эндометрия [1–3].
В настоящее время в качестве основных этиологических агентов ВЗОМТ рассматриваются возбудители инфекций, передающихся половым путем (ИППП), и представители индигенной микробиоты нижних отделов урогенитального тракта. Среди возбудителей ИППП ведущая роль в развитии ВЗОМТ принадлежит Chlamydia trachomatis, Neisseria gonorrhoeae и Mycoplasma genitalium. Однако более 70% случаев ВЗОМТ связаны с разнообразными анаэробными условно-патогенными микроорганизмами, колонизирующими слизистую влагалища и шейки матки: Haemophilus influenzae, энтеробактерии (Escherichia coli, Klebsiella spp., Proteus spp.), Streptococcus spp., микроорганизмы, ассоциированные с бактериальным вагинозом (БВ): Prevotella bivia, Peptostreptococcus spp., Peptococcus spp., Bacteroides spp., Gardnerella vaginalis [4]. Последние исследования c использованием молекулярно-биологических методов показали, что в развитии ВЗОМТ принимают участие такие трудно- и некультивируемые БВ- ассоциированные бактерии, как Sneathia (Leptotrichia) sanguinegens, Sneathia amnionii, Atopobium vaginae и BV-associated bacteria 1 (BVAB1) [5].
Одним из наиболее специфичных для БВ микроорганизмов является Mycoplasma hominis. Несмотря на то, что M. hominis принято относить к условно-патогенным микроорганизмам, в научной литературе описано множество клинических случаев с участием M. hominis как единственного микроорганизма в развитии тяжелых воспалительных процессов в легких, межпозвоночных дисках, почках, печени, сердце и головном мозге [6–10].
В данной работе представлены 2 клинических наблюдения, в которых M. hominis является наиболее вероятным этиологическим агентом развития тяжелых заболеваний органов малого таза: тубоовариального абсцесса и пиосальпинкса у пациенток репродуктивного возраста.
Описание клинических наблюдений
Клиническое наблюдение № 1
Пациентка Б., 32 года, доставлена в стационар машиной «скорой помощи» с жалобами на тянущие боли внизу живота, слабость, недомогание, повышение температуры тела до 38°С. Больной себя считает в течение суток, когда появились тянущие боли внизу живота, повышение температуры тела, в связи с чем принимала но-шпу без эффекта. На следующий день боль усилилась.
Из анамнеза: несколько лет назад амбулаторно лечилась по поводу воспаления придатков матки. Беременностей не было.
При поступлении состояние пациентки средней тяжести: кожные покровы бледные, пульс 84 уд./мин, АД 110/80 мм рт. ст., температура тела 38°С. При пальпации живота выявлена болезненность в нижних отделах, больше в левой подвздошной области. Симптомов раздражения брюшины не было. При гинекологическом исследовании: тело матки в antеflexio/versio, не увеличено, плотное, ограниченно подвижное, чувствительное при пальпации, придатки с обеих сторон увеличены, болезненны при исследовании. Выделения из половых путей гноевидные.
Данные ультразвукового исследования (УЗИ) от 24.03.2013: эхо-признаки двустороннего острого воспалительного процесса с формированием пиосальпинксов.
Результаты лабораторного обследования:
Клинический анализ крови: WBC 11,9×109/л, RBC 4,5×1012/л, HGB 118 г/л, HCT 37,4%, PLT 340×109/л, LYM 1,3×109/л, MON 0,48×109/л, нейтрофилы палочкоядерные 3%, нейтрофилы сегментоядерные 82%, эозинофилы 0, базофилы 0, СОЭ 22 мм/ч.
Микроскопическое исследование: лейкоциты 0–5 (и влагалище, и цервикальный канал), эпителий – много, эритроциты – не обнаружено, микрофлора – кокко-бациллярная, ключевые клетки – обнаружено, трихомонады – не обнаружено, гонококки – не обнаружено.
Диагноз: Обострение двустороннего хронического сальпингоофорита с формированием тубоовариального абсцесса слева и пиосальпинкса справа.
Была начата антибактериальная терапия: цефтриаксон 1,0 2 раза в день в/м, метронидазол 100,0 (500 мг) 3 раза в день в/в, свечи «гексикон» per vaginum, инфузионная терапия в течение 5 дней. Антибактериальная терапия с положительным эффектом, однако при динамическом УЗИ сохранялись эхографические признаки пиосальпинкса, в связи с чем произведена лапароскопия.
Протокол лапароскопии: матка нормальных размеров, серозный покров инъецирован. Слева от матки (интимно спаянный с ребром матки) определяется единый воспалительный конгломерат с наложением фибрина, в состав которого входили левая маточная труба, левый яичник, образующие осумкованную полость с гнойным содержимым. Правая маточная труба воспалительно изменена, инфильтрирована и отечна, серозный покров с инъекцией сосудов, фимбриальный отдел запаян, содержимое – гной. Правый яичник не изменен.
Хирургический диагноз: Обострение двустороннего хронического сальпингоофорита с формированием тубоовариального абсцесса слева и пиосальпинкса справа.
Произведено разделение спаек, сальпингоофорэктомия слева, сальпингэктомия справа, санация и дренирование брюшной полости. Удаленные левые придатки и правая маточная труба были отправлены на гистологическое исследование, бактериологическое исследование и исследование методом полимеразной цепной реакции (ПЦР).
Результаты гистологического исследования: в препаратах выраженная диффузная лимфоплазмацитарная инфильтрация с примесью распадающихся нейтрофилов, очаговой пролиферацией эпителия, отеком мышечной и серозной оболочек, в просвете трубы – эозинофильный инфильтрат с примесью нейтрофильных гранулоцитов и макрофагов.
Результаты бактериологического исследования: роста микрофлоры нет.
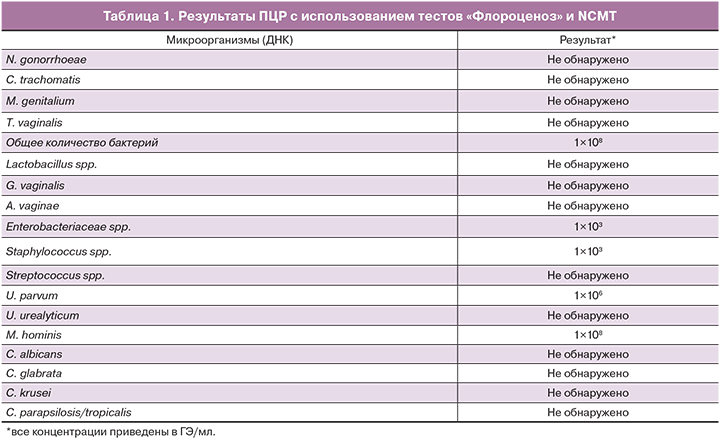
Результаты ПЦР представлены в табл. 1.
В послеоперационном периоде пациентке была продолжена антибактериальная терапия, ультрафиолетовое облучение крови (УФОК) в количестве 10 сеансов. Послеоперационный период протекал без осложнений.
Клиническое наблюдение № 2
Пациентка А., 29 лет. Клиническая картина развивалась быстро. Пациентка почувствовала тянущие боли внизу живота, больше справа. Боли и температура тела (37,8°С) постепенно нарастали, самостоятельно приняла бисептол в количестве 2 таблеток. В связи с отсутствием эффекта и нарастанием болей вызвала бригаду скорой помощи и была доставлена в стационар.
При поступлении состояние средней тяжести, температура тела 38°С. Язык сухой, обложен налетом. Отмечалась болезненность при пальпации нижних отделов живота, больше в правой подвздошной области, перитонеальные симптомы отрицательные.
Из анамнеза: беременность – 1, закончилась своевременными родами без осложнений. Далее при обследовании по поводу вторичного бесплодия при гистеросальпингографии выявлено нарушение проходимости левой маточной трубы.
При гинекологическом осмотре выявлена болезненность в области левых придатков. В области правых придатков определялось образование, ограниченно подвижное, неоднородной консистенции, размерами до 9 см в диаметре, болезненное при исследовании, в едином конгломерате с маткой.
По УЗИ – картина двустороннего острого воспалительного процесса с формированием пиосальпинкса справа.
Результаты лабораторного обследования:
Клинический анализ крови: WBC 13×109/л, RBC 3,9×1012/л, HGB 105 г/л, HCT 26%, PLT 256×109/л, LYM 1,0×109/л, MON 0,2×109/л, GRA 11,8×109/л.
Микроскопическое исследование: лейкоциты 20–30 (влагалище), 10–15 (цервикальный канал), эпителий – много, эритроциты – не обнаружено, микрофлора – смешанная, ключевые клетки – обнаружено, трихомонады – не обнаружено, гонококки – не обнаружено.
Диагноз: Обострение двустороннего хронического сальпингоофорита с формированием пиосальпинкса справа.
С целью предоперационной подготовки проводилась антибиотикотерапия: ципрофлоксацин 200 мг 2 раза в день в/в в сочетании с метронидазолом 100 мг × 3 раза в день в/в. Через 8 часов от поступления в стационар, несмотря на проведение комплексной терапии, появились перитонеальные симптомы, в связи с чем было принято решение произвести диагностическую лапароскопию.
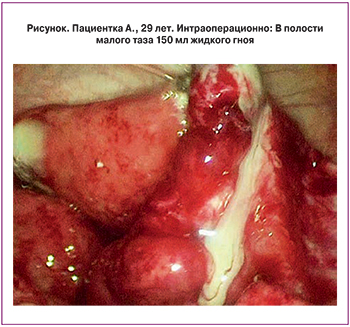 Протокол лапароскопии: тело матки не увеличено. Правая маточная труба утолщена до 3 см, отечна, гиперемирована, фимбриальный отдел запаян. Левая маточная труба не увеличена, отечна, фимбриальный отдел свободный. В полости малого таза 150 мл жидкого гноя. Яичники с обеих сторон не изменены (рисунок).
Протокол лапароскопии: тело матки не увеличено. Правая маточная труба утолщена до 3 см, отечна, гиперемирована, фимбриальный отдел запаян. Левая маточная труба не увеличена, отечна, фимбриальный отдел свободный. В полости малого таза 150 мл жидкого гноя. Яичники с обеих сторон не изменены (рисунок).
Хирургический диагноз: Обострение двустороннего хронического сальпингоофорита с формированием пиосальпинкса справа.
Произведено рассечение спаек, правосторонняя сальпингэктомия, санация и дренирование брюшной полости. Правая маточная труба была отправлена на гистологическое, бактериологическое и ПЦР-исследование. У пациентки был также взят мазок из влагалища для исследования с помощью тестов «Флороценоз».
Гистологическое заключение: выраженное хроническое воспаление маточной трубы с гнойным обострением (стенка маточной трубы утолщена, фиброзирована). Слизистая с большим числом микрососудов. Выраженная диффузная лейкоцитарная инфильтрация всей толщи стенки. В просвете детрит: хронический сальпингит в фазе обострения. Пиосальпинкс.
Бактериологическое исследование: роста микрофлоры нет.
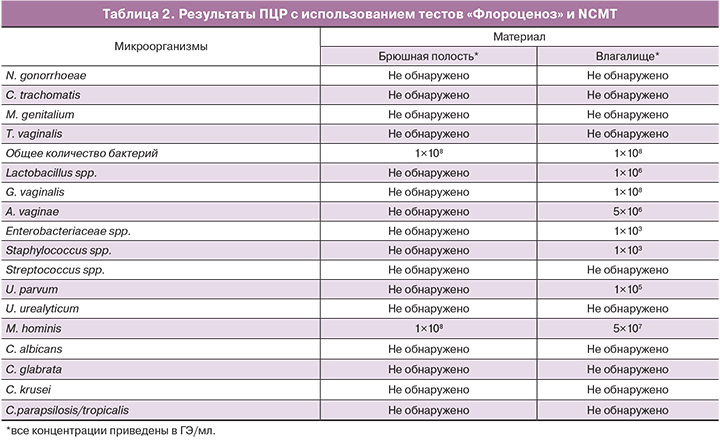
Результаты ПЦР представлены в табл. 2.
Динамика анализа крови после операции: WBC 10,6×109/л, RBC 4,3×1012/л, HGB 116 г/л, HCT 33,7%, PLT 235×109/л, LYM 1,8×109/л, MON 0,4×109/л, GRA 8,4×109/л.
В послеоперационном периоде пациентке была продолжена антибактериальная терапия. Послеоперационный период протекал без осложнений.
Обе пациентки были выписаны из стационара в удовлетворительном состоянии с соответствующими рекомендациями.
Обсуждение
Первые сведения о возможной этиологической роли M. hominis в развитии ВЗОМТ относятся к 50-м годам прошлого века. В работах Randall и соавт., Melen и Gotthardson [11, 12] сообщалось о выделении из материала маточных труб или тубоовариального абсцесса от женщин с ВЗОМТ чистой культуры PPLO (PleuroPneumoniae Like Organisms), которые впоследствии были идентифицированы как M. hominis. Позднее Mardh выделил культуру M. hominis из биопсийного материала маточных труб у 4 из 50 (то есть 8%) пациенток с гистологически подтвержденным сальпингитом [13]. В другом исследовании Mardh наблюдал у пациенток с ВЗОМТ выраженный гуморальный ответ на M. hominis [14]. Развитие сальпингита было смоделировано у лабораторных животных – зеленых мартышек – при инокулировании им в полость маточных труб культуры M. hominis [15].
Однако систематизированное изучение этиологии данной группы заболеваний крайне затруднено по целому ряду обстоятельств:
Лечебные мероприятия назначаются максимально быстро от момента обращения пациентки (начало терапии в течение 72 часов от появления симптомов заболевания значительно снижает риск развития осложнений).
Существующие схемы лечения включают антибиотики широкого спектра действия (цефалоспорины, нитроимидазолы, макролиды, тетрациклины). При этом применяется комбинация из нескольких препаратов с целью перекрывания максимального числа видов возможных возбудителей ВЗОТМ.
Проводимые диагностические мероприятия направлены в первую очередь на установление клинических и лабораторных признаков воспаления и наличия патологических изменений в органах малого таза [1, 2], диагностика же потенциальных возбудителей ВЗОМТ ограничена только N. gonorrhoeae и C. trachomatis.
В нашем исследовании для изучения этиологии ВЗОМТ была использована группа тестов «Флороценоз» производства ФБУН ЦНИИ эпидемиологии Роспотребнадзора.
Диагностические характеристики данных тестов были детально изучены и описаны ранее [16–20]. Помимо использования тестов «Флороценоз» материал был проанализирован на наличие ДНК облигатно-патогенных микроорганизмов: C. trachomatis, N. gonorrhoeae, M. genitalium, T. vaginalis [18]. Сразу следует отметить, что ни в материале из брюшной полости, ни в материале из нижних отделов урогенитального тракта, полученных от обеих пациенток, перечисленных патогенов обнаружено не было. Мы также исследовали материал методом ПЦР на наличие ДНК БВ-ассоциированных микроорганизмов, не включенных в тесты «Флороценоз»: Megasphera, BVAB 1, 2, 3, Leptotrix; перечисленные микроорганизмы также не были выявлены.
В результате было установлено, что доминирующим микроорганизмом в образцах из брюшной полости у обеих пациенток была M. hominis, ее концентрация соответствовала концентрации общей бактериальной ДНК – 108 ГЭ/мл (см. табл. 1, 2).
В случае пациентки 1 лапароскопическая операция, в ходе который был получен материал для лабораторной диагностики, была выполнена через 4 суток после начала приема антибактериальных препаратов. Терапия данной пациентки включала препараты цефтриаксон и метронидазол, которые не активны в отношении M. hominis.
Если предположить, что помимо M. hominis в очаге воспаления присутствовали какие-либо еще микроорганизмы, которые были элиминированы в процессе лечения, необходимо принимать во внимание тот факт, что прошедшего времени недостаточно для разрушения и полной элиминации фрагментов ДНК потенциальных возбудителей. Например, элиминация ДНК C. trachomatis, N. gonorrhoeae, M. genitalium занимает от 2 до 3 недель [1], и в случае их присутствия до начала лечения высока вероятность их обнаружения даже в низкой концентрации.
Для пациентки 2 между первым приемом антибактериального препарата (бисептол однократно) и проведением хирургического вмешательства прошло еще меньше времени – менее 9 часов. За столь короткое время может начаться подавление активности чувствительных к препарату микроорганизмов, но не их разрушение, тем более не разрушение их ДНК. К тому же бисептол не активен в отношении большинства анаэробных микроорганизмов, которые также могут принимать участие в развитии сальпингита. В этом случаем мы можем утверждать, что M. hominis и есть тот микроорганизм, который вызвал патологический процесс.
Исследования последних лет показали существенное значение для активации иммунного ответа и взаимодействия с микробиотой Toll-like рецепторов (TLR) слизистых оболочек (в том числе репродуктивных органов) [21]. В M. hominis был выявлен ряд факторов, взаимодействующих с TLR-2 и обладающих макрофаг-стимулирующей активностью. M.R. Peltier и соавт. выделили 29 кДа липопротеин, взаимодействие которого с макрофагами приводило к повышению экспрессии фактора некроза опухоли (TNF)-α, который, в свою очередь, играет важную патогенетическую роль в преждевременных родах и внутриутробной гибели плода [22]. Позднее из M. hominis был выделен адгезин массой 40 кДа, также представляющий собой TLR2-зависимый активатор макрофагов и, возможно, являющийся одним из ключевых триггеров в каскаде цитокинового ответа при развитии воспаления [23].
Многочисленные клинико-эпидемиологические исследования показывают достоверную ассоциацию M. hominis с БВ [24, 25]. На этом фоне выглядит логичным предположение, что перечисленные выше и множество других описанных ранее факторов вирулентности M. hominis в большей степени реализуются в условиях высокой бактериальной нагрузки и активного размножения микроорганизма, которые обеспечиваются микробным окружением при БВ, в первую очередь, симбиозом с G. vaginalis [25]. Ранее в исследованиях было показано, что концентрация M. hominis на слизистой влагалища при БВ превышает таковую у пациенток с нормальной микрофлорой в 50 000 раз и могут достигать значений 107–108 копий/мл [26, 27]. На этом фоне факторами, способствующим развитию восходящей инфекции и колонизации верхних отделов органов малого таза, являются подавление барьерных функций слизистых оболочек цервикального канала и влагалища в результате сниженной концентрации лактобактерий и увеличения разнообразия и концентрации условно-патогенных микроорганизмов, а также угнетение местного иммунного ответа [26].
Не так давно несколько штаммов M. hominis, обнаруженных в амниотической полости, были проанализированы методом секвенирования нового поколения (NGS). В результате были обнаружены три гена, продукты которых могут принимать участие в обеспечении колонизации микроорганизмом верхних отделов репродуктивного тракта во время беременности с риском развития преждевременных родов [27].
Со стороны человеческого организма некоторые варианты полиморфизма в генах TLR1, TLR2, TLR4, TLR6 проводят к нарушению функционирования местных защитных механизмов, и повышается вероятность развития воспаления в органах репродуктивной системы женщин [28].
Заключение
В описанных в данной работе клинических наблюдениях тубоовариального абсцесса и пиосальпинкса благодаря применению молекулярно-биологического исследования на основе количественной ПЦР было установлено, что наиболее вероятная этиологическая роль принадлежит M. hominis. Частота таких наблюдений в силу сложившейся практики ведения пациенток с ВЗОМТ остается неизвестной и требует дальнейшего изучения.



