Bone metabolism in peri- and postmenopausal women with type 1 diabetes mellitus
Safarova S.S.
Aim. To estimate the effect of type 1 diabetes mellitus on bone mineral density and bone metabolism parameters in women; determine the direction of changes in serum bone remodeling markers and BMD in the peri- and post-menopausal women with this disease. Material and methods. The study comprised 57 peri- and post-menopausal women with type 1diabetes (study group) and 43 women in the control group. Diagnostic evaluation included analysis of BMD using DXA and expressed as T-score, and serum bone remodeling markers (ALP, P1NP and b-CTx). Results. The findings showed the inconsistency of changes in bone remodeling in patients with type 1 diabetes mellitus (35.5% and 16.6%, p <0.001) with a predominant change in bone formation. The duration of diabetes had a positive correlation with serum b-CTX concentration (r = 0.349, p = 0.008). A negative correlation was found between the change in T-score at the lumbar spine and the duration of diabetes (r = -0.239, p = 0.03). There was a statistically significant correlation between T-score at the lumbar spine and serum b-CTX level (r = -0.452, p = 0.002). Conclusion. The findings of this study suggest that changes in bone metabolism in the majority of patients are associated with bone formation inhibition and, to a much lesser extent, bone resorption accelerating during the late perimenopause, continuing at similar rates in the early years of postmenopause, and decreasing bone loss later.
Keywords
Type 1 diabetes mellitus (T1DM) is one of the most common chronic metabolic diseases associated with a high risk of complications, including diabetic osteopathy [1]. Pathophysiological processes linking changes in bone tissue with T1DM may be caused by the direct effects of insulin deficiency and/or hyperglycemia on the bone, by glycation end products that interfere with the production of matrix proteins and thus disrupt the bone collagen synthesis, by the production of inflammatory cytokines and adipokine damaging bone cells, and by impaired neuromuscular regulation [2, 3]. As is known, T1DM predisposes to higher fall rates and lowers bone mineral density (BMD) thus increasing the risk of fractures [4].
Postmenopausal remodeling of bone tissue in women of the older age is aggravated by T1DM, resulting in a 12-fold increase in the risk of hip fractures compared with the general population [5].
The study aimed to estimate the effect of type 1 diabetes mellitus on bone mineral density and bone metabolism parameters in women; determine the direction of changes in serum bone remodeling markers and BMD in the peri- and post-menopausal women with this disease.
Material and methods
This was a randomized cross-sectional study comprising 57 peri-and postmenopausal women with T1DM aged 40 to 68 years (56.3 ± 0.9 years), who did not have a history of bone metabolism disorders or osteoporosis. The mean duration of T1DM was 17.08 ± 0.8 years; the mean glycosylated hemoglobin (HbA1c) was 7.5 ± 0.2%, neuropathy and retinopathy were detected in 23 and 52% of patients, respectively. All patients received long-acting insulin at a total daily dose of 48.7 ± 4.3 U. The control group included 43 women (55.4 ± 1.2 years) without a history of T1DM.
Exclusion criteria were as follows: previous treatment for osteoporosis or a history of fracture, endocrine diseases, non-diabetic liver and kidney diseases, a history of 4-5 stage diabetic nephropathy.
Diagnostic evaluation included history taking, body weight (66.9 ± 0.9 kg) and height (161.1 ± 0.7 cm), body mass index (BMI) in kg/m2 (25.8 ± 0.3 kg / m2). The menopausal status of the study participants was assessed using the menopausal Kupperman index. The duration of menopause averaged 13.4 ± 0.8 years. All patients underwent dual-energy X-ray Absorptiometry (DXA) on a densitometer (DXA HOLOGIC, Discovery QDR 4500A, USA) at the lumbar spine (L1-L4) and the proximal and femoral neck. BMD was assessed according to WHO criteria and categorized as osteoporosis (T-score ≤2.5SD), osteopenia (T-score from -1 to -2.5 SD) and normal BMD (T-score> -1).
Parameters of phosphorus-calcium metabolism were estimated by measuring serum concentrations of total (tCa), ionized calcium (iCa) and inorganic phosphorus (P). Parathyroid hormone (PTH), calcitonin (CT) and vitamin D3 (25 (OH) D) levels were also analyzed. Total alkaline phosphatase (ALP) and the aminoterminal propeptide of type I procollagen (PINP) were determined to estimate bone tissue formation. Bone resorption was evaluated by the level of C-terminal telopeptide (b-CTx). The bone markers were examined on an automatic electro-chemiluminescence Cobas e41 analyzer using the Roche Diagnostics reagents (Germany).
Statistical analysis was carried out using BioStat Pro 6.2.2.0 software according to recommendations for medical and biological research. The statistical significance of the differences was determined by the Mann-Whitney U test. The data are presented as a mean ± standard error of the mean (M ± m) and 95% confidence interval for the mean (95% CI). Differences were considered statistically significant at p <0.05. To investigate the relationship between two variables, the Spearman correlation analysis (r) was used.
Results
The mean levels of tCa did not differ statistically significantly between women with T1DM and in the control group (p> 0.05) though had a certain tendency to lower values in women with T1DM; in both groups they were within the age-specific reference range (8.4-10, 2 mg/dL) with a tendency to decrease in postmenopause. The values of iCa in the patients with T1DM were statistically significantly lower than the control values (p <0.05); the postmenopausal women with T1DM had the lowest iCa concentrations of 1.05 ± 0.01 mmol / l (p <0.05). In postmenopausal women in the control group, mean serum P concentrations were statistically significantly lower than in patients with T1DM (p <0.05). The results are shown in the table.
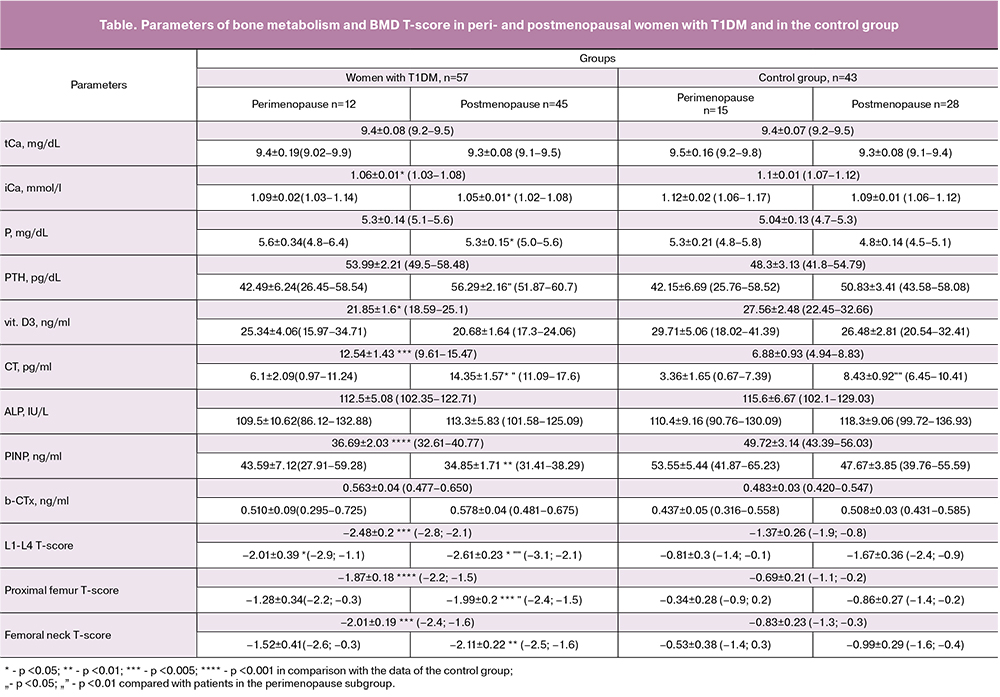
In women with long-term T1DM, the PTH level was statistically significantly different from that in women having T1DM for less than ten years (p <0.05). The patients with T1DM had a greater decrease in vitamin D3 below the reference values compared with the control group (p <0.05), with a tendency to decrease in postmenopause in both groups. Nineteen (33%) patients with T1DM had vitamin D3 deficiency (below10ng/ml). An increase in the duration of T1DM was accompanied by a decline in the level of vitamin D3 with a negative correlation between the two parameters (r = -0.397, p = 0.01). Also, changes in vitamin D3 were found to depend on serum iСа concentrations. The two variables had a positive, statistically significant correlation (r = 0.391, p <0.05). In postmenopausal women in the control group, the mean CT level was statistically significantly lower than in patients with T1DM (p <0.05).
The findings of the study on the increase in PTH and CT concentrations along with the decline in the calcium concentration are indicative of the violation of impaired secretion of calcium-regulating hormones and their relationship with pathological bone remodeling in T1DM. It should be noted that with the increase in the disease duration and the stage of decompensation, the extent of these changes is increasing.
35.5% of patients with T1DM demonstrated a statistically significant decrease in bone formation markers (ALP and PINP), (p> 0.05 and p <0.001, respectively) and 16.6% of patients had an increase in the bone resorption marker (b-CTx), (p> 0, 05).
The mean PINP concentrations in the T1DM group were 36.69 ng / ml (32.61-40.77 ng / ml) and were lower compared to 49.72 ng/ml (43.39-56.03 ng / ml, p <0.001) in the control group. The mean PINP index in the subgroup of postmenopausal T1DM patients was also under the age-specific range (p <0.01). At the same time, the concentrations of the bone resorption marker b-CTx were statistically significantly different from those in the control group (p> 0.05). In postmenopausal patients with T1DM, the mean CTx was slightly higher than that of perimenopausal women. However, it was within the age-specific reference range. A part of women with T1DM showed a decrease in the bone formation marker PINP while having unchanged bone resorption. Previously published studies on the on the estimation of bone remodeling in patients with T1DM reported a decrease mainly in bone formation markers [5, 6], while bone resorption markers in most studies did not differ statistically from the control subjects.
Levels of CTX and P1NP had a statistically significant correlation with the duration of diabetes (r = 0.349, p = 0.008) and (r = -0.210, p = 0.03), respectively. The P1NP level was negatively correlated with HbA1c (r = -0.328, p = 0.03). Among women with T1DM, there was a negative correlation between b-CTx concentration and the glomerular filtration rate (eGFR: r = -0.207, p = 0.04). A correlation was also found between bilirubin, albumin, BMI and b-CTx concentration (bilirubin: r = 0.284, p = 0.03; albumin: r = -0.542; p = 0.003; BMI: r = 0.219; p = 0.03).
Compared with the control group, the women with T1DM had marked and statistically significant (p <0.005) changes in the BMD T-scores at the L1-L4 vertebrae and the femoral neck. Among the patients with T1DM, 30%, 20% and 32.5% of women had osteoporosis in L1-L4vertebrae, proximal femur, and femoral neck, respectively. Osteopenia in the vertebrae, proximal femur, and femoral neck was found in 35%, 48.5% and 25% of women with T1DM, respectively. In 31 and 13 of 57 women with T1DM, T-score changes were detected only in the lumbar spine and the femur, respectively. Ten women had a combination of changes in the two regions. Thus, a part of women (n = 44) who had changes in only one of the studied sites could be at risk of an incorrect diagnosis if BMD measurements were made only in one region.
In the control group, 14%, 2.3% and 7% of women had osteoporosis in the L1-L4vertebrae, proximal femur, and femoral neck, respectively. Osteopenia in the vertebrae, proximal femur, and femoral neck was found in 23%, 26% and 28% of women, respectively.
The change in the T-score at the lumbar region had a negative correlation with the duration of diabetes (r = -0.239, p = 0.03). A negative correlation was found between the duration of diabetes and the changes in the T-scores measured at proximal femur, and femoral neck, respectively (r = -0.568, p = 0.001 and r = -0.460, p = 0.01). A statistically significant negative correlation was also found in the subgroup of postmenopausal women (r = -0.515, p = 0.01 and r = -0.416, p = 0.04).
In postmenopausal women with T1DM, a decrease in BMD in this area occurs in parallel with an increase in the duration of the disease with concomitant age-related changes. A negative correlation was found between the T-score measured at the lumbar spine and the concentration of bone metabolism marker b-CTX (r = -0.452, p = 0.002). Statistically significant negative correlation was also noted in the subgroup of postmenopausal women (r = -0.489, p = 0.003). These findings imply that a history of T1DM exacerbates the disorders of bone metabolism, thereby contributing to the development of osteoporosis in late postmenopause.
Analysis of bone metabolism markers in women showed a statistically significant association between T1DM duration with the level of b-CTX and the T-score measured at the lumbar spine. This implies that both bone metabolism markers and DXA are independent factors indicating bone tissue changes, which can be of great importance for early diagnosis and evaluation of the treatment effectiveness [6, 7].
Development of bone remodeling disorders cannot be explained only by an early onset of T1DM when patients do not have enough time to accumulate a high peak bone mass since a decrease in BMD is still determined in some patients with T1DM with the aт onset of the disease after the age of 30 [1]. Taking into account a significant decrease in BMD, it is possible to hypothesize [7] that in patients with T1DM disturbances of bone remodeling have an autoimmune origin.
Conclusion
The findings of this study suggest that changes in bone metabolism in the majority of patients are associated with bone formation inhibition and, to a much lesser extent, bone resorption accelerating during the late perimenopause, continuing at similar rates in the early years of postmenopause, and decreasing bone loss later.
References
- Vestergaard P., Rejnmark L., Mosekilde L. Diabetes and its complications and their relationship with risk of fractures in type 1 and 2 diabetes. Calcif. Tissue Int. 2009; 84(1): 45-55.
- Pramojanee S.N., Phimphilai M., Chattipakorn N., Chattipakorn S.C. Possible roles of insulin signaling in osteoblasts. Endocr. Res. 2014; 39(4): 144-51.
- Soloveva-Savoyarova G.E., Drozhzhina V.A. Estrogens and non-carious lesions of teeth. St. Petersburg: I.I. Mechnikov SZGMU; 2012. (in Russian)
- Farr J.N., Khosla S. Determinants of bone strength and quality in diabetes mellitus in humans. Bone. 2016; 82: 28-34.
- Hough F.S., Pierroz D.D., Cooper C., Ferrari S.L.; IOF CSA Bone and Diabetes Working Group. Mechanisms in endocrinology: mechanisms and evaluation of bone fragility in type 1 diabetes mellitus. Eur. J. Endocrinol. 2016; 174(4): R127-38.
- AL-Hariri M. Sweet bones: the pathogenesis of bone alteration in diabetes. J. Diabetes Res. 2016; 2016: ID 6969040.
- Starup-Linde J., Vestergaard P. Biochemical bone turnover markers in diabetes mellitus - a systematic review. Bone. 2016; 82: 69-78.
Received 09.11.2017
Accepted 22.12.2017
About the Authors
Safarova, Sain S., MD, associate professor of the Department of Internal Diseases III, medical-prophylactic faculty II, Azerbaijan Medical University.AZ1000, Republic of Azerbaijan, Baku, S. Vurgun str. 23. Tel.: +994505200555. E-mail: sainsafarova@gmail.com. http:// orcid.org/0000-0002-7131-3878
For citations: Safarova S.S. Bone metabolism in peri- and postmenopausal women with type 1 diabetes mellitus. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2018; (9): 80-4. (in Russian)
https://dx.doi.org/10.18565/aig.2018.9.80-84



