Clinical and anamnestic features of women with preterm premature rupture of membranes
Objective. To identify clinical and anamnestic risk factors in pregnant women with preterm premature rupture of membranesGuseinova G.E., Khodzhaeva Z.S.
Subjects and methods. Examinations were made in 150 women aged 18 to 40 years, who were divided into 3 groups: 1) 50 pregnant women with premature rupture of membranes (PROM); 2) 50 pregnant women with intact membranes, who delivered at 22-36 weeks’ gestation; 3) 50 pregnant women who delivered spontaneously at term (at ≥ 37 weeks). The age indicators, the history of somatic and gynecological diseases, the outcomes of previous pregnancies, the features of the course of this pregnancy, childbirth and neonatal outcomes were studied in detail.
Results. The risk of PROM significantly more often occurred at the most active reproductive age (25-30 years) in primigravida primiparous women who were somatically burdened with frequent respiratory and odontogenic inflammatory diseases. Pregnant women with PROM had several risk factors that occurred during this pregnancy. Cervical diseases and uterine fibroid were statistically significantly more common. The gestational age for PROM varied within 22-36 weeks’ gestation, and the statistically significantly more common method of delivery was cesarean section. In the same group of women, the newborn infants had a birth weight of less than 2500 g, which affected neonatal outcomes.
Conclusion. The etiology of PROM during preterm pregnancy is multifactorial; there is a need for timely detection, the correction of one or another condition not only during, but also before pregnancy, and the elaboration of strategies for improving the outcomes, by predicting, preventing, and treating this situation.
Keywords
Preterm birth (PB) is the leading cause of perinatal morbidity and mortality in the world, where most newborns die at gestational age less than 32 weeks [1, 2, 3]. Prematurity and low birth weight largely determine the prognosis of survival and the quality of subsequent life of the newborn. In general, 15 million cases of PB are reported annually worldwide [2]. The rate of preterm premature rupture of membranes (PPROM) is between 40% and 50% of all preterm births [4].
Pregnant women with PB, both with intact fetal membranes and PPROM have a number of risk factors: age, anamnestic data, complications during current pregnancy, somatic and infectious diseases.
PPROM is the fetal membranes defect due to inflammatory changes leading to weakening of membranes. It has been suggested that microcracks in the fetal membranes lead to the disruption of membrane integrity and are a source of penetration of microorganisms into the uterine cavity [5].
The purpose of this study is to identify clinical and anamnestic risk factors in pregnant women with PPROM.
Materials and Methods
We conducted a one-step comparative study. In accordance with the study objective, 150 women were examined. All patients were divided into three groups: group I consisted of 50 patients with PPROM at the gestational age up to 36 weeks, group II consisted of 50 patients with intact fetal membranes at the gestational age up to 36 weeks and group III included 50 somatically healthy pregnant women with unremarkable obstetric and gynecological history and spontaneous term delivery (≥37 weeks).
We studied anamnestic data in all women: age, prior somatic and gynecological diseases, surgical interventions, and the state of reproductive function. The course of the current pregnancy, modes of delivery, neonatal outcomes have been analyzed.
The criteria for inclusion in groups I and II were spontaneous singleton pregnancy, which ended with PB at 22-36 weeks with PPROM (group I) or spontaneous PB with intact fetal membranes (group II). Criterion for inclusion in group III was spontaneous singleton pregnancy, resulting in spontaneous term delivery (≥37 weeks). Informed consent to participate in the study was obtained from all patients.
Exclusion criteria were multiple pregnancy, assisted reproductive technology-related pregnancy, structural and chromosomal anomalies, severe somatic pathology in the pregnant woman, development of preeclampsia during the current pregnancy, malformations of the uterus.
Statistical processing of the received data was performed on personal computer using the IPM SPSS Statistics version 22.0 software package. For the description of the quantitative parameter the data were presented as arithmetic mean and standard deviation M (SD). To compare groups by qualitative binary values method χ2 was used, if one of the values was less than 5, Fisher’s exact test was used. Differences with the probability of error p < 0.05 were considered as statistically significant.
Results
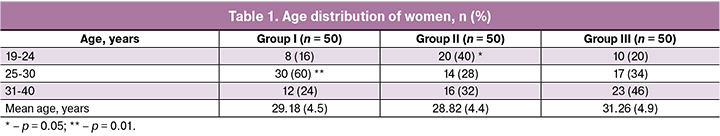
The age of the women studied varied from 19 to 40 years and averaged 29.54 (5.0) years: group I – 29.18 (4.5) years, group II - 28.82 (4.4) years, group III – 31.26 (4.9) years. In the analysis of the age distribution of women it was revealed that in group II the patients at the age of 19-24 years were statistically more frequent – 20 (40%) women, compared to group I and III – 8 and 10 women (16% and 20%), respectively, p = 0.01. At the same time, in group I, women in the most active reproductive age of 25-30 years (60%) were statistically more frequent than in groups II and III – 14 and 17 women (28% and 34%), respectively, p = 0.003. There were no statistically significant differences in all three groups within the age range of 31-40 years. The age distribution of the examined pregnant women by groups is presented in Table 1.
Almost all women lived in the same climatic and geographical conditions, predominantly in Moscow and the Moscow region. There were no statistically significant differences in the level of education among the examined women.
In the course of the study we analyzed the state of health of pregnant women, as well as the peculiarities of somatic and gynecological history.
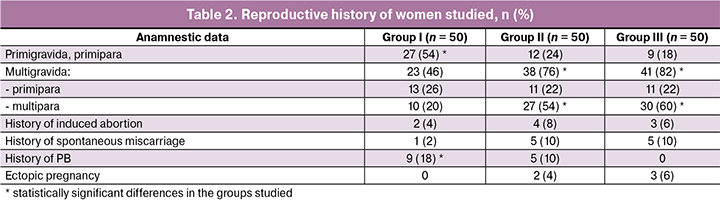
According to the data presented in Table 2, primiparous women were statistically more frequent in group I – 27 patients (54%), compared to groups II and III – 12 and 9 women (24% and 18%), respectively, p < 0.001. There were no statistically significant differences between multigravidas and primiparas in the compared groups. Multiparas and multigravidas were statistically more common in groups II and III – 27 and 30 women (54% and 60%), compared to group I – 10 women (20%), p < 0.001. There were no statistically significant differences in the incidence of induced abortions, spontaneous miscarriages and ectopic pregnancies in all three groups. It should be noted that PB in the history took place more often in group I – 9 women (18%), in comparison with group II — 5 women (10%) and they were absent in group III, p = 0.009.
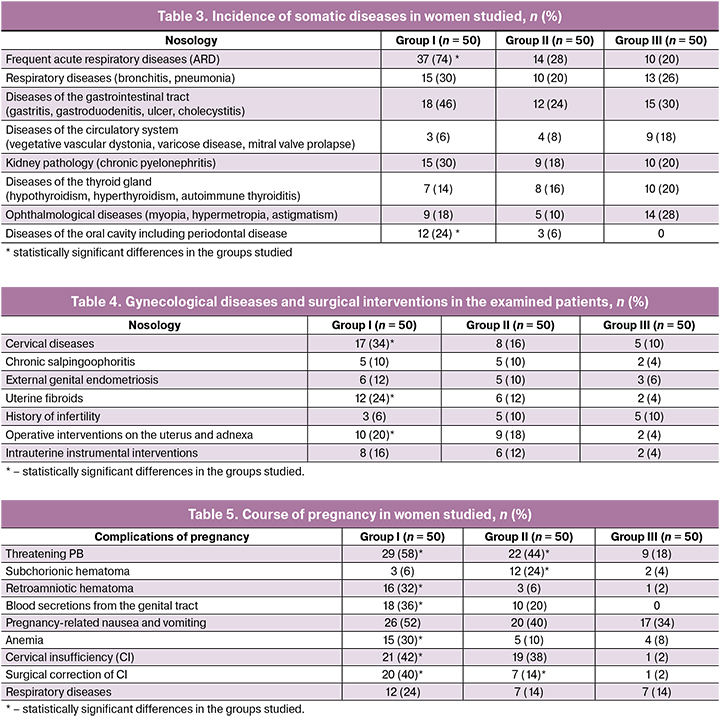
When studying the frequency of somatic diseases, statistically significant differences were revealed in three groups. For example, acute respiratory diseases were statistically more frequent in the history of group I, namely 37, 14 and 10 cases (74%, 28% and 20%), respectively, p < 0.001. There were no statistically significant differences in the frequency distribution of nosologies such as diseases of respiratory tract, cardiovascular system, kidneys, thyroid gland and vision. Periodontal diseases were observed among patients with PB, but statistically more often in group I – in 12 women (24%) compared to group II – 3 women (6%) and were absent in group III with term delivery, p < 0.001. The incidence of somatic diseases is shown in Table 3.
Comparative analysis of menstrual function peculiarities (age of menarche, nature and duration of menstrual cycle) in the studied three groups showed no statistically significant differences, the values corresponded to the average population indicators.
The analysis of gynecological diseases in the women studied, presented in Table 4, revealed that cervical diseases were the most common in all three groups – 17, 8 and 5 women (34%, 16% and 10%), respectively, with statistically significant cross-group differences, p = 0.008. Among infectious and inflammatory diseases of the pelvic organs, chronic salpingoophoritis was noted with the same frequency in groups I and II: 5 and 5 patients (10% and 10%), compared to group III – 2 women (4%), but statistically significant differences were not found. Uterine fibroids were statistically more frequent in group I in 12 women (24%) compared to groups II and III — 6 and 2 women (12% and 4%), respectively, p = 0.01. Endometriosis, infertility history were found in all three groups studied, and in the comparative analysis no statistically significant differences were revealed among them. The frequency of surgical interventions on pelvic organs was statistically significantly higher in the group of pregnant women with PB: in 10 and 9 women (20% and 18%), respectively, compared to the control group – 2 women (4%), p = 0.04.
Analysis of the course of this pregnancy showed that the most common complications presented in Table 5 were threatening PB, which were more common in women with PB in groups I and II – in 29 and 12 women (58% and 44%), respectively, compared to the control group III – 9 women (18%), p < 0.001.
Subchorionic hematoma in early pregnancy was statistically more common in pregnant women of group II – 12 women (24%) compared to groups I and III – 3 and 2 women (6% and 4%), respectively, p < 0.001. In turn, retroamniotic hematoma from the end of the first or the beginning of the second trimester of pregnancy was statistically more common in group I – in 16 pregnant women (32%), compared to groups II and III – 3 and 1 women (6% and 2%), respectively, p < 0.001. The threat of termination of pregnancy with the presence of blood spotting was statistically more frequent in group I – in 18 women (36%), compared to group II - in 10 women (20%) and it was not noted in group III, p < 0.001.
Pregnancy-related nausea and vomiting were noted almost equally in all three groups and amounted to 26, 20 and 17 women (52%, 40% and 34%), respectively; there were no statistically significant differences. Predominantly mild anemia was detected more often in group I – in 15 women (30%) compared to groups II and III – in 5 and 4 pregnant women (10% and 8%), respectively, p = 0.05. CI was statistically more likely in groups I and II – 21 and 19 women (42% and 38%), respectively, compared to group III – 1 woman (2%), p < 0.001. In group I, 20 women (40%) with CI underwent surgical correction, compared to women of groups II and III — where correction was done in 7 and 1 cases (14% and 2%, respectively, p < 0.001. In early pregnancy there were ARD events in 12 women (24%) in group I, while in groups II-III the incidence of ARD in the early stages of pregnancy was the same and was 7 and 7 cases (14% and 14%), respectively.
Outcomes of childbirth and the course of the early neonatal period
In the comparative analysis of the peculiarities of childbirth at different gestational age presented in Table 6, the absence of statistically significant differences depending on the mode of delivery at 22-27 weeks of pregnancy in groups of women with PB, regardless of their clinical phenotype (I and II groups) was noted. At the same time, the frequency of cesarean section at 28-33 weeks of pregnancy was statistically significantly higher in group II. On the contrary, spontaneous delivery at 34-36 weeks of pregnancy statistically significantly more often prevailed in group I. The average gestational period for delivery in group I was 32.84 (3.3) weeks, in group II it was 32.16 (2.4) weeks and in group III it was 39.4 (3.5) weeks of gestation.

The duration of the period between membrane rupture and delivery was statistically significant in group I and ranged from 50 minutes to 105 hours 40 minutes and averaged 21.16 hours (40 minutes), compared to group II and III — 2.3 hours (21 minutes) and 3.6 hours (26 minutes), respectively, p < 0.001.
The most frequent indications for surgical delivery in group I with PPROM included anhydramnios (with the period between membrane rupture and delivery over 24 hours), absence of regular uterine contractions, impaired fetal condition according to ultrasound and Doppler velocimetry; in group II with PB the indications were deterioration of fetal condition, poor uterine contractions, acute fetal hypoxia. Indications for surgical delivery by cesarean section in the groups studied are presented in Table 7.
Mean infant birth weight in group I was 2000.0 (605.0) grams, in group II - 1917.0 (485.0) grams, in group III – 3308.0 (209.0) grams, p = 0.01 (Table 8). Examining the newborns’ condition according to Apgar scale, we have identified two main reasonable points: dependence on gestational period at birth and mode of delivery. In group I, the average assessment of the newborn’s condition according to the Apgar scale was 6.5 (1.2) points in the 1st minute, 7.4 (1.0) points in the 5th minute, in group II – 5.3 (2.5) points in the 1st minute, and 6.2 (2.7) points in the 5th minute; in group III – 7.9 (0.3) points in the 1st minute, 8.7 (0.4) points in the 5th minute, respectively. According to the results of the analysis, in group I delivery by cesarean section increased the score of newborns according to Apgar scale in the 1st minute by 6.1±1.2 points and in the 5th minute by 7.1±0.9 points, in comparison with the vaginal delivery – 4.5±3.5 points and 6.9±2.8 points, respectively. Groups I and III did not have any significant differences in the assessment of the newborn’s condition depending on the mode of delivery. The mode of delivery at 22-27 weeks gestation did not affect the newborn’s condition (according to Apgar scale). Some tendency to improve the newborn’s condition (the 5-minute Apgar) was observed in operative delivery at 34-36 weeks gestation.

Thus, neonatal outcomes were severe in group II due to delivery at the early stages of pregnancy, compared to group I. Central nervous system depression syndrome was detected in groups I and II — 9 and 18 cases (18% and 36%) and statistically significantly differed in group II compared to group I (p = 0.04). In group II, statistical differences with group I by the frequency of hemolytic disease development (p = 0.01) were also revealed. The development of sepsis in newborns in groups with PB was 8 (16%) and 5 (10%) cases, but no statistically significant differences were found. The probable causes of sepsis were prematurity, (long period between membrane rupture and delivery – 21, 16 hours (40 min) in the group with PPROM), presence of intrauterine infection.
Discussion
PPROM is an important problem in obstetric practice. The research conducted by us allowed to establish the multifactorality of this pregnancy complication.
The obtained results revealed a higher incidence of PPROM at 22-36 weeks gestation in first-time pregnant women, whereas PB with intact fetal membranes and term delivery were found among multiparous women. Most pregnant women with PB as a result of PPROM were in the most active reproductive age of 25-30 years (60%). According to the results of the study of foreign scientists it was revealed that the peak of occurrence of PPROM in incomplete pregnancy in 43% of cases was noted at the age of 26-30 years [6—8].
In our study, in the analysis of the reproductive function of women, it was found that the risk of PPROM during pregnancy up to 36 weeks is higher in women with history of PB. The American Association of Obstetricians and Gynecologists cited data according to which PPROM with history of PB increases the risk of “recurrence” of PPROM by 16-32% [9].
Pregnancy of women with PPROM was complicated by the threat of termination since the early stage of pregnancy, which in turn increased the threatening premature labor. It was also complicated by the formation of retroamniotic hematoma in 32% of cases, while pregnant women with PB and intact fetal membranes had subchorionic hematoma and anemia from early pregnancy in 24% of cases [10-14].
According to meta-analysis on the relationship of subchorionic hematoma with adverse outcomes of pregnancy, pregnant women with subchorionic hematoma have an increased risk of abortion (with 0.7% to 3.6%), PB (from 10.1% to 13.6%) [15-19]. There is evidence that the localization of retroamniotic hematoma at the edge of the placenta can lead to local irritation of amnion, thus, provoking PB with PPROM [19, 20].
The frequency of PPROM did not have statistically significant differences between groups I and II, but in group I women with PPROM underwent surgical correction more frequently. This association is confirmed by the results of foreign trials [21]. In addition, according to Cochran’s library, a number of factors increase the risk of PPROM: history of PB, development of CI, cerclage, bleeding during pregnancy [22].
The development of periodontitis and oral infections is a predisposing risk factor for PB and adverse pregnancy outcomes. In our study, the presence of oral diseases was found in 24% of cases with PPROM. According to the received data, gynecological diseases (uterine fibroids, cervical diseases) and surgical interventions on pelvic organs play an important role in the development of PPROM, which can be consistent with literature data [23].
In our study, the most frequent mode of delivery of pregnant women with PB was cesarean section, which is consistent with the literature data [24-26]. According to the received data, the delivery period for the majority of patients in group I (60%) was 34-366 weeks gestation. There is evidence that 59.5% of PPROM cases occur during gestational period of 35-36 weeks [27]. The results of multicenter studies did not reveal the benefits of emergency delivery in PPROM up to 34 weeks of gestation in comparison with the wait-and-see tactics for neonatal complications [28]. According to the results of a prospective cohort study conducted in 2018, the most common neonatal complications in preterm newborns in the group of women with PPROM are respiratory disorders, intrauterine infection and sepsis [29].
Conclusion
PPROM in incomplete pregnancy is a clinical phenotype of PB, and its outcome depends on the gestation period during which the complication occurred. The study of clinical and anamnestic peculiarities of patients indicates its preventability in a number of cases. At the same time, a comprehensive study of this problem will make it possible to develop approaches to prevent PPROM before pregnancy and to predict it in the first trimester of pregnancy.
References
- Offiah I., O’Donoghue K., Kenny L. Clinical risk factors for preterm birth. In: Morrison J., ed. Preterm birth-mother and child. InTech; 2012: 73-95.
- Blencowe H., Cousens S., Chou D., Oestergaard M., Say L., Moller A. et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod. Health. 2013; 10(Suppl. 1): S2.
- Goldenberg R.L., Culhane J.F., Iams J.D., Romero R. Epidemiology and causes of preterm birth. Lancet. 2008; 371(9606): 75-84.
- Mercer B.M., Crouse D.T., Goldenberg R.L., Miodovnik M., Mapp D.C., Meis P.J., Dombrowski M.P. The antibiotic treatment of PPROM study: systemic maternal and fetal markers and perinatal outcomes. Am. J. Obstet. Gynecol. 2012; 206(2): 145. e1-9.
- Menon R., Richardson L.S. Preterm prelabor rupture of the membranes: a disease of the fetal membranes. Semin. Perinatol. 2017; 41(7): 409-19.
- Hailemariam Segni, Takele Digafe Diribaand, Elias Ali. Incidence, maternal and perinatal outcome of premature rupture of fetal membrane cases in Jimma University Teaching Hospital, South West Ethiopia. EC Gynaecology. 2017; September 11.
- Ibishi V.A., Isjanovska R.D. Prelabour rupture of membranes: mode of delivery and outcome. Open Access Maced. J. Med. Sci. 2015; 3(2): 237-40.
- Zhang L.X., Sun Y., Zhao H., Zhu N., Sun X.D., Jin X. et al. A Byesian stepwise discriminant model for predicting risk factors of preterm premature rupture of membranes: a case-control study. Chin. Med. J. (Engl.). 2017; 130(20): 2416-22.
- ACOG Committee on Practice Bulletins-Obstetrics. Clinical management guidelines for obstetrician-gynecologists. Practice Bulletin No. 160: Premature rupture of membranes. Obstet. Gynecol. 2016; 127(1): e39-51.
- Manisha Choudhary, Samta Bali Rathore, Jai Chowdhary, Swati Garg.Pre and post conception risk factors in PROM. Int. J. Res. Med. Sci. 2015; 3(10): 2594-8.
- Endale T., Fentahun N., Gemada D., Hussen M.A. Maternal and fetal outcomes in term premature rupture of membrane.World J. Emerg. Med. 2016; 7(2): 147-52.
- Jaiswal A.A., Hariharan C., Dewani D.K.C. Study of maternal and fetal outcomes in premature rupture of membrane in central rural India. Int. J. Reprod. Contracept. Obstet. Gynecol. 2017; 6(4): 1409-12.
- Janhavi Mukharya, Simmi Mukharya. Comparative study of fetal and maternal outcomes of prelabour rupture of membranes at term. Int. J. Reprod. Contracept. Obstet. Gynecol. 2017; 6(1): 149-63.
- Hexia Xia, Xilian Li, Xiaotian Li, Huan Liang, Huan Xu. The clinical management and outcome of term premature rupture of membrane in East China: results from a retrospective multicenter study. Int. J. Clin. Exp. Med. 2015; 8(4): 6212-17.
- Tuuli M.G., Norman S.M., Odibo A.O., Macones G.A., Cahill A.G. Perinatal outcomes in women with subchorionic hematoma: a systematic review and meta-analysis. Obstet. Gynecol. 2011; 117(5): 1205-12
- Li Q., Zhu J., Hua K. Effects of subchorionic hematoma on pregnancy outcome: a meta-analysis. Zhonghua Yi Xue Za Zhi. 2016; 96(17): 1383-5.
- Mitchell-Jones N., Coad A., ClaytonSmith P., Bottomley C. OP05.03: Mid‐trimester subchorionic hematoma; maternal and neonatal morbidity data. Ultrasound Obstet. Gynecol. 2016; 48(Suppl. 1): 64.
- Heller H.T., Asch E.A., Durfee S.M., Goldenson R.P., Peters H.E., Ginsburg E.S. et al. Subchorionic hematoma: correlation of grading techniques with first‐trimester pregnancy outcome. J. Ultrasound Med. 2018; 37(7): 1725–32.
- Ott J., Pecnik P., Promberger R., Pils S., Binder J., Chalubinski K.M. Intra-versus retroplacental hematomas:a retrospective case-control study on pregnancy outcomes. BMC Pregnancy and Childbirth. 2017;17(1): 366.
- Bakalis S., David A.L. Successful outcome after spontaneous first trimester intra-amniotic haematoma and early preterm premature rupture of membranes. BMJ Case Rep. 2018; 11(1). pii: e224596.
- Monson M.A., Gibbins K.J., Esplin M.S., Varner M.W., Manuck T.A. Pregnancy Outcomes in women with a history of previable, preterm prelabor rupture of membranes. Obstet. Gynecol. 2016; 128(5): 976-82.
- Abou El Senoun G., Dowswell T., Mousa H.A. Planned home versus hospital care for preterm prelabour rupture of the membranes (PPROM) prior to 37 weeks’ gestation. Cochrane Database Syst. Rev. 2014; (4): CD008053.
- Михайлов А.В., Дятлова Л.И., Рогожина И.Е., Глухова Т.Н., Панина О.С. Ведение беременности, осложненной преждевременным излитием околоплодных вод при недоношенной беременности. Акушерство и гинекология. 2014; 2: 67-73.[Mikhailov A.V., Dyatlova L.I., Rogozhina I.E., Glukhova T.N., Panina O.S. Conducting pregnancy complicated by premature rupture of amniotic fluid in preterm pregnancy//Obstetrics and Gynecology. 2014; 2: 67-73.(In Russ.).
- Hussin A.K., Jamil U., Nanu D. Early rupture of membrane a risk factor for cesarean section in term pregnancy. ANALELE UNIVERSITĂŢII “DUNĂREA DE JOS” GALAŢI. 2013; Fascicula XVII(nr.2): 95-9.
- Chakraborty B., Mandal T., Chakraborty S. Outcome of prelabor rupture of membranes in a tertiary care center in west Bengal. Indian J. Clin. Pract. 2013; 24(7): 657-62.
- Kunze M., Hart J.E., Lynch A.M., Gibbs RS. Intrapartum management of premature rupture of membranes:effect on cesarean delivery rate. Obstet. Gynecol. 2011; 118(6): 1247-54.
- Okeke T.C., Enwereji J.O., Okoro O.S., Adiri C.O., Ezugwu E.C., Agu P.U. The incidence and management outcome of preterm premature rupture of membranes (PPROM) in a tertiary hospital in Nigeria. Am. J. Clin. Med. Res. 2014; 2(1): 14-7.
- Lynch T.A., Olson-Chen C., Colihan S., Meyers J., Holloman C., Li D. et al. Preterm prelabor rupture of membranes: outcomes with expectant management until 34 versus 35 weeks. Am. J. Perinatol. 2019; 36(7): 659-68.
- Ramesh T.V., Bineet Panigrahi, Pranaya P., Hima Bindu P. Outcome of neonates born to mothers with premature rupture of membranes. Int. J. Contemp. Pediatr. 2018; 5(4): 1190-4.
Received 09.02.2019
Accepted 22.02.2019
About the Authors
Guseynova Gulnara E., graduate student in the Department of Pregnancy Pathology, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Healthcare of Russian Federation. Phone: +7 (967) 153-18-81. E-mail: marysca666@rambler.ru117997 Russia, Moscow, Akademika Oparina str., 4.
Khodzhaeva Zulfiya S., M.D., Professor, Head of High Risk Pregnancy Dept, National Medical Research Center for Obstetrics, Gynecology and Perinatology
named after Academician V.I. Kulakov Ministry of Healthcare of Russian Federation. Phone: +7 (916) 407-75-67. E-mail: zkhodjaeva@mail.ru
117997 Russia, Moscow, Akademika Oparina str., 4
For citation: Guseinova G.E., Khodzhaeva Z.S. Clinical and anamnestic features of women with preterm premature rupture of membranes.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; (8): 54-61(in Russian).
http://dx.doi.org/10.18565/aig.2019.8.54-61



