Clinical manifestations and recurrence rates of various forms of extragenital endometriosis
Pronina V.A., Sokolova A.V., Chernukha G.E.
Objective: This study aimed to evaluate the clinical and anamnestic data of patients with different forms of extragenital endometriosis (EGE), considering previous surgical interventions.
Materials and methods: A cross-sectional study was conducted at the V.I. Kulakov NMRC for OG&P from 2021 to 2023. The study involved 200 patients (mean age: 32.03 (7.15) years) with EGE, diagnosed using pelvic ultrasound (US) and magnetic resonance imaging (MRI). Patients were categorized into 3 groups based on the form of endometriosis: peritoneal endometriosis (PE), endometrioid cysts (EC), and deep endometriosis (DE). In cases of combined pathology, inclusion in a specific group was determined based on the most severe form of EGE. Women’s complaints were obtained through interviews, questionnaires, and comprehensive analysis of clinical and anamnestic data, including previous surgical and drug treatments.
Results: The study revealed that one in every three patients had complaints not typically associated with endometriosis. In every third case, the EC was incidentally identified using ultrasonography. When specifically questioned, 23.4% of patients with EC did not experience the characteristic pelvic pain associated with endometriosis, compared to 10.0% in the PE group and only 2.7% in the DE group. One in every three patients had undergone at least one previous surgical intervention, with 30.2% of them not receiving suppressive hormone therapy and 39.7% receiving short courses. Subsequently, the recurrence rate was 94.7% among patients who did not receive hormone therapy and 92% among those who received a short course of suppressive hormone therapy, irrespective of hormone therapy type. After previous surgical treatment for PE and EC, 48.7% of patients were subsequently diagnosed with DE. Among patients with previous surgery for EC, 41.9% experienced EC recurrence and 54.8% progressed to DE, whereas DE recurred to the same form of EGE in 85.7% of cases.
Conclusion: In one in every three cases, patients with EGE presented with complaints that were not typical of endometriosis. A targeted collection of complaints and medical history can aid in suspecting endometriosis as early as the initial outpatient visit, thereby reducing the time before diagnosis and treatment. Short courses of suppressive hormone therapy, regardless of type, do not prevent recurrence or progression of endometriosis after surgical treatment.
Authors’ contributions: Chernukha G.E., Pronina V.A. – conception of the study; Pronina V.A., Sokolova A.V. – data collection and analysis, drafting and editing of the manuscript; Chernukha G.E. – editing of the manuscript and approval for submission.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Acknowledgment: The authors would like to thank Simich-Lafitsky N.D., Ph.D. for statistical analysis and mathematical description of the data obtained during the study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient Сonsent for Publication: All patients provided informed consent for the publication of their data.
Authors’ Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Pronina V.A., Sokolova A.V., Chernukha G.E. Clinical manifestations and recurrence rates of various forms of extragenital endometriosis.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (12): 134-142 (in Russian)
https://dx.doi.org/10.18565/aig.2023.234
Keywords
Endometriosis, a prevalent gynecological disease, affects approximately 40–60% of women with chronic pelvic pain (CPP) and approximately 50% of those experiencing infertility [1–4]. Although nearly 90% of women with endometriosis experience some level of pain, there is an average diagnostic delay of 6–8 years. Statistics reveal that a woman may consult around 5 doctors before receiving a confirmed diagnosis and commencing treatment [5–7].
This delay can be attributed partly to the limitations of ultrasound diagnostics (USD) in accurately detecting endometriosis, especially peritoneal (PE) and deep endometriosis (DE), which rely heavily on the expertise of the practitioner [8–14]. Additionally, it stems from the broad spectrum of symptoms that often lack focus on the primary clinical signs at the time of the doctor's visit. A cross-sectional study conducted in France from 2005 to 2021 identified 41 symptom categories in women with endometriosis, including uncommon symptoms, such as fever, nausea, or vaginal itching [15]. Similarly, Chapron et al., in their study involving 1685 patients, noted that 58.3% of women with surgically excluded endometriosis still reported dysmenorrhea [16].
Although existing literature presents conflicting data, there are indications supporting the potential identification of endometriosis and its specific forms based on clinical manifestations [7, 17, 18]. For instance, Chen et al. demonstrated that intense pain in two or more locations coupled with gastrointestinal symptoms increases the likelihood of endometriosis by 15 times [17]. Another study by Chapron et al. highlighted the associations between different forms of endometriosis and specific symptoms: uterosacral ligament endometriosis with severe CPP and profound dyspareunia, vaginal endometriosis with lower urinary tract symptoms, and colorectal endometriosis (CRE) with severe dysmenorrhea and gastrointestinal issues [18].
Furthermore, studies indicate that endometriotic cyst (EC) typically lack prominent clinical manifestations, and the presence of pain is often linked to concurrent forms of endometriosis, particularly PE and DE [19, 20].
An essential aspect of extragenital endometriosis (EGE) is its progressive and recurrent nature. Previously, laparoscopy with histological confirmation was the gold standard for diagnosis [9]. However, this approach has been reconsidered as surgery alone without subsequent suppressive hormone therapy and does not offer a long-term solution. Studies suggest that every second patient might experience a relapse within 5–7 years, especially those with severe forms, such as DE [21–23].
Given the limited research on the risk of postoperative progression of endometriosis, this study aimed to evaluate the clinical and anamnestic data of patients with various forms of extragenital endometriosis, taking into account their prior surgical interventions.
Materials and methods
This cross-sectional study involved 200 patients aged 18–49 years (mean age – 32.03 (7.15) years; mean body mass index – 21.35 (3.35) kg/m2) with EGE, diagnosed using pelvic ultrasound (US) and magnetic resonance imaging (MRI).
In 54% of the patients, EGE was laparoscopically and histologically verified, in 63/200 (31.5%) patients during a previous surgical intervention before inclusion in the study, and in 45/200 (22.5%) during the subsequent first elective surgery.
The study was conducted at Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation from 2021 to 2023.
Complaints and medical history, including previous surgical treatment and hormone therapy, as well as patient questionnaires on the presence of various manifestations of pain syndrome and associated non-gynecological diseases were collected. The exclusion criteria were current or past oncologic diseases of the female reproductive system, pregnancy and lactation, and absence of EGE signs according to expert MRI performed or reviewed at the Center. Patients with adenomyosis alone were excluded from the study to reduce the likelihood of systematic errors.
Pelvic MRI was performed using GE Signa Excite 1.5T and GE Signa Architect 3.0T MRI devices. Contrast enhancement was performed in cases that required differential diagnosis of endometrioid lesions and as an adjunct tool for visualization of extragenital forms of endometriosis. MRI was used to evaluate signal intensities on T1-weighted (T1WI) and T2-weighted images (T2WI) obtained in the sagittal, axial, and coronal planes, as well as on diffusion-weighted images, using 0.3 cm slice thickness and 32–42 cm field of view. The visual diagnosis of EC, DE, and EGE was performed according to the European Society of Urogenital Radiology (ESUR) guidelines [24]. A presumptive diagnosis of PE was made if there were MR signs of endometrial heterotopias less than 0.5 cm in size, with hypointense signal on T2VI, with pickle-shaped inhomogeneous contours on the uterine serosa, ligaments, including the sacroiliac-uterine ligaments, as well as on the peritoneum of other sites, and vesicoureteral and parametrial tissues in the pelvic region [10–14].
The patients were categorized into three groups based on the form of endometriosis: PE, EC, and DE. In cases of combined pathology, inclusion in a specific group was determined on the basis of the most severe form of EGE.
The main complaint that the patient presented with was included in the analysis of the reason for referral. CPP was defined as the presence of acyclic lower abdominal pain for at least six months and was not associated with menstruation, ovulation, or sexual intercourse. Dyspareunia was assessed only in women who were sexually active, and infertility was assessed only in women who were planning a pregnancy. All data were anonymized before calculating the material obtained. The study was reviewed and approved by the Research Ethics Committee of V.I. Kulakov NMRC for OG&P of the Ministry of Health of Russia.
Statistical analysis
The sample size was calculated based on the prevalence rate of dysmenorrhea as the most significant clinical manifestation of endometriosis [9]; with a type I error α=0.05, and a power of 80%, we estimated that at least 51 participants would be required. Initially, 261 patients with suspected genital endometriosis based on pelvic ultrasound were selected from the general population for this study. At follow-up, 48 patients did not come for follow-up, nine did not undergo pelvic MRI, and four did not have EGE detected by MRI. The final sample size was 200 patients, which was a representative sample overall and for each subgroup separately.
For continuous variables, the mean (M), standard deviation (SD), median (Me), and interquartile range (Q1, Q3) were calculated. Categorical variables are presented as counts and percentages (%). The normality of the distribution was tested using the Shapiro–Wilk test. Continuous variables showing normal distribution were expressed as mean (M) and standard deviation (SD); otherwise, the median (Me) with interquartile range (Q1; Q3) was reported. The Kruskal–Wallis test was used to compare non-normally distributed data among three or more groups. Statistical analysis was performed using IBM SPSS Statistics (v.26). The critical level of significance when testing the statistical hypotheses was set at p<0.05.
Results
PE was observed in 68/200 women (34%), EC in 53/200 (26.5%), and DE in 79/200 (39.5%). Isolated EC and DE were observed in only 9/200 (4.5%) and 4/200 (2%) cases, respectively, most of which had combinations of EGE forms (Fig. 1). It should be noted that every 4th patient had concomitant colorectal endometriosis, every 13th patient had EGE at other sites (bladder, scar, appendix), and EGE was more often observed in combination with DE. EGE was combined with adenomyosis in 182/200 (91%) cases. The clinical characteristics of the subgroups of patients with the different forms of EGE are shown in Table 1.
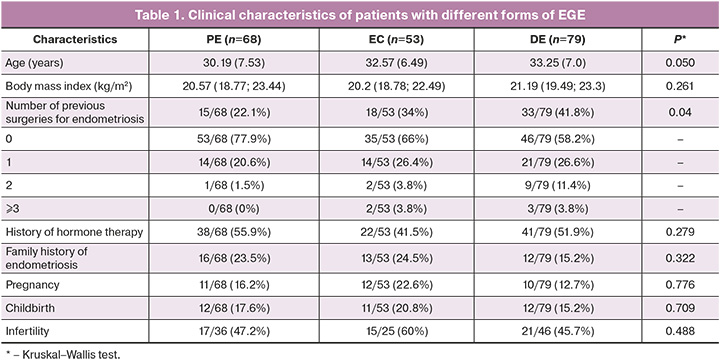
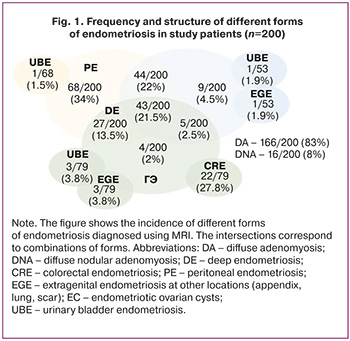
According to these data, more severe forms of endometriosis tend to develop with increasing age. Thus, among women ≤ 30 years of age, PE was diagnosed in 38/87 (43.7%), EC in 22/87 (25.3%), and DE in 27/87 (31%), while in patients > 30 years of age, the incidence of these forms of endometriosis was 30/113 (26.5%), 31/113 (27.4%), and 52/113 (46.1%), respectively (Table 2).
It is important to note that between the ages of 18 and 24 years, one in three patients had DE, with primary DE noted in 90.9% of cases. This cohort of patients was characterized by low (5/11, 45.5%) or normal body mass index (19.38 (1.46) kg/m2), the presence of clinical hyperandrogenism (8/11, 72.7%), and dysmenorrhea, predominantly primary (8/11, 72.7%) of high intensity (8.27 (1.34) points), in more than 90% of cases combined with other manifestations of pain syndrome (CPP, dyspareunia, and premenstrual and ovulatory pain). One in three women had a history of ovarian apoplexy (4/11, 36.4%), which was twice as frequent as that in patients of the same age group with PE (3/16, 18.8%).
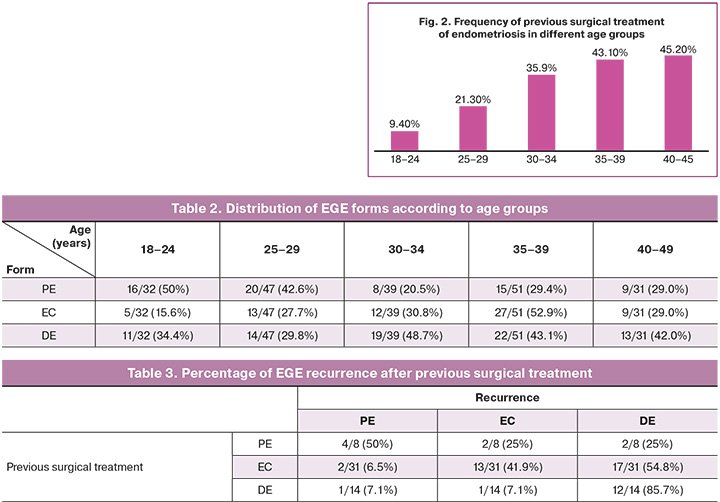
As the age of patients increased, the percentage of women with a history of surgical intervention for endometriosis also increased. For example, among the 63 patients with prior surgical treatment, 1 in 10 women underwent surgery at age 18–24 years, approximately 1 in 5 at age 25–29 years, and almost 1 in 2 at age 30 years or older (Fig. 2). Previously, 47/63 (74.6%) patients had a history of one surgery, 11/63 (17.5%) of two surgeries, and a history of 3 or more surgeries had 5/63 (7.9%) of three or more surgeries.
DE was subsequently diagnosed in 19/39 (48.7%) patients who had previously undergone surgery for PE and EC. Among the patients who underwent prior surgery for EC, 41.9% had recurrence of endometriomas and 54.8% had progression to DE. DE recurred to the same form of EGE in 85.7% of cases and only 14.2% recurred to EC and PE. Among the patients with a history of surgery for EGE, 3/5 (60%) had subsequent DE and 2/5 (40%) had recurrence of EGE (Table 3).
It should be noted that 1 in 3 patients with a history of surgery (19/63, 30.2%) did not receive suppressive hormone therapy after the surgical intervention; the recurrence rate in this group of women was 94.7% (18/19). The mean duration of postoperative treatment with oral progestins was 9.73 (5.46) months, combined oral contraceptives 7.07 (3.67) months, and gonadotropin-releasing hormone agonists 4.0 (1.56) months, respectively. It is important to note that in 56.8% (25/44) of the cases, the therapy was administered in short courses of 3–6 months, and in 23/25 patients (92%), recurrence occurred 3 or more months after its termination. Only four cases of EGE recurrence occurred against the background of hormone therapy: two cases of EC recurrence against the background of 9 and 36 months of dienogest; one case, 12 months of combined oral contraceptives; and one case, 24 months of levonorgestrel-releasing intrauterine system (LNG-IUS) 20.
Analysis of the reasons for referral showed that every third woman visited a gynecologist with complaints that were not typical of EGE (70/200, 35%) (Fig. 3). Every 10th patient underwent routine annual examination (23/200, 11.5%) and every 13th for selection of therapy, including contraception. Rarer reasons for referral include acne, vulvovaginitis, mastodynia, and others. Endometriosis-specific complaints included pain syndrome including dysmenorrhea and CPP (86/200, 43%), abnormal uterine bleeding (AUB) (26/200, 13%), and, in rarer cases, infertility (18/200, 9%).
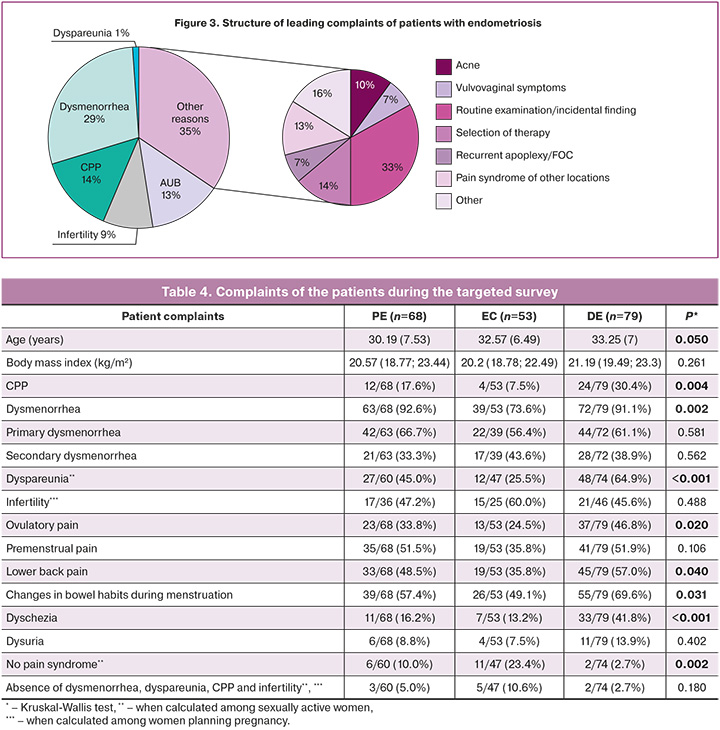
The complaints varied depending on the type of endometriosis. Thus, 23/68 (33.8%) patients with PE, 16/53 (30.2%) with EC and 47/79 (59.5%) with DE presented with dysmenorrhea, dyspareunia or CPP. Every second patient with EC (26/53, 49.1%), every third with PE (26/68, 38.2%), and only every 5th patient with DE (18/79, 22.8%) presented with complaints not typical of genital endometriosis (p=0.007). In every third patient, ECs were incidentally detected on ultrasound (19/53, 35.8%).
In the targeted interview, 108/200 (54.0%) women had dysmenorrhea with menarche and 66/200 (33.0%) women had secondary dysmenorrhea. In the entire cohort of patients, 89.5% had at least one manifestation of pain syndrome despite the absence of such complaints at the time of treatment (Table 4). It is important to note that a number of manifestations typical of endometriosis, such as dyspareunia, dyschezia, and changes in stool during menstruation, were significantly more often observed in patients with DE. In patients with EC, approximately one in five cases was characterized by the absence of any classic symptoms of endometriosis.
Despite the relatively high frequency of pain syndrome, the mean time from the onset of symptoms, especially dysmenorrhea, to the diagnosis of endometriosis was 10 (3.5;15) years, and for CPP it was 1 (0;3) years. Endometriosis manifested itself in 78/200 (39%) patients aged <18 years, almost every second before the age of 20 years (96/200, 48%), and in 79.5% of women before the age of 30 years (159/200). After the age of 30 years, the tendency for clinical manifestations of endometriosis decreased: at the age of 30–34 years, the frequency was 13.5% (27/200), at 35–39 years – 4.5% (9/200); and at 40 years and older, only 2.5% (5/200).
After initial diagnosis of EGE or confirmed recurrence, 105/200 patients (52.5%) were recommended hormone therapy, including dienogest in 79/105 (75.2%) patients, combined oral contraceptives in 18/105 (17.1%) patients, gonadotropin-releasing hormone agonists in 3/105 (2.9%) patients, and LNG-IUS 20 in 5/105 (4.8%) patients.
No therapy was prescribed to the remaining patients, including due to pregnancy planning (18/200, 11.1%), need for surgical intervention (59/200, 29.5%), and failure to attend follow-up visits (18/200, 11%). Indications for surgical treatment were the presence of DE in combination with EC and/or DE in 40/59 (67.8%) patients, EC > 4 cm in diameter in 12/59 (20.3%) patients, and EGE in combination with infertility or severe pain syndrome in 7/59 (11.8%) patients.
Discussion
Endometriosis, characterized by pronounced clinical symptoms, often remains undiagnosed for an extended period of time. Our study data revealed that one-third of the patients with endometriosis presented with complaints unrelated to the condition. Notably, the manifestations of EGE tended to vary depending on the specific form of the ailment. For instance, patients with ECs report less frequent pain than those with pelvic or deep endometriosis. Surprisingly, one-third of the patients had ECs that were incidentally discovered during the ultrasound examinations.
It's essential to highlight that women with ECs were significantly less likely to complain of dysmenorrhea, dyspareunia, and chronic pelvic pain (CPP) compared to patients with pelvic or deep endometriosis. About one in four patients with ECs did not exhibit all three classical pain syndrome manifestations, while the absence of characteristic endometriosis-related complaints in women with pelvic and deep endometriosis was 10.0% and 2.7%, respectively.
Interestingly, literature suggests that pain syndrome in ECs is more commonly associated with other concomitant forms of EGE. Studies indicate that only 38.3% of cases with isolated ECs presented pain syndrome, while 61.7% had no active complaints [19, 20]. The distinction between pelvic and deep endometriosis may not be immediately apparent. Our study showed that while CPP (17.6% and 30.4%) and dyschezia (16.2% and 41.8%) were less prevalent in PE than in DE, dyspareunia (45.0% and 64.9%) and ovulatory pain (33.8% and 46.8%) were quite common. Furthermore, the percentage of women lacking endometriosis-associated complaints, including infertility, was comparable in both forms (5.0% and 2.7%, respectively).
These findings suggest the importance of MRI in suspected cases of EGE, especially in patients with endometriomas who experience pain, to assess the extent of the condition and to determine appropriate management strategies. Notably, in the 18 to 24 years age group, DE was diagnosed in one out of every three cases, with signs of primary DE found in 90% of those cases. This finding challenges the notion that this form primarily affects older women. Consequently, our findings advocate MRI evaluation in young females with pain symptoms, even in the absence of evident genital endometriosis on ultrasound.
While ECs constitute one of the most prevalent forms of endometriosis, occurring in 50.5% of our study cases, isolated endometriomas were observed in only 4.5% of the cases. These results align with Theobald et al.'s study, which reported intraoperatively verified ECs in 40–50% of cases, PE in 20–30%, colorectal endometriosis (CRE) in 10-20%, and extragenital müllerianosis (EME) in less than 10% [25]. Additionally, Blum et al. [26] and Piriyev et al. [27] also showed a relatively low occurrence of isolated endometriotic cysts, at 3.7% and 2.3%, respectively. Considering that half of the patients with ECs did not present characteristic complaints during gynecological appointments, assessing comorbidity becomes pivotal in identifying the risk group for EGE, as highlighted in our previous study [28]. The frequent co-occurrence of multiple forms of endometriosis warrants MRI assessments for patients with ECs to clarify cyst genesis and size and to evaluate the extent of EGE.
It is recognized that previous surgical interventions may correlate with the emergence of more severe types of endometriosis [21, 22]. Our study revealed that 48.7% of patients previously operated on for PE and ECs and 85.7% with prior DE experienced progression or recurrence of DE during follow-up. Interpreting the recurrence rates of ECs and DE in PE requires caution because of the frequent combination of forms (59.5% of cases in our study) and inability to excise all superficial foci intraoperatively. Thus, we can only refer to this as clinical relapse in such cases. These findings are consistent with those in the existing literature. Nirgianakis K. et al. demonstrated that women previously operated on for ECs underwent repeat ovarian resection for endometriomas in 46.8% of cases and repeat surgery for DE in 39.5%. Patients initially diagnosed with DE tend to develop the same form of EGE (53.7%) during subsequent surgeries [29]. Xu B. et al. reported an increased prevalence of endometriosis and a statistically significant escalation in pain severity among patients with recurrent ECs compared to those initially operated on (p<0.001) [30].
The tendency of endometriosis to recur and progress underscores the need for careful assessment of surgical indications and, if necessary, long-term postoperative suppressive hormone therapy. Notably, one-third of the post-surgical patients in our study did not receive suppressive hormone therapy, while it was prescribed for only 56.8% of cases for a duration of 3–6 months. This may be due to a lack of awareness among physicians regarding the feasibility of long-term drug therapy for endometriosis. Furthermore, there is a dearth of studies assessing the safety of progestagens, particularly dienogest, beyond a 7-year period [31]. The recurrence rate after the cessation of the 6-month therapy course was 92%. Therefore, the administration of short-term hormone therapy, irrespective of type, should not be considered as a secondary prevention of EGE.
In summary, the management of young patients diagnosed with endometriosis who lack surgical indications necessitates further investigation into the efficacy and safety of hormone therapy throughout the reproductive period. Additionally, exploring new drugs for the conservative therapy of EGE is crucial.
Conclusion
Numerous unresolved issues regarding the diagnosis and treatment of various forms of endometriosis persist. Considering that one-third of patients lack characteristic endometriosis-related complaints during their initial visit, targeted interviews and comprehensive history collection are advisable. This approach may aid in the early diagnosis of endometriosis, leading to further evaluation with imaging diagnostic modalities and timely initiation of therapy. This becomes crucial as severe forms, such as DE, manifest before the age of 25 in one-third of cases. Furthermore, the tendency of endometriosis to recur and progress after previous surgical treatment highlights the ineffectiveness of short courses of suppressive hormone therapy, irrespective of the type. Therefore, research aimed at studying the efficacy and safety of long-term hormone therapy and exploring new conservative treatment approaches for endometriosis is essential.
References
- Moradi Y., Shams-Beyranvand M., Khateri S., Gharahjeh S., Tehrani S., Varse F. et al. A systematic review on the prevalence of endometriosis in women. Indian J. Med. Res. 2021; 154(3): 446-54. https://dx.doi.org/10.4103/ijmr.IJMR_817_18.
- Bernuit D., Ebert A.D., Halis G., Strothmann A., Gerlinger C., Geppert K., Faustmann T. Female perspectives on endometriosis: findings from the uterine bleeding and pain women’s research study. Journal of Endometriosis. 2011; 3(2): 73-85. https://dx.doi.org/10.5301/je.2011.8525.
- Fuldeore M.J., Soliman A.M. Prevalence and symptomatic burden of diagnosed endometriosis in the United States: national estimates from a cross-sectional survey of 59,411 women. Gynecol. Obstet. Invest. 2017; 82(5): 453-61.https://dx.doi.org/10.1159/000452660.
- Pereira A., Herrero-Trujillano M., Vaquero G., Fuentes L., Gonzalez S., Mendiola A., Perez-Medina T. Clinical management of chronic pelvic pain in endometriosis unresponsive to conventional therapy. J. Pers. Med. 2022; 12(1):101. doi: 10.3390/jpm12010101.
- Agarwal S.K., Antunez-Flores O., Foster W.G., Hermes A., Golshan S., Soliman A.M. et al. Real-world characteristics of women with endometriosis-related pain entering a multidisciplinary endometriosis program. BMC Womens Health. 2021; 21(1): 19. https://dx.doi.org/10.1186/s12905-020-01139-7.
- Urteaga I., McKillop M., Elhadad N. Learning endometriosis phenotypes from patient-generated data. NPJ Digit. Med. 2020; 3: 88. doi: 10.1038/s41746-020-0292-9.
- Sinaii N., Plumb K., Cotton L., Lambert A., Kennedy S., Zondervan K., Stratton P. Differences in characteristics among 1,000 women with endometriosis based on extent of disease. Fertil. Steril. 2008; 89(3): 538-45. https://dx.doi.org/10.1016/j.fertnstert.2007.03.069.
- Nisenblat V., Bossuyt P.M., Farquhar C., Johnson N., Hull M.L. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst. Rev. 2016; 2(2): CD009591. https://dx.doi.org/10.1002/14651858.CD009591.
- Becker C.M., Bokor A., Heikinheimo O., Horne A., Jansen F., Kiesel L. et al.; ESHRE Endometriosis Guideline Group. ESHRE guideline: endometriosis. Hum. Reprod. Open. 2022; 2022(2): hoac009. doi: 10.1093/hropen/hoac009.
- Méndez Fernández R., Barrera Ortega J. Magnetic resonance imaging of pelvic endometriosis. Radiologia. 2017; 59(4): 286-96. https://dx.doi.org/10.1016/j.rx.2017.02.002.
- Khashchenko E.P., Uvarova E.V., Fatkhudinov T.K., Chuprynin V.D., Asaturova A.V., Kulabukhova E.A. et al. Endometriosis in adolescents: diagnostics, clinical and laparoscopic features. J. Clin. Med. 2023; 12(4): 1678. https://dx.doi.org/10.3390/jcm12041678.
- Maciel C., Ferreira H., Djokovic D., Kyaw Tun J., Keckstein J., Rizzo S., Manganaro L. MRI of endometriosis in correlation with the #Enzian classification: applicability and structured report. Insights Imaging. 2023; 14(1): 120. https://dx.doi.org/10.1186/s13244-023-01466-x.
- Manganaro L., Fierro F., Tomei A., Irimia D., Lodise P., Sergi M.E. et al. Feasibility of 3.0T pelvic MR imaging in the evaluation of endometriosis. Eur. J. Radiol. 2012; 81(6): 1381-7. https://dx.doi.org/10.1016/j.ejrad.2011.03.049.
- Thomeer M.G., Steensma A.B., van Santbrink E.J., Willemssen F.E., Wielopolski P.A., Hunink M.G. et al. Can magnetic resonance imaging at 3.0-Tesla reliably detect patients with endometriosis? Initial results. J. Obstet. Gynaecol. Res. 2014; 40(4): 1051-8. https://dx.doi.org/10.1111/jog.12290.
- Fruchart M., El Idrissi F., Lamer A., Belarbi K., Lemdani M., Zitouni D., Guinhouya B.C. Identification of early symptoms of endometriosis through the analysis of online social networks: A social media study. Digit. Health. 2023; 9: 20552076231176114. https://dx.doi.org/10.1177/20552076231176114.
- Chapron C., Lafay-Pillet M.C., Santulli P., Bourdon M., Maignien C., Gaudet-Chardonnet A. et al. A new validated screening method for endometriosis diagnosis based on patient questionnaires. EClinicalMedicine. 2022; 44: 101263. https://dx.doi.org/10.1016/j.eclinm.2021.101263.
- Chen C.X., Carpenter J.S., Gao X., Toh E., Dong Q., Nelson D.E. et al. Associations between dysmenorrhea symptom-based phenotypes and vaginal microbiome: a pilot study. Nurs. Res. 2021; 70(4): 248-55. https://dx.doi.org/10.1097/NNR.0000000000000510.
- Chapron C., Santulli P., de Ziegler D., Noel J.C., Anaf V., Streuli I. et al. Ovarian endometrioma: severe pelvic pain is associated with deeply infiltrating endometriosis. Hum. Reprod. 2012; 27(3): 702-11. https://dx.doi.org/10.1093/humrep/der462.
- Khan K.N., Kitajima M., Fujishita A., Hiraki K., Matsumoto A., Nakashima M., Masuzaki H. Pelvic pain in women with ovarian endometrioma is mostly associated with coexisting peritoneal lesions. Hum. Reprod. 2013; 28(1): 109-18. https://dx.doi.org/10.1093/humrep/des364.
- Perelló M., Martínez-Zamora M.A., Torres X., Munrós J., Llecha S., De Lazzari E. et al. Markers of deep infiltrating endometriosis in patients with ovarian endometrioma: a predictive model. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017; 209: 55-60. https://dx.doi.org/10.1016/j.ejogrb.2015.11.024.
- Nirgianakis K., Ma L., McKinnon B., Mueller M.D. Recurrence patterns after surgery in patients with different endometriosis subtypes: a long-term hospital-based cohort study. J. Clin. Med. 2020; 9(2): 496. https://dx.doi.org/10.3390/jcm9020496.
- Sibiude J., Santulli P., Marcellin L., Borghese B., Dousset B., Chapron C. Association of history of surgery for endometriosis with severity of deeply infiltrating endometriosis. Obstet. Gynecol. 2014; 124(4): 709-17. doi: 10.1097/AOG.0000000000000464.
- Shakiba K., Bena J.F., McGill K.M., Minger J., Falcone T. Surgical treatment of endometriosis: a 7-year follow-up on the requirement for further surgery. Obstet. Gynecol. 2008; 111(6): 1285-92. https://dx.doi.org/10.1097/AOG.0b013e3181758ec6.
- Bazot M., Bharwani N., Huchon C., Kinkel K., Cunha T.M., Guerra A. et al. European society of urogenital radiology (ESUR) guidelines: MR imaging of pelvic endometriosis. Eur. Radiol. 2017; 27(7): 2765-75.https://dx.doi.org/10.1007/s00330-016-4673-z.
- von Theobald P., Cottenet J., Iacobelli S., Quantin C. Epidemiology of endometriosis in France: a large, nation-wide study based on hospital discharge data. Biomed. Res. Int. 2016; 2016: 3260952. https://dx.doi.org/10.1155/2016/3260952.
- Blum S., Fasching P.A., Hildebrandt T., Lermann J., Heindl F., Born T. et al. Comprehensive characterization of endometriosis patients and disease patterns in a large clinical cohort. Arch. Gynecol. Obstet. 2022; 305(4): 977-84.https://dx.doi.org/10.1007/s00404-021-06200-w.
- Piriyev E., Schiermeier S., Römer T. Coexistence of endometriomas with extraovarian endometriosis and adhesions. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021; 263: 20-4. doi: 10.1016/j.ejogrb.2021.05.044.
- Пронина В.А., Думановская М.Р., Чернуха Г.Е. Оптимизация принципов ранней диагностики эндометриоза на основе оценки коморбидности и клинической манифестации. Акушерство и гинекология. 2023; 4: 87-96. [Pronina V.A., Dumanovskaya M.R., Chernukha G.E. Principles of early diagnosis of endometriosis based on the assessment of comorbidity and clinical manifestations. Obstetrics and Gynecology. 2023; (4): 87-96. (in Russian)]. https://dx.doi.org/10.18565/aig.2023.9.
- Nirgianakis K., Ma L., McKinnon B., Mueller M.D. Recurrence patterns after surgery in patients with different endometriosis subtypes: a long-term hospital-based cohort study. J. Clin. Med. 2020; 9(2): 496. https://dx.doi.org/10.3390/jcm9020496
- Xu B., Lin L., Pan Y., Chen P., Ye C., Zhao L. et al. The clinical picture and fecundity of primary and recurrent ovarian endometriosis with family history: a retrospective analysis. J. Clin. Med. 2023; 12(5): 1758. https://dx.doi.org/10.3390/jcm12051758.
- Heinemann K., Imthurn B., Marions L., Gerlinger C., Becker K., Moehner S., Faustmann T. Safety of dienogest and other hormonal treatments for endometriosis in real-world clinical practice (VIPOS): a large noninterventional study. Adv. Ther. 2020; 37(5): 2528-37. https://dx.doi.org/10.1007/s12325-020-01331-z.
Received 09.10.2023
Accepted 11.12.2023
About the Authors
Veronika A. Pronina, obstetrician-gynecologist, PhD student at the Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia, +7(916)025-86-26, ver22595@yandex.ru, https://orcid.org/0000-0003-4566-4065, 117997, Russia, Moscow, Ac. Oparin str., 4.Anastasia V. Sokolova, PhD, obstetrician-gynecologist at the Department of Gynecological Endocrinology, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia, +7(915)455-31-32, stasia0590@mail.ru, https://orcid.org/0000-0002-1197-3575,
117997, Russia, Moscow, Ac. Oparin str., 4.
Galina E. Chernukha, Dr. Med. Sci., Professor, Chief Researcher, obstetrician-gynecologist at the Department of Gynecological Endocrinology, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia, +7(985)999-60-00, c-galina1@yandex.ru,
https://orcid.org/0000-0002-9065-5689, 117997, Russia, Moscow, Ac. Oparin str., 4.
Corresponding author: Veronika A. Pronina, ver22595@yandex.ru



