Acid-base balance and gas composition of umbilical cord blood in relation to the use of epidural analgesia in vaginal delivery
Objective. To study the parameters of umbilical cord blood acid-base balance and gas composition in relation to the use of epidural analgesia (EA) in childbirth.Tysyachnyy O.V., Baev O.R., Evgrafova A.V., Prikhodko A.M., Pismensky S.V.
Subjects and methods. The investigation enrolled 380 women; a study group (n = 188) received no EA; a comparison group (n = 192) had EA. The parameters of umbilical cord blood acid-base balance and gas composition were determined using a gas analyzer.
Results. The pH values in the EA and non-EA groups were 7.28 (0.06) and 7.31 (0.07), respectively (p = 0.02). In these groups, Base Excess (BE) was 8.3 (2.3) and 6.7 (1.7), respectively (p = 0.004). The partial pressure of oxygen and carbon dioxide did not differ.
Conclusion. EA in childbirth does not have a clinically significant negative effect on the fetus and newborn, but is combined with a decrease in pH (p = 0.02) and an increase in the level of BE deficiency (p = 0.004)
Keywords
Labor is known to be the only physiological process that is accompanied by significant pain. According to the available literature, up to 25–30% of women describe their labor pain as severe, excessive [1]. On giving analgesia during delivery it is necessary to take into account not only the patient’s condition, but also the course of labor, presence of obstetric and extragenital pathology, and especially the condition of the fetus. Nowadays epidural anesthesia (EA) being a common method of pain relief during labor is the most effective method of anesthesia [2], and it is used in every third woman [3]. Special attention is paid to the assessment of the fetus since it is known that the use of EA can cause deterioration of fetal status [4]. Studies have shown that the onset of EA during labor is accompanied by transient changes in cardiotocography, indicating changes in the fetal heart rhythm [5]. The nature of these changes has not yet been fully elucidated. It is assumed that a temporary imbalance of catecholamines causes a change in uterine tone and, secondarily, in fetal heart rate. The significance of these changes for determining the labor management strategy depends on whether they are physiological in nature or indicate the suffering of the fetus.
Fetal assessment is usually conducted with cardiotocography, but this method suffers from a lack of specificity. In this regard, fetal hypoxia could be established more accurately by determining the acid-base status of the fetal blood immediately after birth. However, there are sparse data pertinent to the acid-base status of a newborn in case of intrapartum EA.
The objective of the investigation was to study changes in acid-base and gas parameters in samples of umbilical blood depending on the use of intrapartum EA.
Materials and Methods
This was a study of 380 healthy nulliparous and multiparous women from 2017 to 2018. They were divided into two groups: a study group (n = 188) who were not given EA and a control group (n = 192) with EA. Inclusion criteria were maternal age of 18-40 years, spontaneous singleton pregnancy, nulliparous and multiparous women, cephalic presentation, term labor, spontaneous onset of labor, informed consent.
The study was approved by the local ethics committee (National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov, Ministry of Health of Russia, Moscow).
Exclusion criteria were autoimmune pathology, pregnancy complication, operative vaginal delivery, cesarean delivery, acute fetal hypoxia, labor dystocia, the use of oxytocin augmentation during labor, congenital uterine abnormality, fetal abnormalities, inflammatory agents during the pregnancy.
Puncture of the epidural space was performed according to the standard technique at levels L2 – L3, L3 – L4 in the position of the woman on the left side. The epidural bolus was injected after the cervix was dilated at least 3 cm. The following doses were administered as needed, depending on the intensity of the pain and the obstetric situation. The solution of 0.2% ropivacaine hydrochloride (Naropin) at 10 ml was used for anesthesia.
Umbilical artery blood was used to determine the indicators of the umbilical blood gas and acid-base status. One minute later after delivery, the umbilical cord segment was clamped in three places. The first was done at a distance of 10 cm from the umbilical retraction, the second at 3 cm from the first one, the third at 15 cm from the second. The umbilical cord was cut between the first two clips. Blood sampling was performed from the umbilical artery between the second and third clamps. Blood was collected into the vacutainer (Monovette 1 ml LH, catalog number 05.1146.020, Germany) with an anticoagulant. This tube was filled with a solution of calcium-balanced heparin, which guaranteed fast and optimal mixing of blood and anticoagulant.
Studies of samples from the artery of the umbilical cord blood were performed within 5 minutes after sampling in the blood gas analyzer ABL 700 (Radiometer). This gas analyzer can determine the acid-base status of the blood, oxygen status, electrolytes, glucose, lactate, hematocrit, fetal and other derivatives, hemoglobin, bilirubin. Cord blood sampling was carried out with special syringes (spacers).
Newborn with cord blood pH <7.15 were excluded from the study, because, according to modern literature, the pH value below the specified value is a criterion for the diagnosis of “asphyxia in labor” [6–8].
Statistical analysis was performed using IBM SPSS software, version 22 for Windows. Shapiro-Wilk test was used to determine the normal distribution. Data presented as median / interquartile ranges (Q1 – Q3) (for abnormally distributed data - Mann–Whitney U test), mean ± standard deviation (for normally distributed data – T-test). Fisher test was used for qualitative data. The p-values of ≤0.05 were considered significant.
Results
There were no differences in mean age between groups without EA and with EA; it was 29.3 (4.2) and 28.9 (4.7) years, respectively, (р = 0.07).
No significant differences in mean pre-pregnancy body mass index between groups without EA and with EA were noted, 26.6 (5.8) kg/m2 versus 26.3 (3.6) kg/m2 (р = 0.9).
Analysis of rates for extragenital diseases (Table 1) showed that there were no significant differences in incidence of diseases (vision organs, ENT organs, gastrointestinal tract), but diseases of the cardiovascular system and urinary system occur more often in group without EA (р = 0.005 and р = 0.01, respectively).
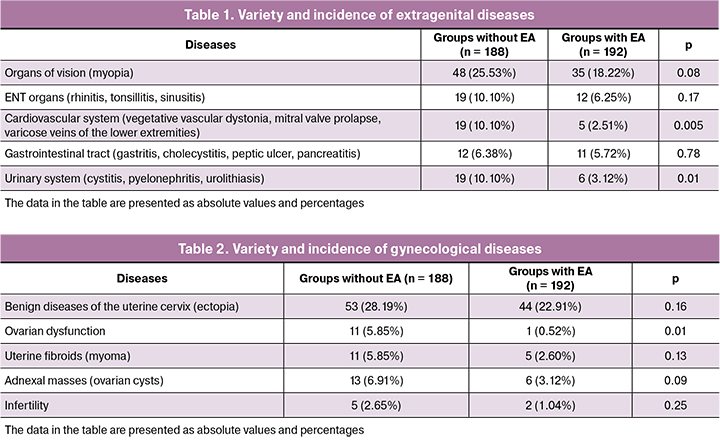
Gynecological history shows that there were no significant differences in incidence of diseases (Table 2), but ovarian dysfunction occurs more often in group without EA; 5.85% versus 0.52% (р = 0.01).
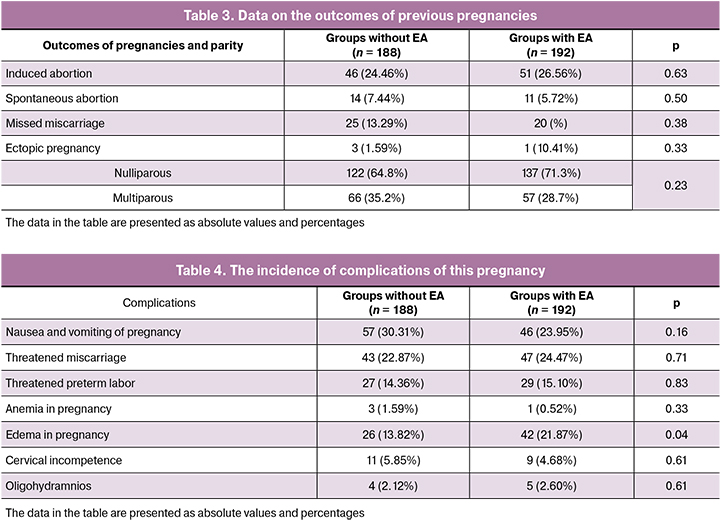
Obstetric history shows that there were no significant differences in incidence of induced abortion, spontaneous abortion, missed miscarriage and ectopic pregnancy, as well as in number of nulliparous and multiparous women (Table 3).
The analysis of the course of pregnancy did not reveal differences between the groups (Table 4), however, the edemas in pregnant women were significantly more common in the group with EA.
Gestational age did not differ between groups at the time of delivery and was as follows: 281.5 (6.8) days (40 weeks 1 day) in the study group versus 282.1 (6.5) days (40 weeks 2 days) in the control group, p < 0.53.
The analysis of labor duration showed that there was no difference in duration of the first stage of labor between the groups: 391.1 (158.3) min in the study group versus 415.9 (165.8) min in the control group (p = 0.21). The duration of the second stage of labor was 15 min shorter in the group without analgesia and lasted 49.7 (39.2) min versus 65.1 (37.8) min in the control group (p = 0.0001). The total duration of labor did not differ between groups, but there was a tendency to a shorter (36 min) duration of this stage of labor in the group without the use of analgesia, 442.1 (170.7) min versus 478.6 (169.1) min in the EA group (p = 0.06). Detailed analysis of the course of labor showed that the duration of the first stage did not differ between groups, and in the subgroup of nulliparous women in the group without EA, it lasted 458.1 (159.5) min versus 450.7 (156.5) min in the EA group (p = 0.91). In the subgroup of multiparous women, the duration was 306.8 (109.9) min versus 335.3 (160.5) min, respectively (p = 0.59). The second stage of labor in the group without analgesia in nulliparous women lasted 11 min shorter and amounted to 65.7 (40.5) min versus 77.3 (38.2) min ( p=0.02), and in multiparous women it was shorter by 7 min: 29.6 (26.5) min versus 36.5 (14.6) min (p=0.001). The total duration of labor did not differ between the groups, and in nulliparous groups in the group without analgesia, it amounted to 524.8 (163.2) min versus 524.7 (149.7) min in the EA group (p = 0.75). The labor lasted 337.6 (113.9) min versus 371.8 (164.7) min among multiparous women, respectively (p = 0.43).
It should be noted that in the EA group in 92.8% of cases, the epidural injection of anesthetic was administered once, it was necessary to give anesthetic for the second time in the same dose in 7.2% of cases.
Condition of all newborns was satisfactory with Apgar scores ranging from 7 to 10 points. Despite no differences in the numerical value of medians of the Apgar score, which in both groups were equal to 8 (8 - 8 points), statistical analysis revealed a tendency of the first-minute score to be higher in the group without EA (p = 0.02). At 5 minute, this parameter did not differ and amounted to 9 (9–9 points) versus 9 (9–9 points), respectively (p = 0.21).
Newborn anthropometric data analysis also revealed no differences between the groups. The average body weight among newborns in the study group was 3488.4 (377.8) grams versus 3484.8 (365.6) grams in the control group (p = 0.93), the average height was 52.1 (1.9) cm versus 51.7 (2.1) cm (p = 0.12), respectively.
 Acid-base and gas analysis of the umbilical blood revealed that its pH in the group of women who received EA was 7.28 (0.06), which is significantly lower compared to the group of women without EA - 7.31 (0.07), (p = 0.02) (Fig. 1). At the same time, the average base excess (BE) parameter with the use of EA was 8.3 (2.3), which is significantly higher compared to the group without analgesia - 6.7 (1.7) (p = 0.004) (Fig. 2). The partial pressure of oxygen and carbon dioxide did not differ between the two groups and was 23.8 (10.3) versus 24.1 (12.2), (p = 0.42) and 37.7 (8.1) versus 36.2 (9.7), (p = 0.63).
Acid-base and gas analysis of the umbilical blood revealed that its pH in the group of women who received EA was 7.28 (0.06), which is significantly lower compared to the group of women without EA - 7.31 (0.07), (p = 0.02) (Fig. 1). At the same time, the average base excess (BE) parameter with the use of EA was 8.3 (2.3), which is significantly higher compared to the group without analgesia - 6.7 (1.7) (p = 0.004) (Fig. 2). The partial pressure of oxygen and carbon dioxide did not differ between the two groups and was 23.8 (10.3) versus 24.1 (12.2), (p = 0.42) and 37.7 (8.1) versus 36.2 (9.7), (p = 0.63).
Discussion
Regional methods of labor analgesia, EA in particular, is widely used in modern obstetrical practice, and in some countries, it is performed in 77% of cases of vaginal delivery [9]. Despite the widespread application, the effects of intrapartum EA on the condition of the fetus remain understudied. In order to further elucidate them, we evaluated the acid-base status and gas composition of the umbilical arterial blood in cases of uncomplicated vaginal delivery with EA, since these indicators are more informative for the newborn status assessment than the Apgar score [7].
According to the literature, the mechanism of action of EA is associated with a decrease in catecholamine levels in mother’s blood, leading to vasodilation, which improves the placental blood flow and the acid-base status of both the mother and the fetus. Analgesia and improved oxygen status contribute to improved coordination of uterine contractions, but there is a possibility of their complete cessation due to deep analgesia [10]. Labor also causes massive catecholamine release in the fetus, especially during the second stage of labor, which helps maintain blood flow to its vital organs. Reynolds F. in his study showed that the fetus experiences less stress during labor with EA, which is confirmed by absent acidosis according to the results of the acid-base status analysis [11].
Our data showed that intrapartum EA does not have a clinically significant negative effect on the fetus and newborn, but is associated with pH decrease (p = 0.02) and base deficit (p = 0.004). Consequently, this type of analgesia directly or indirectly affects fetal metabolism. Hasegawa et al. (2013) provide similar data, also showing that administration of EA is associated with lower pH values (7.27 ± 0.10 versus 7.31 ± 0.09, p < 0.001) [12].
Medications administered to a woman in labor are known to enter fetal circulation via the placenta, but the risk of a direct toxic effect of local anesthetics on the fetus is low due to binding to the maternal plasma proteins. The revealed differences may be due to the effects of EA on the maternal organism. These effects can be facilitated by a change in uterine and placental blood supply, as evidenced by the increase in the uterine artery pulsatility index within 30 min after its administration [13].
According to Hasegawa J. et al. (2013), EA itself does not affect the condition of the newborn, but it is associated with an increase in the cesarean section rate and instrumental delivery, leading in turn to lower Apgar scores, deterioration of acid-base status and gas composition of umbilical blood [12]. Herrera-Gómez A. et al. (2018) presented similar data showing a fourfold increase in the frequency of operative vaginal delivery with the use of EA (OR = 4.085; 95% CI = 3.091, 5.401) [14]. The same team of authors (2017) showed an increase in cesarean sections when using this type of analgesia (OR = 2.673; p = 0.0001), including cesarean sections due to intrapartum fetal deterioration (OR = 1.739; p = 0.0012) [15].
Another possible cause of the differences in the studied parameters may be a longer second period of labor in the EA group. In our study, it was 65.1 (37.8) min versus 49.7 (39.2) min (p = 0.0001) in the group without EA, which correlates with the results of other studies [16, 17]. Meta-analysis has shown that the use of EA does not affect the duration of the first stage of labor, but it extends the duration of the second stage on average by 14 min [18].
In addition to the above factors, there are also available data on the association of the duration of the second stage of labor with the change in the acid-base status of the newborn. Thus, studies conducted in previous years showed that an increase in the duration of the second stage of labor significantly affects neonatal morbidity [19] and correlates with a decrease in umbilical blood pH [20]. However, there are data suggesting opposite trends. Suzuki S. et al. indicate that the second stage of labor lasting less than three hours does not affect lipid peroxidation in fetal blood [21]. It should be noted that this study has a number of drawbacks, the major among them being the small sample size (58 patients selected in three groups), which may affect the clinical significance of the obtained results.
As for the absent difference in the partial pressure of carbon dioxide - pCO2 and oxygen pO2 in the blood, our data correspond to the literature data, which show no effect of this method of analgesia on the mentioned parameters [22, 23].
Conclusion
Despite the fact that EA is the most effective method of pain relief during labor, it can affect the fetal condition and lead to decompensation when there is a prior decrease in compensatory capacity.
References
- Николаева О.А., Гречканев Г.О., Семенников М.В., Николаев И.И., Варшавер И.М., Симкина А.Ю. Акушерские аспекты обезболивания родов. Медицинский альманах. 2018; 3: 131-3. [Nikolaeva O.A., Grechkanev G.O., Semennikov M.V., Nikolaev I.I., Varshaver I.M., Simkina A.Yu. Obstetric aspects of labor pain relief. Medical Almanac. 2018; 3: 131-3. (in Russian)].
- Jepsen I., Keller K.D. The experience of giving birth with epidural analgesia. Women Birth. 2014; 27(2): 98-103. https://dx.doi.org/10.1016/j.wombi.2014.01.005.
- Petruschke I., Ramsauer B., Borde T., David M. Differences in the Frequency of Use of epidural analgesia between immigrant women of Turkish origin and non-immigrant women in Germany - explanatory approaches and conclusions of a qualitative study. Geburtshilfe Frauenheilkd. 2016; 76(9): 972-7.
- Herrera-Gómez A., García-Martínez O., Ramos-Torrecillas J., De Luna-Bertos E., Ruiz C., Ocaña-Peinado F.M. Retrospective study of the association between epidural analgesia during labour and complications for the newborn. Midwifery. 2015; 31(6): 613-6. https://dx.doi.org/10.1016/j.midw.2015.02.013.
- Korb D., Bonnin M., Michel J., Oury J.F., Sibony O. Analysis of fetal heart rate abnormalities occurring within one hour after laying of epidural analgesia. J. Gynecol. Obstet. Biol. Reprod. (Paris). 2013; 42(6): 564-9. https://dx.doi.org/10.1016/j.jgyn.2013.02.006.
- Garabedian C., De Jonckheere J., Butruille L., Deruelle P., Storme L., Houfflin-Debarge V. Understanding fetal physiology and second line monitoring during labor. J. Gynecol. Obstet. Hum. Reprod. 2017; 46(2): 113-7. https://dx.doi.org/10.1016/j.jogoh.2016.11.005.
- Приходько А.М., Баев О.Р. Определение кислотно-основного состояния пуповинной крови. Показания и техника. Акушерство и гинекология. 2018; 5: 127-31. [Prikhodko A.M., Baev О.R. Determination of the acid-base state of umbilical cord blood. Indications and equipment. Obstetrics and gynecology. 2018; 5: 127-31. (in Russian)].
- Приходько А.М., Романов А.Ю., Евграфова А.В., Шуклина Д.А. Определение уровня pH и лактата крови из предлежащей части плода для оценки его состояния в родах. Вопросы гинекологии, акушерства и перинатологии. 2017; 16(6): 96-9. [Prikhodko A.M., Romanov A.Yu., Evgrafova A.V., Shuklina D.A. Determination of pH and blood lactate from the presenting part of the fetus to assess its condition at birth. Issues of gynecology, obstetrics and perinatology. 2017; 16 (6): 96-9. (in Russian)].
- Burkle C.M., Olsen D.A., Sviggum H.P., Jacob A.K. Parturient recall of neuraxial analgesia risks: Impact of labor pain vs no labor pain. J. Clin. Anesth. 2017; 36: 158-63. https://dx.doi.org/10.1016/j.jclinane.2016.10.033.
- Reynolds F. Labour analgesia and the baby: good news is no news. Int. J. Obstet. Anesth. 2011; 20(1): 38-50. https://dx.doi.org/10.1016/j.ijoa.2010.08.004.
- Reynolds F. The effects of maternal labour analgesia on the fetus. Best Pract. Res. Clin. Obstet. Gynaecol. 2010; 24(3): 289-302. https://dx.doi.org/10.1016/j.bpobgyn.2009.11.003.
- Hasegawa J., Farina A., Turchi G., Hasegawa Y., Zanello M., Baroncini S. Effects of epidural analgesia on labor length, instrumental delivery, and neonatal short-term outcome. J. Anesth. 2013; 27(1): 43-7. https://dx.doi.org/10.1007/s00540-012-1480-9.
- Fratelli N., Prefumo F., Andrico S., Lorandi A., Recupero D., Tomasoni G., Frusca T. Effects of epidural analgesia on uterine artery Doppler in labour. Br. J. Anaesth. 2011; 106(2): 221-4. https://dx.doi.org/10.1093/bja/aeq317.
- Herrera-Gómez A., De Luna-Bertos E., Ramos-Torrecillas J., Ocaña-Peinado F.M., Ruiz C., García-Martínez O. Risk assessments of epidural analgesia during labor and delivery. Clin. Nurs. Res. 2018; 27(7): 841-52. https://dx.doi.org/10.1177/1054773817722689.
- Herrera-Gómez A., Luna-Bertos E., Ramos-Torrecillas J., Ocaña-Peinado F.M., García-Martínez O., Ruiz C. The effect of epidural analgesia alone and in association with other variables on the risk of cesarean section. Biol. Res. Nurs. 2017; 19(4): 393-8. https://dx.doi.org/10.1177/1099800417706023.
- Баев О.Р., Козлова О.А., Рубцова С.В., Румянцева В.П., Пырегов А.В. Влияние эпидуральной аналгезии на продолжительность родов, частоту слабости родовой деятельности и кесарева сечения. Акушерство и гинекология. 2014; 6: 41-6. [Baev O.R., Kozlova O.A., Rubtsova S.V., Rumyantseva V.P., Pyregov A.V. The effect of epidural analgesia on the duration of labor, the frequency of weakness of labor and cesarean section. Obstetrics and gynecology. 2014; 6: 41-6. (in Russian)].
- Shmueli A., Salman L., Orbach-Zinger S., Aviram A., Hiersch L., Chen R., Gabbay-Benziv R. The impact of epidural analgesia on the duration of the second stage of labor. Birth. 2018; 45(4): 377-84. https://dx.doi.org/10.1111/birt.12355.
- Anim-Somuah M., Smyth R.M., Jones L. Epidural versus non-epidural or no analgesia in labour. Cochrane Database Syst. Rev. 2011; (12): CD000331. https://dx.doi.org/10.1002/14651858.CD000331.pub3.
- Grantz K.L., Sundaram R., Ma L., Hinkle S., Berghella V., Hoffman M.K., Reddy U.M. Reassessing the duration of the second stage of labor in relation to maternal and neonatal morbidity. Obstet. Gynecol. 2018; 131(2): 345-53. https://dx.doi.org/10.1097/AOG.0000000000002431.
- Yoon B.H., Kim S.W. The effect of labor on the normal values of umbilical blood acid-base status. Acta Obstet. Gynecol. Scand. 1994; 73(7): 555-61.
- Suzuki S., Okudaira S. Influence of the duration of the second stage of labor on fetal pH levels and oxidative status in uncomplicated pregnancies. J. Matern. Fetal Neonatal Med. 2004; 15(2): 100-3.
- Caliskan E., Ozdamar D., Doger E., Cakiroglu Y., Kus A., Corakci A. Prospective case control comparison of fetal intrapartum oxygen saturations during epidural analgesia. Int. J. Obstet. Anesth. 2010; 19(1): 77-81. https://dx.doi.org/10.1016/j.ijoa.2009.04.012.
- Kutlesić M., Kutlesić R. Epidural analgesia in labor: specific characteristics, dilemmas and controversies. Med. Pregl. 2012; 65(9-10): 441-7.
Received 05.12.2018
Accepted 07.12.2018
About the Authors
Tysyachnyy, Oleg V., PhD, scientific researcher of the first maternity departments, National Medical Research Center for Obstetrics, Gynecology and Perinatology namedafter Academician V.I. Kulakov Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +74954381188. E-mail: olti23@mail.ru
Baev, Oleg R., MD, professor, head of the first Maternity Department, National Medical Research Center for Obstetrics, Gynecology and Perinatology named
after Academician V.I. Kulakov Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +74954381188. E-mail: o_baev@oparina4.ru
I.M. Sechenov First Moscow State Medical University of the Ministry of Health of the Russian Federation (Sechenov University),
Department of Obstetrics, Gynecology, Perinatology and Reproduction.
Evgrafova, Aleksandra V., second year postgraduate student of the first Maternity Department, National Medical Research Center for Obstetrics,
Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. Tel.: +7495438-11-88. E-mail: a_evgrafova@oparina4.ru
Prikhodko, Andrey M., PhD, physician of the first Maternity Department, National Medical Research Center for Obstetrics, Gynecology and Perinatology named
after Academician V.I. Kulakov of Ministry of Healthcare of Russian Federation.
117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +74954381188. E-mail: a_prikhodko@oparina4.ru
Pismensky, Sergey V., assistant of the Department of Anesthesiology and Resuscitation, National Medical Research Center for Obstetrics,
Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. Tel.: +74954381188. E-mail: s_pismensky@oparina4.ru
For citations: Tysyachnyy O.V., Baev O.R., Evgrafova A.V., Prikhodko A.M., Pismensky S.V. Umbilical cord blood acid-base balance and gas composition in relation to the use of epidural analgesia in vaginal delivery. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; (5): 70-6. (in Russian)
http://dx.doi.org/10.18565/aig.2019.5.70-76



