To the question of predictors for the development of preeclampsia in gestational diabetes
Objective. To investigate the relationship between the level of C-peptide, the severity of arterial stiffness and the presence of obesity in preeclampsia and gestational diabetes. Materials and methods. The study comprised 114 pregnant women at more than 24 weeks’ gestation, including patients with gestational diabetes and preeclampsia (n = 22), preeclampsia (n = 23), gestational diabetes (n = 36), and control subjects (n = 33). The patient evaluation included fasting serum level of C-peptide, augmentation index, and body mass index. The statistical analysis involved correlation coefficients, analysis of variance, and empirical ROC curve. Results. There was an independent association between C-peptide level and preeclampsia (p = 0.04) and obesity (p <0.001), but not concurrent gestational diabetes and preeclampsia. The augmentation index threshold was determined at -45% in relation to the presence of preeclampsia among patients with a C-peptide level above 3.1 ng/ml (sensitivity 0.92; specificity 0.59; OR = 17.14; 95%CI = [1.80; 163.8]). No statistically significant correlation was found between augmentation index and body mass index (r = -0.01; p = 0.9). Conclusion. The study established the values of C-peptide and augmentation index that may be suggested for further evaluation as possible predictors for preeclampsia and cardiovascular diseases after preeclampsia, regardless of the presence of gestational diabetes.Bettikher O.A., Zazerskaya I.E., Popova P.V., Vasiljeva E.Yu., Bart V.A.
Keywords
Over the last decades, there has been a dramatic increase in the incidence of gestational diabetes mellitus (GDM). According to numerous studies, including the HAPO multicenter study, women with GDM are more likely to develop preeclampsia compared to those without GDM (2.6% versus 1.2%) [1-3]. These complications are associated with high maternal and perinatal morbidity and mortality. At the same time, the causes of the high incidence of PE in patients with GDM remain unclear, and their common underlying pathogenesis is doubtful. The HAPO multi-center study involving thousands of women provided evidence of strong, independent association of hyperinsulinemia (measured by fasting C-peptide) with PE [4].
The C-peptide connects alpha and beta chains of proinsulin and is secreted into the portal bloodstream in equimolar concentrations with insulin. For many years, C-peptide was considered an inert side product of insulin synthesis and for a long time (until the 1990s) was used exclusively as a marker for the function of pancreatic β-cells. C-peptide has become the focus of considerable attention after the discovery of its diverse biological functions independent of insulin. Detection of its binding sites on the membranes (G-bound receptor) confirms the possibility of independent biological activity of the C-peptide. Recent experimental data suggest that high concentrations of C-peptide (more than 3.1 ng/ml) exert a pro-inflammatory atherogenic effect [5–10].
A history of both GDM and PE is associated with an increased risk of developing type 2 diabetes and cardiovascular diseases. Increased arterial stiffness in women with preeclampsia can be measured, including, by the oscillometric method. This method allows for the measurement of several parameters of the brachial artery elastic properties by analyzing the pulse wave. The literature on the impact of GDM on this indicator remains controversial. Currently, insufficient evidence is available to evaluate the contribution of concurrent GDM and PE during pregnancy to the change in the elastic properties of the peripheral arteries, and, therefore, to cardiovascular prognosis [11, 12]. Investigating the relationship between arterial stiffness and the level of C-peptide in concurrent GDM and PE during pregnancy may help advance understanding of PE pathogenesis in patients with GDM.
This study aimed to investigate the relationship between the level of C-peptide, the severity of arterial stiffness and obesity in patients with PE and GDM.
Material and methods
The study comprised 114 singleton pregnant women aged 20 to 40 years at more than 24 weeks’ gestation (37.1 ± 2.8 weeks). All patients signed informed consent to take part in the study, which was approved by the ethics committee of Almazov National Medical Research Centre of Minzdrav of Russia. The participants were divided in 4 groups, including women with GDM and PE (group 1; n = 22), only PE (group 2; n = 23), only GDM (group 3; n = 36), and control subjects (group 4; n = 33).
PE was defined as hypertension occurring after 20 weeks’ gestation with proteinuria of at least 0.3 g/l in a 24-hour urine collection; GDM was diagnosed based on the criteria of the 2013Russian national consensus. The observation, treatment, and delivery were carried out by the Protocols for the Management of Pregnant Women with GDM, PE of the Almazov National Medical Research Centre of Minzdrav of Russia. PE management included blood pressure control, magnesium sulfate, preservation of circulating blood volume, control of electrolyte and protein balance, normalization of the rheological and coagulation properties of blood. The severity of PE was assessed by PE severity scale as presented in the protocols of the Institute of Perinatology and Pediatrics of Almazov National Medical Research Centre of Minzdrav of Russia, based on the Russian Guidelines on Diagnosis and Management of Cardiovascular Diseases in Pregnancy (Table 1).
Patients in group 2 were statistically significantly (11/23) more likely to have severe PE compared with group 1 (1/22). The rate of early PE (> 34 weeks) did not differ statistically significantly (p = 0.82) between group 1 (59.09%, n = 13) and group 2 (65.57%, n = 16). In patients with GDM, glycemia was controlled by diet, and in some by insulin (18.1% of patients in group 1, 4/22; 58.3% of patients in group 3, 21/36). The patients received basal and basal-bolus insulin therapy.
Exclusion criteria in the study were as follows: a history of hypertension before pregnancy, moderate to severe anemia, history or current systemic connective tissue disorders, antiphospholipid syndrome, glomerulonephritis, adrenal gland diseases, heart diseases (including hemodynamically significant heart defects, myocarditis), history or current malignancies; decompensated hypo- or hyperthyroidism; history of organ transplantation; antiretroviral therapy; drug addiction; fetal chromosomal abnormalities; systemic corticosteroid therapy and long-term use of nonsteroidal anti-inflammatory drugs; other severe somatic diseases; pregnancy achieved by assisted reproductive technologies.
Body mass index (BMI) calculation and criteria for the diagnosis of obesity (BMI = weight (kg) /height (m2); BMI ≥ 30) were adapted from the WHO classification. The level of fasting serum C-peptide was measured by immunochemical assay (Elecsys Immunochemistry analyzer, Roche, Switzerland). The arterial stiffness of the brachial artery was estimated with the patient in the sitting position after a 5-minute rest using the oscillometric method (BPLab 24-hour blood pressure monitor with Vasotens software, Petr Telegin, 2011). Arterial stiffness was expressed in augmentation index (AI, reflected wave index), reduced to heart rate = 75 beats/min.
The association between factors under study was examined using correlation coefficients and one-way and three-way analysis of variance (Factorial ANOVA). The normality of the distribution was tested by the Kolmogorov-Smirnov and Lilliefors tests. The values of C-peptide and BMI were logarithmically transformed before analyses. When constructing a prognostic decision rule, an empirical ROC curve was used to isolate patients with PE in terms of C-peptide level and arterial stiffness. Sensitivity, odds ratios and other characteristics of this rule were evaluated based on the classification matrix. Statistical analysis was performed using STATISTICA 10.0 (StatSoft, Inc.).
Results
There were statistically significant differences between the groups regarding the levels of fasting serum C-peptide (p = 0.001). The highest mean level of log C-peptide was observed in group 1 (Table 2). Post-hoc comparisons (Scheffe test) showed statistically significant differences between groups 1 and 3 (p = 0.004), and groups 1 and 4 (p = 0.02). The absence of differences in log C-peptide between groups 3 and 4 was probably due to a compensated course of GDM at the time of the examination of pregnant women.
There were statistically significant differences between the groups in terms of BMI (p <0.001); obesity rates in group 1, 2, 3, and 4 were 72.7%, 43.5%, 33, 3%, and 12.1%, respectively. At the same time, grade III obesity was diagnosed in 16.67% in group 1 and 2.8% in group 3; grade II obesity was found in 11.11%, 36.4%, and 16.67% of women in group 1, 2, and 3, respectively. Grade I obesity was observed in 72.22% and 63.6% of women in groups 1 and 2, respectively, and in 27.78% and 12.1% of women in group 3 and control group (p < 0.0001).
Associations between the variables under study were determined using a three-way analysis of variance. A direct positive and independent association was identified between the BMI and the development of GDM, BMI, and PE. No statistically significant association was found between BMI and concurrent GDM and PE (p = 0.45). PE (p = 0.04) and obesity (p <0.001) had a direct and independent association with the level of C-peptide; on the contrary, GDM did not affect the relationship between PE and the C-peptide level. There were statistically significant differences between the groups regarding arterial stiffness measured by AI (p = 0.002); the highest mean AI was found in group 2 (Table 2). At the same time, differences in AI between group 2 and 3 (p = 0.003), and group 2 and 4 (p = 0.0095) were significant in post-hoc comparisons (Sheffe test).
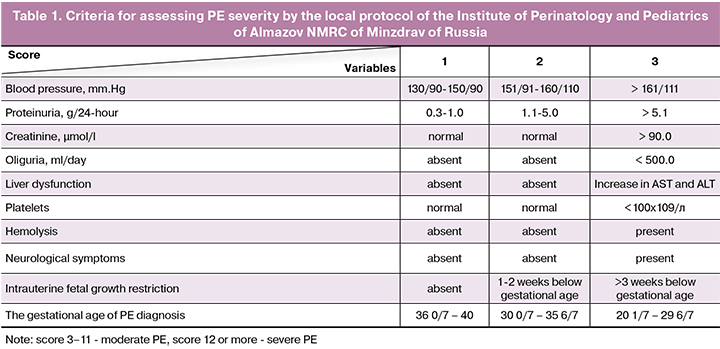
The analysis of variance showed a positive association between AI level and PE (p <0.001), and PE and high level (> 3.1 ng/ml) of C-peptide (p = 0.006), and a negative relationship with a combination of GDM and PE (p = 0.004) (Table 3). Arterial stiffness was lower in patients with concurrent GDM, PE and high C-peptide than without GDM, which is presumably due to the difference in the severity of PE between groups and, possibly, pathogenesis, which requires further study. At the same time, AI was not statistically significantly correlated with BMI (r = -0.01; p = 0.9).
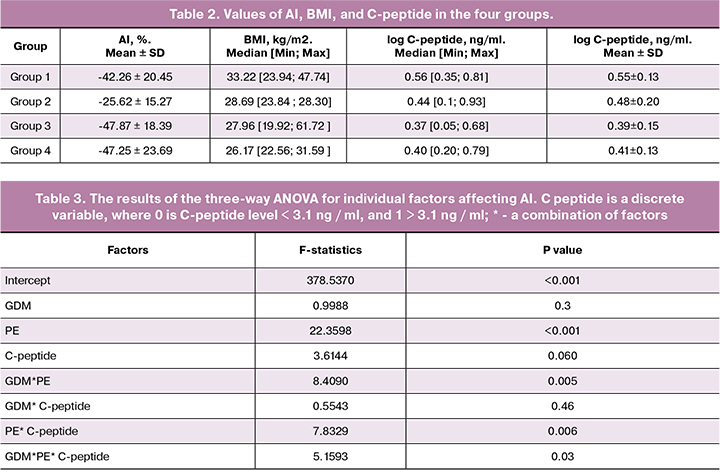
The three-way analysis of variance (Table 3) identified no statistically significant effect of C-peptide on AI, but it was statistically significant with PE. This “behavior” of the C-peptide may be associated with the different relationship between PE and AI index in patients, whose C-peptide level was above or below the 3.1 ng/ml threshold. This effect is well illustrated by the diagrams in Figures 1–3. Figure 1 shows the confidence intervals for the mean AI value in patients with and without PE. If the C - peptide level was above the 3.1 ng/ml threshold (0.49 is log C-peptide level), a large, statistically significant difference appeared between the groups, while with lower C-peptide levels, the difference was nonsignificant (Fig. 1, 2). In Figure 3, the gap between the two modes on the C-peptide level histogram (top) corresponding to the 3.1 ng/ml threshold is visible. Also noticeable is the difference in the scattering pattern to the left and right of the dashed line. Higher scattering density on the right side also demonstrates significant differences in patients with different levels of C-peptide.
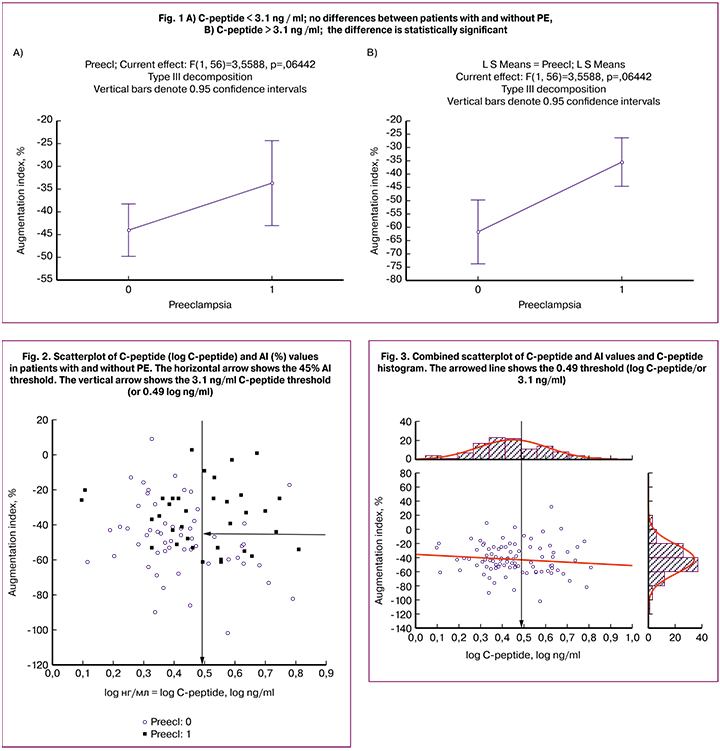
As part of this study, the significance of AI and high C-peptide was evaluated together concerning PE. In an isolated group of patients with a C-peptide level above 3.1 ng/ml, the AI threshold was determined = -45% in relation to the presence of PE based on an empirical ROC curve. For the obtained decision rule, the sensitivity (Sen) = 0.92, the specificity (Spe) = 0.59, the odds ratio (OR) = 17.14, the confidence interval for the odds ratio (95%CI) = [1.80; 163.8]. Using the threshold values of C-peptide and AI helps identify patients with PE with high accuracy, and given the literature data on the prognostic value of the studied markers, may help select a high-risk group before symptoms manifest. Further studies are needed to identify the relationship between PE and C-peptide level in patients with C-peptide levels below 3.1 ng/ml [2, 4].
Discussion
Our findings continue the discussion about the common origin of GDM and PE, and the contribution of obesity to this relationship. Risk factors for developing both GDM and PE are obesity and insulin resistance. The literature is lacking consensus on the relationship between GDM and PE. According to the results of Schneider S. et al. (2012), Hauser M. et al. (2011), GDM itself is an independent risk factor for PE, and the degree of obesity does not affect this relationship. Ostlund I. et al. (2004) concluded that obesity is the main interfering factor in these relationships, which, nevertheless, cannot fully explain the risk of developing PE in patients with GDM [13–15]. Our findings suggest that obesity is a risk factor for both GDM and PE, but is not associated with the co-occurrence of both complications.
According to modern studies (Framingham heart study, 2010), vascular stiffness is one of the factors determining the prognosis for cardiovascular morbidity [11]. Our data on the relationship of AI with PE and the combination of GDM and PE complement the available research evidence. Some studies have found that AI is a predictor of PE and can serve as a clinical marker for the diagnosis of the disease. Also, according to some data, pathological changes in AI in patients with PE may continue in the postpartum period, which suggests its role as a predictor of long-term cardiovascular morbidity. However, the current literature is lacking sufficient coverage of the evidence presented to date regarding the characteristics of arterial stiffness in patients with GDM and concurrent GDM and PE. Phan K et al. (2015) reported a study with a very small sample that the combination of GDM and PE was associated with increased arterial stiffness compared with these complications taken separately, which contradicts our data [11, 12, 16, 17].
There are considerable differences in the classical understanding of the relationship between insulin resistance and hyperinsulinemia, on the one hand, and vascular stiffness, blood pressure, and the outcome of primary non-pregnancy hypertension on the other. According to some authors, the role of insulin in the early development of hypertension is insignificant, and the effects of insulin on blood pressure are minimal; however, this has been refuted by a large meta-analysis: hyperinsulinemia and insulin resistance are associated with an increased risk of hypertension in the general population. Other authors note that insulin levels and insulin resistance are associated with carotid stiffness in middle-aged non-diabetic patients with hypertension. Also, an experimental study has shown that prolonged administration of insulin into the renal artery of rats leads to a persistent increase in blood pressure. According to the latest research, there are beneficial effects of metformin therapy at 32 to 34 weeks of gestation on arterial stiffness in patients with GDM, but the authors note the need to further study the issue. Nevertheless, the authors are unanimous in the opinion that the mechanism of the relationship between hyperinsulinemia and hypertension is not clear enough and warrants further studies [18–25]. Probably, the newly discovered biological properties of C-peptide could serve as a “key” for understanding the link.
In our study, levels of C-peptide, AI, and BMI significantly differed between groups, which confirm the validity of participant allocation into study groups, as well as the differences in the pathogeneses of the pregnancy complications under study. Our findings suggest that obesity and high levels of C-peptide are independent risk factors for the development of PE, which is consistent with some literature data. Even though obesity is closely related to the development of GDM, although it cannot explain all the risks of its development, BMI is not associated with concurrent GDM and PE.
The study findings confirm the role of AI as a recognized marker of preeclampsia, reflecting the elastic properties of blood vessels. Given that, according to the extensive Framingham Heart Study, arterial stiffness is recognized as a risk factor for cardiovascular events, we consider it possible to suggest this indicator in combination with the C-peptide as a predictor of cardiovascular morbidity after PE. The study results showed for the first time the high value of combined use of C-peptide and AI as a marker for PE. It should be noted that AI level is significantly lower in concurrent GDM and PE than in isolated PE, which probably reflects the difference in the pathogenesis of PE under these conditions, as well as the degree of damage to the vascular elastic properties. And what is most significant is the absence of association between AI and obesity, which makes it an independent and universal predictor suitable for further study.
Conclusion
The present study demonstrated an independent association of C-peptide level with obesity and PE. The increase in the C-peptide level can occur in two independent ways: one of them is associated with obesity, while the other is likely to be linked to insulin resistance without obesity. Despite the identified associations between obesity and GDM, obesity and PE, a high level of C-peptide is not associated with the combination of these pregnancy complications. Our findings do not confirm the assumption that there is a single trigger for the development of concurrent PE and GDM.
The results of the study also indirectly indicate the difference in the pathogenesis of isolated PE and concurrent PE and GDM. The combination of these pregnancy complications does not lead to a decrease in arterial elasticity, compared with isolated GDM or PE.
The strong correlation between PE, the C-peptide level of more than 3.1 ng/ml and AI above -45% suggest that these indicators may serve not only as PE predictors but also as possible markers of long-term cardiovascular morbidity after PE. These findings lend support for further studies investigating the pathogenic pathway of the damaging effects of hyperinsulinemia.
References
- Feig D.S., Hwee J., Shah B.R., Booth G.L., Bierman A.S., Lipscombe L.L. Trends in incidence of diabetes in pregnancy and serious perinatal outcomes: a large, population-based study in Ontario, Canada, 1996-2010. Diabetes Care. 2014; 37(6): 1590-6. https://dx.doi.org/10.2337/dc13-2717.
- Lowe L.P., Metzger B.E., Dyer A.R., Coustan D.R., Hadden D.R., Moshe Hod. et al. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: an overview. In: Kim C., Ferrara A., eds. Gestational diabetes during and after pregnancy. London: Springer; 2010: 17-34.
- Nerenberg K.A., Johnson J.A., Leung B., Savu A., Ryan E.A., Chik C.L., Kaul P. Risks of gestational diabetes and preeclampsia over the last decade in a cohort of Alberta women. J. Obstet. Gynaecol. Can. 2013; 35(11): 986-94. https://dx.doi.org/10.1016/S1701-2163(15)30786-6.
- The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study Cooperative Research Group. Hyperglycemia and Adverse Pregnancy
- Outcome (HAPO) study: preeclampsia. Am. J. Obstet. Gynecol. 2010; 202: 255. e1-7. https://dx.doi.org/10.1016/j.ajog.2010.01.024.
- Walcher D., Marx N. C-peptide in the vessel wall. Rev. Diabet. Stud. 2009 Fall; 6(3): 180-6. https://dx.doi.org/10.1900/RDS.2009.6.180.
- Vasic D., Spyrantis A., Durst R., Bach H., Vogt S., Rottbauer W., Walcher D.
- C-peptide induces human renal mesangial cell proliferation in vitro, activating Src-kinase, PI-3 kinase and ERK1/2. Mol. Cell. Endocrinol. 2012; 351(2): 337-41. https://dx.doi.org/10.1016/j.mce.2012.01.011.
- Wahren J. C-peptide and the pathophysiology of microvascular complications of diabetes. J. Intern. Med. 2017; 281(1): 3-6. https://dx.doi.org/10.1111/joim.12541.
- Vasic D., Marx N., Sukhova G., Bach H., Durst R., Grüb M. et al. C-peptide promotes lesion development in a mouse model of arteriosclerosis. J. Cell. Mol. Med. 2012; 16(4): 927-35. https://dx.doi.org/10.1111/j.1582-4934.2011.01365.x.
- Vasic D., Walcher D. C-peptide: a new mediator of atherosclerosis in diabetes. Mediators Inflamm. 2012; 2012: 858692. https://dx.doi.org/10.1155/2012/858692.
- Vasic D., Walcher D. Proinflammatory effects of C-peptide in different tissues. Int. J. Inflam. 2012; 2012: 932725. https://dx.doi.org/10.1155/2012/932725.
- Ahmed R., Dunford J., Mehran R., Robson S., Kunadian V. Preeclampsia and future cardiovascular risk among women: a review. J. Am. Coll. Cardiol. 2014; 63(18):1815-22. https://dx.doi.org/10.1016/j.jacc.2014.02.529.
- Phan K., Gomez Y., Gorgui J., Elbaz L., El-Messidi A., Gagnon R., Daskalopoulou S.S. Arterial stiffness and hemodynamics in women with hypertensive disorders of pregnancy and gestational diabetes: a prospective longitudinal study. Can. J. Cardiol. 2015; 31(10, Suppl.): S58.
- Schneider S., Freerksen N., Röhrig S., Hoeft B., Maul H. Gestational diabetes and preeclampsia - similar risk factor profiles? Early Hum. Dev. 2012; 88(3): 179-84. https://dx.doi.org/10.1016/j.earlhumdev.2011.08.004.
- Houser M., Tuuli M., Macones G., Odibo A. 272: Is the association between gestational diabetes and preeclampsia modified by obesity? Am. J. Obstet. Gynecol. 2011; 204(1): S115. https://dx.doi.org/10.1016/j.ajog.2010.10.290.
- Ostlund I., Haglund B., Hanson U. Gestational diabetes and preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004; 113(1): 12-6. https://dx.doi.org/10.1016/j.ejogrb.2003.07.001.
- Mehra S., Brennan-Prescod S.F., Gavard J. A., Goldkamp J., Ashraf M. 333: Central aortic pressure and arterial stiffness as early predictors of gestational hypertension and preeclampsia. Am. J. Obstet. Gynecol. 2016; (1, Suppl.): S187-8.
- Рябоконь Н.Р., Зазерская И.Е., Большакова О.О. Особенности жесткости сосудов при преэклампсии и после родов. Журнал акушерства и женских болезней. 2016; 65(5): 49-55. [Riabokon N.R., Zazerskaya I.E., Bolshakova O.O. Features of vascular stiffness in pregnancy complicated by preeclampsia and postpartum. Journal of Obstetrics and Women’s Diseases. 2016;65(5):49-55. (In Russ.)]. https://dx.doi.org/10.17816/J0WD65549-55.
- Seven E. Overweight, hypertension and cardiovascular disease: focus on adipocytokines, insulin, weight changes and natriuretic peptides. Dan. Med. J. 2015; 62(11): B5163.
- Wang F., Han L., Hu D. Fasting insulin, insulin resistance and risk of hypertension in the general population: A meta-analysis. Clin. Chim. Acta. 2017; 464: 57-63. https://dx.doi.org/10.1016/j.cca.2016.11.009.
- Salzer L., Tenenbaum-Gavish K., Hod M. Metabolic disorder of pregnancy (understanding pathophysiology of diabetes and preeclampsia). Best Pract. Res. Clin. Obstet. Gynaecol. 2015; 29(3): 328-38.
- Catena C., Colussi G., Frangipane A., Russo A., Verheyen N.D., Sechi L.A. Carotid artery stiffness is related to hyperinsulinemia and insulin-resistance in middle-aged, non-diabetic hypertensive patients. Nutr. Metab. Cardiovasc. Dis. 2015; 25(10): 968-74. https://dx.doi.org/10.1016/j.numecd.2015.06.009.
- Irsik D.L., Chen J.K., Brands M.W. Chronic renal artery insulin infusion increases mean arterial pressure in male Sprague-Dawley rats. Am. J. Physiol. Renal Physiol. 2018; 314 (1): F81-8. https://dx.doi.org/10.1152/ajprenal.00374.2017.
- Osman M.W., Nath M., Khalil A., Webb D.R., Robinson T.G., Mousa H.A. The effects of metformin on maternal haemodynamics in gestational diabetes mellitus: A pilot study. Diabetes Res. Clin. Pract. 2018; 139: 170-8.
- Симоненко В.Б., Горюцкий В.Н., Дулин П.А. Роль инсулинорезистентности в патогенезе артериальной гипертонии. Клиническая медицина. 2014; 92(9): 27-33. [Simonenko VB, Goriutskii VN, Dulin PA. The role of insulin resistance in pathogenesis of arterial hypertension]. Klin Med (Mosk). 2014;92(9):27-33. Review. (in Russian)].
- Джиджихия К.М., Каде А.Х., Занин С.А., Джиджихия З.М., Соловьева М.Р., Джикия Т.Г., Согомонян К.А. Роль гиперинсулинемии в развитии артериальной гипертензии при метаболическом синдроме 2. Международный журнал прикладных и фундаментальных исследований. 2013; 5: 102. [Dzhidzhikhiya K.M., Kade A.H., Zanin S.A., Dzhidzhikhiya Z.M., Solovjeva M.R., Dzhikiya T.G., et al. International journal of applied and fundamental research. 2013;5:102 (in Russian)]
Received 11.07.2018
Accepted 21.09.2018
About the Authors
Bettikher, Ofelia A., PhD student at the Department of Obstetrics and Gynecology,Almazov National Medical Research Centre of Minzdrav of Russia.
197341, Russia, Saint Petersburg, Akkuratova str. 2b. Tel.: +79516640248. E-mail: ophelia.bettikher@gmail.com ORCiD ID: 0000-0002-1161-1558
Zazerskaya, Irina E., MD, head of the Department of Obstetrics and Gynecology, Almazov National Medical Research Centre of Minzdrav of Russia; head of the Research Laboratory of Women’s Health and Reproduction. 197341, Russia, Saint Petersburg, Akkuratova str. 2b. Tel.: +79219488340. E-mal: zazera@almazovcentre.com
Popova, Polina V., PhD, Head of the Scientific Laboratory of Endocrine Diseases in Pregnancy; Almazov National Medical Research Centre of Minzdrav of Russia.
197341, Russia, Saint Petersburg, Akkuratova str. 2. Tel.: +79217424404. E-mail: pvpopova@yandex.ru
Vasiljeva, Elena Yu., head of the Central Laboratory, Almazov National Medical Research Centre of Minzdrav of Russia.
197341, Russia, Saint Petersburg, Akkuratova str. 2. E-mail: elena-almazlab@yandex.ru. ORCiD ID 0000-0002-2115-8873
Bart Victor A., PhD, head of the Scientific Laboratory of Biostatistics,
Almazov National Medical Research Centre of Minzdrav of Russia.
197341, Russia, Saint Petersburg, Akkuratova str. 2. Tel.: +79215831658. E-mail: vbartvit@mail.ru
For citation: Bettikher O.A., Zazerskaya I.E., Popova P.V., Vasiljeva E.Yu., Bart V.A. To the question of predictors for the development of preeclampsia in gestational diabetes. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; (5): 50-6. (in Russian)
https://dx.doi.org/10.18565/aig.2019.5.50-56



