Immunohistochemical markers of uterine myoma recurrence
Objective: To identify immunohistochemical markers of uterine myoma recurrence.Tonoyan N.M., Kozachenko I.F., Asaturova A.V., Kometova V.V., Magnaeva A.S., Tevryukova N.S., Frankevich V.E., Adamyan L.V.
Materials and methods: The study involved 13 patients (Group 1) with newly diagnosed uterine myomas (UM) and 18 patients (Group 2) with recurrent uterine myomas (RUM). All participants underwent laparoscopic reconstructive surgery at the Department of Operative Gynecology of the V.I. Kulakov NMRC for OG&P. Myomatous tissue and nodules of patients who underwent organ-sparing surgery were submitted for pathomorphological and immunohistochemical examination. Analysis included evaluation of the expression of a proliferation marker (Ki-67), vascular endothelial growth factor (VEGF), progesterone (PgR), and estrogen (ER) receptors, p16 proto-oncogene, and p53 anti-oncogene.
Results: VEGF expression was higher in tumor tissue compared to myometrial samples in patients in both groups. The level of Ki-67 in myomatous nodules in Group 2 patients was higher than in Group 1 (p=0.031), which may reflect the proliferative potential of the tumor most susceptible to recurrence. Higher levels of ER and PgR in the myomatous tissue of patients in Group 2 than in Group 1 probably reflect the potential for tumor growth. The expression level of p16 was higher in the myomatous nodules in patients in Group 2 (p=0.027). Apparently, changes in p53 protein expression are not the leading factor in the pathogenesis of uterine myoma, as no statistically significant differences were found in the expression of this protein between the patients of the study groups. Proteins and growth factors identified in tissue samples may be considered potential markers of the disease and its recurrence.
Conclusion: High levels of Ki-67, VEGF, p16, ER, and PgR in uterine myoma nodules are pathogenetic factors of myoma recurrence. Morphological evaluation of myometrial tissues and myomatous nodules in patients undergoing surgery for uterine myoma for the first time and for recurrent myoma allows identification of molecular and biological mechanisms of uterine myoma development and recurrence.
Keywords
Uterine myoma (UM) is the most common benign tumor of uterine smooth muscle cells [1, 2]. To provide the opportunity to women who wish to preserve surgeons, surgeons must expand the indications for fertility-sparing surgery, improve and rationalize existing methods of surgical treatment.
The rate of recurrence after organ-sparing surgery is rather high, and repeated surgical interventions are associated with high risk and complexity due to adhesions, tissue infiltration, reactive changes in the myometrium, uterine anatomical structure [3].
UM undergo autonomous growth due to the influence of growth factors, inhibitors and activators of apoptosis, proliferation, and intensification of neoangiogenesis [4]. The growth and appearance of new fibroid nodules depend on the relationship between proliferation and apoptosis (decrease in the rate of cell death by apoptosis, increase in proliferation) [5, 6].
The WHO classification of non-epithelial tumors of the uterine body (2014) distinguishes cellular, mitotically active, epithelioid, myxoid, atypical, lipoleiomyoma, etc. histological variants of UM [7]. According to various authors, mitotically active and cellular myomas are the most common (70%) among recurrent myomas [5, 8].
The present study aimed to identify immunohistochemical markers of uterine myoma recurrence.
Materials and methods
The study involved 13 patients (Group 1, mean age 37.9±5.5 years) with newly diagnosed uterine myomas (UM) and 18 patients (Group 2, mean age 39.9±5.2 years) with recurrent uterine myomas (RUM). All patients were treated in the Department of Operative Gynecology of the V.I. Kulakov NMRC for OG&P.
Morphological examination of surgical specimens (myometrium and fibroid tissues) was performed in the 1st Department of Anatomic Pathology of the V.I. Kulakov NMRC for OG&P.
Histological and IHC preparations were prepared according to the standard technique. The material was fixed in formol-ethanol and buffered (phosphate) 10% neutral formalin. Subsequently, the preparations were processed using a histological tissue processing machine and embedded in paraffin. The total duration of fixation, guiding and embedding the material in paraffin did not exceed one day. For the morphological study, at least 10 staged sections were obtained from each block. The 5 µm thick sections were stained with hematoxylin and eosin according to conventional methods [9].
IHC study was performed on the sections obtained after microtomy and subjected to further staining. IHC reactions were performed on 4 µm thick paraffin sections on L-polyzine-coated slides. The demasking of antigens for IHC was performed on a retriever using citrate buffer (pH 6.0) and at 600 W. Monoclonal and polyclonal antibodies to the proliferation marker (Ki-67), progesterone receptor (PgR), estrogen (ER), proto-oncogene (p16), tumor growth suppressor (p53) (clone Ventana), vascular endothelial growth factor (VEGF) (abcam 1:100) were used in the study [10].
The expression of Ki-67, VEGF, p16, p53 was assessed as a percentage of positively stained cells to the total number of smooth muscle cells. Expression PgR, and ER were assessed using Histo-score, which was calculated using the formula: HS=1a+1b+3c; where a is the percentage of weakly stained cells, b is the percentage of moderately stained cells, c is the percentage of strongly stained cells; 1, 2, 3 are the staining intensity, expressed in points. The results were evaluated on the following scale: 0–10 points, no expression, 11–100 points – weak expression, 101–200 points – moderate expression, 201–300 points – strong expression. The result of p16, p53 was evaluated as a percentage (moderate and pronounced staining intensity was taken into account).
Statistical analysis
Statistical analysis was performed using StatTech v. 2.6.3 (Stattech Ltd., Russia) and Microsoft Excel spreadsheets. The normality of the distribution was tested by the Shapiro–Wilk test. Quantitative variables that showed normal distribution were expressed as means (M) and standard deviation (SD), 95% confidence interval (95% CI). Variables not meeting normality assumptions were reported as the median (Me) and interquartile range (Q1; Q3). Normally distributed continuous variables were compared between two groups with a Student’s t test. Variables that did not show a Gaussian distribution were compared using the Mann–Whitney U test. Differences were considered statistically significant at p<0.05.
Results and discussion
UM was diagnosed by bimanual pelvic examination and ultrasound, confirmed by laparoscopy, and finally verified by morphologic examination. Among histologically confirmed uterine leiomyomas, simple uterine leiomyomas (n=11, 85%) and cellular leiomyomas (n=2, 15%) were verified. Among recurrent uterine leiomyomas, there were simple uterine corpus leiomyomas (n=12, 67%), cellular uterine leiomyomas (n=5, 27%), and mitotically active leiomyoma (n=1, 10%).
All women underwent laparoscopy and myomectomy. The indications for surgical treatment were heavy menstrual bleeding leading to anemia, pronounced pain syndrome, failed earlier conservative therapy, and infertility.
An analysis of patients in Group 2 revealed that a repeated myomectomy was necessary after an average of 5.6 (4.4) years.
The follow-up assessment was performed at 12, 18 and 24 months after the operation. The recurrence rate at 12 months was 7.9% and 15.7% in the primary and recurrent UM groups and 15.8 and 31.2% at 24 months, respectively.
The pathogenesis of UM remains unknown, and the causes of the tumor as well as its recurrence are still the subject of debate [3]. Currently, there are no precise criteria to assess the probability of UM recurrence, which makes it difficult to choose an individualized rehabilitation program for patients.
In this study, we performed pathomorphological and IHC analysis of myometrium and fibroid nodules with markers Ki-67, VEGF, PgR, ER, p16, p53, which are interrelated components of the signaling pathway (classical nuclear estrogen receptor pathway). The results are shown in Table 1 and Figures 1–3.
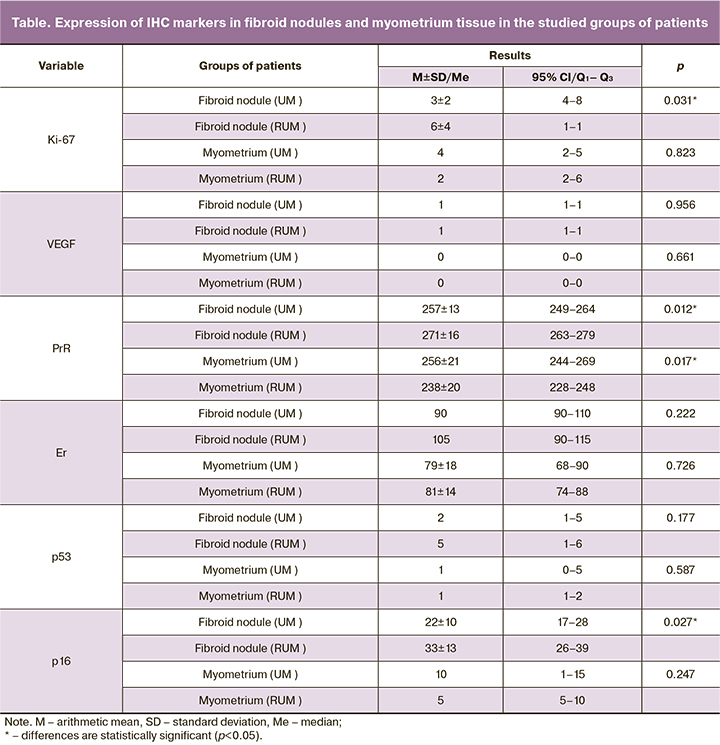
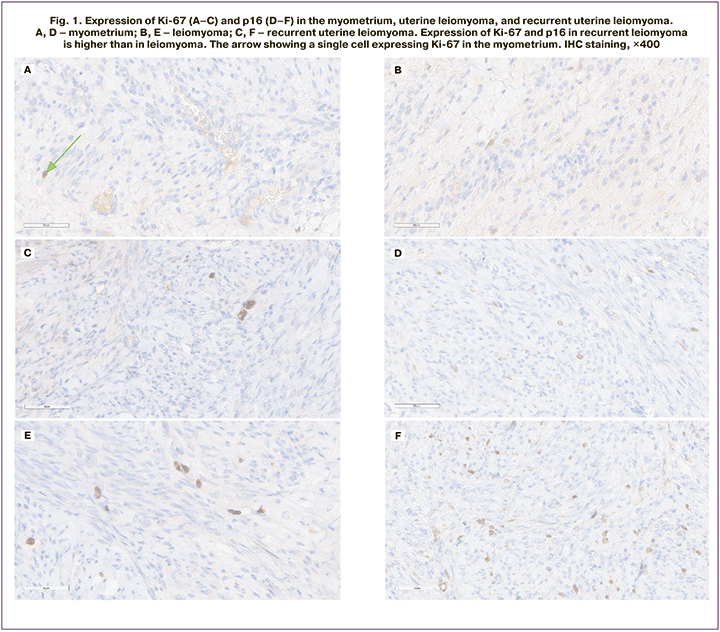
Several studies have investigated some of these markers and reported their value in predicting tumor recurrence (Ki-67, VEGF, and PgR) [5, 12]. Regarding Ki-67 and PgR the results obtained in these works were similar to our findings. However, we could not confirm the value of VEGF as a prognostic marker, as well as with regard to the marker p53, which was investigated in this study (Fig. 3).
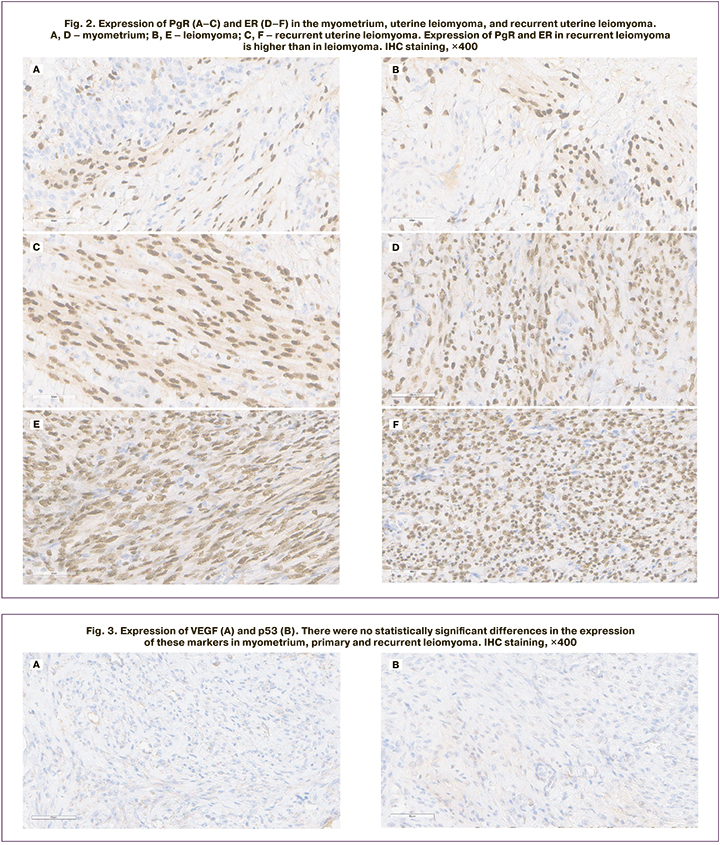
Imbalance between apoptosis and proliferation is the main mechanism regulating the rate and specific features of the disease recurrence [11].
According to the literature, the Ki-67 protein reflects the number of cells in the active phase of the cell cycle and is involved in proliferative cell activity [11, 12].
As a result, we found statistically significant differences (p=0.031) between the levels of Ki-67 expression in primary diagnosed and recurrent leiomyomas. The mean level of proliferative activity in fibroid nodules in Group 1 patients was 3±2%, in Group 2 – 6±4%, while in myometrial tissue in Group 1 and 2 patients no statistically significant differences were found (p=0.823). Detected increased levels of Ki-67 in myoma samples of patients in Group 2 compared to Group 1 may reflect the proliferative potential of the tumor that is most prone to recurrence. Ki-67 may be an indirect marker of disease recurrence.
Xing-Hua Liao et al. found a high expression of Ki-67 in UM compared to myometrial smooth muscle cells [13]. According to Sidorova I.S., Ki-67 content increased in the series "normal myometrium -> simple myoma -> proliferating myoma -> leiomyosarcoma", reaching an expression level many times higher in malignant tumor than in normal myometrium [14]. A study by M. Nisolle revealed an insignificant increase in Ki-67 expression in fibroid nodules compared to myometrial tissue [15].
VEGF, which influences the development of new blood vessels (angiogenesis), plays an important role in tumor angiogenesis. When assessing the level of VEGF expression in leiomyoma and myometrial tissue samples in the study groups, we failed to establish statistically significant differences (p=0.956 and p=0.661, respectively). VEGF levels were higher in fibroid nodules than in myometrial samples in both Group 1 and Group 2 patients. Similar data were reported by Kononenkov et al. who found higher VEGF expression in leiomyomas than in adjacent myometrium, and VEGF expression was higher in leiomyosarcomas than in leiomyomas [16].
Estrogens, progesterone, and their receptors play an essential role in the pathogenesis of UM. Estrogens are known to increase PgR expression, while progesterone increases the synthesis of some growth factors (e.g., epidermal growth factor, EGF) and inhibits the synthesis of others (e.g., insulin-like growth factor type I (IGF I)), affecting growth and proliferation of smooth muscle fibers of leiomyoma depending on tumor microenvironment [17, 18].
There are two isoforms of PgR, namely, PgR-A and PgR-B. Some researchers claim that the level of PgR expression in the myoma does not change during the menstrual cycle and that the expression of both receptor isoforms is elevated in myoma tissue compared to normal myometrium [19]. Our study examined the expression of the PgR-A isoform. The expression level of PgR-A was 5.1% lower in the patients in Group 1 compared to the patients in Group 2 (p=0.012). The expression of PgR-A in myometrial tissue was 256±21 in Group 1 patients and 238256±20 in Group 2 (by H score).
The expression of ER-α in fibroid nodules was 93 (90; 110) in Group 1 patients, and 101 (90; 115) in Group 2. In myometrial tissue, it was 79±18 in Group 1 patients and 81±14 in Group 2 (based on the H score). Thus, the detected increase in ER and PgR levels in the leiomyoma tissue of Group 2 patients, compared to Group 1 patients, probably reflects the tumor growth potential. According to M. Nisolle, high ER levels were also higher in myoma, but only during the proliferation phase [15].
One of the most well known tumor growth suppressor genes is the TP53 gene. p53 is a DNA-binding protein that stimulates apoptosis. Mutations in the TP53 gene are more often responsible for a decrease in tumor suppressor function of this gene and affect adjacent signaling pathways, leading to the activation of cell proliferative activity, cell migration, and invasion [20].
No statistically significant differences (p=0.177 and p=0.587, respectively) were found in p53 expression in fibroid nodules and myometrial tissue samples from Group 1 and Group 2 patients. These data are consistent with previously published results suggesting that mutations in the TP53 gene and, consequently, changes in p53 protein expression are not the main factors in UM pathogenesis [21, 22].
P16 is a tumor growth suppressor protein playing an essential role in cell cycle regulation by inhibiting the transition from G1 cell cycle phase to S phase. Mutation of this gene results in loss of control over the rate and order of cell division phases, changes in sensitivity to growth inhibitory signals, and the cell loses the ability to enter a quiescent state and acquires uncontrolled proliferation properties. According to Atkins et al., p16 is predominantly expressed in leiomyosarcomas. This marker may be useful in the differential diagnosis of STUMP (uterine smooth muscle tumor of uncertain malignant potential) and leiomyosarcoma [23].
Based on the data obtained in the analysis of p16 expression in samples of primary and recurrent leiomyomas, we found statistically significant differences (p=0.027). The expression of p16 in fibroid nodules of Group 1 patients was 34% lower compared to Group 2. It should also be noted that in myometrial tissue, the median of p16 in Group 1 patients was twice higher than in Group 2 patients, but no statistically significant difference was found (p=0.247).
According to E.A. Kogan, the formation of new fibroid nodules occurs in the so-called growth zones, where proliferating cell with stem cell properties and capable of producing various growth factors are concentrated [12].
According to Yu.E. Karavaev, recurrent leiomyomas have higher proliferative activity, and the histological structure in this group reveals a higher proportion of mitotically active and cellular myomas compared to primary leiomyomas. The author also suggests that high levels of Ki-67 and VEGF, both in the leiomyoma and in the surrounding myometrium, are pathogenetic factors of leiomyoma recurrence. High TIMP-1 levels in leiomyoma inhibit its growth and increase the time to leiomyoma recurrence [5].
An analysis of different molecular genetic subtypes of uterine leiomyomas (with MED12 mutations, HMGA2 overexpression, FH inactivation) revealed that ER and PgR were highly expressed in all types of UM, but lower expression of ER and higher PgR expression were detected in myomas with FH inactivation. Myomas with overexpression had significantly higher levels of proliferation (Ki-67) and significantly lower levels of p16 expression. This type of UM shows rapid growth and reaches large sizes [24].
Conclusion
Myometrial tissue and fibroid nodules of patients undergoing surgery for newly diagnosed and recurrent UM were examined using morphological and IGC analysis.
Ki-67 levels are higher in myoma samples from patients with recurrent UM, which may reflect the proliferative potential of the tumor most prone to recurrence. Ki-67 may be an indirect marker of disease recurrence. VEGF levels are higher in fibroid nodules compared to myometrial tissue in patients with both newly diagnosed and recurrent UM. ER and PgR levels were higher in fibroid nodule tissue in patients with recurrent UM, reflecting the potential for tumor growth. Changes in p53 protein expression are not associated with UM pathogenesis. The level of p16 is higher in fibroid nodes of patients with recurrent UM.
With increased proliferation and decreased apoptosis, UM recurrence rates are high, demonstrating the biological basis of growth and providing a rationale for targeted therapy. The IHC factor of favorable prognosis for recurrence is low Ki-67, VEGF, ER, PgR, p16 in fibroid nodules.
These results suggest the need for further studies on UM recurrence. The problem of UM recurrence is inextricably linked to etiological factors and cannot be solved by surgery alone.
References
- Поротикова И.Е., Адамян Л.В., Гаврилова Т.Ю., Демура Т.А., Козаченко И.Ф., Доброхотова Ю.Э., Асатурова А.В. Особенности хирургического лечения больных миомой матки после ранее перенесенной неэффективной эмболизации маточных артерий и ФУЗ-МРТ абляции. Проблемы репродукции. 2016; 22(3): 45-52. [Porotikova I.E., Adamyan L.V., Gavrilova T.Yu., Demura T.A., Kozachenko I.Ph., Dobrokhotova Iu.É., Asaturova A.V. Surgical treatment of uterine myoma after ineffective UAE and MRgFUS ablation. Russian Journal of Human Reproduction. 2016; 22(3): 45-52. (in Russian)]. https://dx.doi.org/10.17116/repro201622345-52.
- Donnez J., Donnez O., Dolmans M.M. With the advent of selective progesterone receptor modulators, what is the place of myoma surgery in current practice? Fertil. Steril. 2014; 102(3): 640-8. https://dx.doi.org/10.1016/j.fertnstert.2014.06.041.
- Тоноян Н.М., Козаченко И.Ф., Франкевич В.Е., Чаговец В.В., Адамян Л.В. Рецидивы миомы матки. Современный взгляд на проблемы диагностики, лечения и прогнозирования. Акушерство и гинекология. 2019; 3: 32-8. [Tonoyan N.M., Kozachenko I.F., Frankevich V.E., Chagovets V.V., Adamyan L.V. Recurrences of uterine fibroids. The modern view on the problems of diagnosis, treatment, and prognosis. Obstetrics and Gynecology. 2019; 3: 32-8 (in Russian)]. https://dx.doi.org/10.18565/aig.2019.3.32-38.
- Малышкина А.И., Воскресенская Д.Л., Воронин Д.Н., Анциферова Ю.С., Сотникова Н.Ю., Малышкина Д.А. Иммунные механизмы регуляции роста миомы матки. Акушерство и гинекология. 2020; 2: 111-5. [Malyshkina A.I., Voskresenskaya D.L., Voronin D.N., Antsiferova Yu.S., Sotnikova N.Yu., Malyshkina D.A. Immune mechanisms of regulating the growth of uterine leiomyoma. Obstetrics and Gynecology. 2020; 2: 111-5. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.2.111-115.
- Караваев Ю.Е., Аскольская С.И., Коган Е.А., Арсланян К.Н., Бурыкина П.Н. Прогностические критерии рецидива лейомиомы матки после реконструктивно-пластических операций. Акушерство и гинекология. 2013; 5: 54-7. [Karavayev Yu.E., Askolskaya S.I., Kogan E.A., Arslanyan K.N., Burykina P.N. Prognostic criteria for recurrent uterine leiomyoma after reconstructive plastic surgeries. Obstetrics and Gynecology. 2013; 5: 54-7. (in Russian)].
- Бурлев В.А. Локальный и системный ангиогенез у больных с миомой матки. Проблемы репродукции. 2007; 1: 26-33. [Burlev V.A. Local and systemic angiogenesis in patients with uterine fibroids. Problems of Reproduction. 2007; 1: 26-33. (in Russian)].
- Munro M.G., Critchley H.O., Fraser I.S.; FIGO Menstrual Disorders Working Group. The FIGO classification of causes of abnormal uterine bleeding in the reproductive years. Fertil. Steril. 2011; 95(7): 2204-8.e3. https://dx.doi.org/10.1016/j.fertnstert.2011.03.079.
- Баширов Э.В., Чуприненко Л.М., Крутова В.А. Пролиферативная активность и экспрессия рецепторов стероидных гормонов как предиктор рецидива лейомиомы после органосохраняющих вмешательств. Медицинский вестник Северного Кавказа. 2018; 13: 35-8. [Bashirov E.V., Chuprinenko L.M., Krutova V.A. Molecular biological predictors of myoma growth after organ-saving surgery. Medical News of North Caucasis. 2018; 13: 35-8. (in Russian)]. https://dx.doi.org/10.14300/mnnc.2018.13010.
- Мальков П.Г., Франк Г.А., ред. Основы обеспечения качества в гистологической лабораторной технике. Руководство. М.; 2011. 108 с. [Malkov P.G., Frank G.A., ed. Fundamentals of quality assurance in histological laboratory equipment. Manual. M.; 2011. 108 p. (in Russian)].
- Сидорова И.С., Коган Е.А., Унанян А.Л., Киселев В.И., Агеев М.Б. Клинико-морфологические параллели различных вариантов роста миомы матки. Российский вестник акушера-гинеколога. 2019; 19(3): 29-36. [Sidorova I.S., Kogan E.A., Unanian A.L., Kiselev V.I., Ageev M.B. Clinical and morphological correlations for different types of uterine myoma growth. Russian Bulletin of Obstetrician-Gynecologist. 2019; 19(3): 29-36. (in Russian)]. https://dx.doi.org/10.17116/rosakush20191903129.
- Шрамко С.В., Бондарев О.И., Коваль Е.Ю., Лоскутова Е.Ю., Подтуркина Т.К., Шишея Е.Ю., Станков А.И., Елдинова О.Г. Биологические маркеры клеточного цикла Ki-67 и Bcl-2 при миоме, аденомиозе и лейомиосаркоме матки. Медицина в Кузбассе. 2019; 18(3): 20-4. [Shramko S.V., Bondarev O.I., Koval E.Yu., Loskutova E.Yu.,Podturkina T.K., Shisheya E.Yu., Stankov A.I., Eldinova O.G. Biological markers of the Ki-67 cell cycle and Bcl-2 in myoma, adenomyisis and leymyosarcoma uteri. Medicine in Kuzbass. 2019; 18(3): 20-4. (in Russian)].
- Коган Е.А., Аскольская С.И., Попов Ю.В., Соломахина М.А., Файзуллина Н.М. Лейомиомы матки больших размеров: патогенетические механизмы роста. Клиническая практика. 2016; 7(1): 22-8. [Kogan E.A., Askolskaya S.I., Popov Yu.V., Solomakhina M.A., Fayzullina N.M. Large uterine leiomyomas: pathogenetic mechanisms of grows. Klinical Practice. 2016; 7(1): 22-8. (in Russian)].
- Liao X.H., Li J.Y., Dong X.M., Wang X., Xiang Y., Li H. et al. ERα inhibited myocardin-induced differentiation in uterine fibroids. Exp. Cell Res. 2017; 350(1): 73-82. https://dx.doi.org/10.1016/j.yexcr.2016.11.007.
- Сидорова И.С., Рыжова О.В., Репин А.Б. Роль апоптоза и клеточной пролиферации в патогенезе гладкомышечных опухолей матки. Российский медико-биологический вестник имени академика И.П. Павлова. 2001; 3-4: 26-31. [Sidorova I.S., Ruzhova O.V., Repin A.B. The role of apoptosis and cell proliferation in the pathogenesis of uterus smooth muscle tumors. Russian Medical and Biological Bulletin named after Academician I.P. Pavlov. 2001; 3-4: 26-31. (in Russian)].
- Nisolle M., Gillerot S., Casanas-Roux F., Squifflet J., Berliere M., Donnez J. Immunohistochemical study of the proliferation index, oestrogen receptors and progesterone receptors A and B in leiomyomata and normal myometrium during the menstrual cycle and under gonadotrophin-releasing hormone agonist therapy. Hum. Reprod. 1999; 14(11): 2844-50. https://dx.doi.org/10.1093/humrep/14.11.2844.
- Коненков В.И., Королева Е.Г., Орлов Н.Б., Прокофьев В.Ф., Шевченко А.В., Новиков А.М. Сывороточные уровни факторов роста гемопоэза и ангиогенеза (IL-5, IL-7, IL-9, FGF-β, G-CSF, VEGF И PDGF) у женщин с миомой матки. Медицинская иммунология. 2018; 20(5): 691-8. [Konenkov V.I., Koroleva E.G., Orlov N.B., Prokofiev V.F., Shevchenko A.V., Novikov A.M. Serum levels of hemopoietic and angiogenesis growth factors (IL-5, IL-7, IL-9, FGF-β, G-CSF, VEGF И PDGF) in momen with uterine myoma. Meditsinskaya Immunologiya/Medical Immunology (Russia). 2018; 20(5): 691-8. (in Russian)]. https://dx.doi.org/10.15789/1563-0625-2018-5-691-698.
- Maruo T., Ohara N., Wang J., Matduo H. Sex steroidal regulation of uterine leiomyoma growth and apoptosis. Hum. Reprod. Update. 2004; 10(3): 207-20. https://dx.doi.org/10.1093/humupd/dmh019.
- Тюрина А.А., Ящук А.Г., Имельбаева А.Г., Яковлева О.В. Роль прогестерона и тканевых факторов роста в патогенезе миомы матки. Практическая медицина. 2018; 16(6): 124-9. [Tyurina A.A., Yaschuk A.G., Imelbaeva A.G., Yakovleva O.V. The role of progesterone and tissue growth factors in pathogenesis of uterine myomas. Practical Medicine. 2018; 16(6): 124-9. (in Russian)]. https://dx.doi.org/10.32000/2072-1757-2018-16-6-124-129.
- Robinson-Rechavi M., Garcia H.E., Laudet V. The nuclear receptor superfamily. J. Cell Sci. 2003; 116(4): 585-6. https://dx.doi.org/10.1242/jcs.00247.
- Kirsch D.G., Kastan M.B. Tumor-suppressor p53: implications for tumor development and prognosis. J. Clin. Oncol. 1998; 16(9): 3158-68. https://dx.doi.org/10.1200/JCO.1998.16.9.3158.
- Kuhn E., Yemelyanova A., Wang T.L., Kurman R.J. Abstract 5536: TP53 and MED12 mutations in uterine smooth muscle tumors. Canser Res. 2012; 72(8, Suppl.): 5536. https://dx.doi.org/10.1158/1538-7445.AM2012-5536.
- Hakverdi S., Demirhan O., Tunc E., Inandiklioglu N., Uslu IN., Gungoren A. et al. Chromosome imbalances and alterations in the p53 gene in uterine myomas from the same family members: familial leiomyomatosis in Turkey. Asian Pac. J. Cancer Prev. 2013; 14(2): 651-8. https://dx.doi.org/10.7314/apjcp.2013.14.2.651.
- Atkins K.A., Arronte N., Darus C.J., Rice L.W. The use of p16 in enhancing the histologic classification of uterine smooth muscle tumors. Am. J. Surg. Pathol. 2008; 32(1): 98-102. https://dx.doi.org/10.1097/PAS.0b013e3181574d1e.
- Xie J., Ubango J., Ban Y., Chakravarti D., Kim J.J., Wei J.J. Comparative analysis of AKT and the related biomarkers in uterine leiomyomas with MED12, HMGA2, and FH mutations. Genes Chromosomes Cancer. 2018; 57(10):485-94. https://dx.doi.org/10.1002/gcc.22643.
Received 24.02.2022
Accepted 28.03.2022
About the Authors
Narine M. Tonoyan, Obstetrician-Gynecologist at the Department of Operative Gynecology, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(913)393-31-13, tonnar.13@bk.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.Irena F. Kozachenko, Dr. Med. Sci., Senior Researcher at the Department of Operative Gynecology, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4.
Aleksandra V. Asaturova, Dr. Med. Sci., Head of the 1st Pathology Department, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
a_asaturova@oparina4.ru, https://orcid.org/0000-0001-8739-5209, 117997, Russia, Moscow, Ac. Oparina str., 4.
Vlada V. Kometova, PhD, Head of Oncopathology Department, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, v_kometova@oparina4.ru,
https://orcid.org/0000-0001-9666-6875, 117997, Russia, Moscow, Ac. Oparina, 4.
Alina S. Magnaeva, Junior Researcher at the 1st Pathology Department, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
alinamagnaeva03@gmail.com, https://orcid.org/0000-0001-5223-9767, 117997, Russia, Moscow, Ac. Oparina str., 4.
Nadezda S. Tevrukova, PhD, Senior Researcher at the 1st Pathology Department, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
n.s.tevrukova@yandex.ru, https://orcid.org/0000-0003-3305-8543, 117997, Russia, Moscow, Ac. Oparina str., 4.
Vladimir E. Frankevich, PhD (Physics and Mathematics), Head of the Department of Systems Biology in Reproduction, Academician V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia, +7(495)438-07-88, v_frankevich@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Leyla V. Adamyan, Dr. Med. Sci., Professor, Academician of RAS, Head of the Department of Operative Gynecology, Academician V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia, +7(495)222-37-37, adamyanleila@gmail.com, 117997, Russia, Moscow, Ac. Oparina str., 4.
Authors' contributions: Adamyan L.V., Frankevich V.E. – topic setting and general scientific management, generalization and discussion of the obtained data; Tonoyan N.M., Kozachenko I.F., Asaturova A.V., Kometova V.V., Magnaeva A.V. – conception and design of the study, material collection and processing, statistical analysis, manuscript drafting and editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The work was conducted within the framework of the state assignment А 21-121032500124-9.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Tonoyan N.M., Kozachenko I.F., Asaturova A.V., Kometova V.V.,
Magnaeva A.S., Tevryukova N.S., Frankevich V.E., Adamyan L.V.
Immunohistochemical markers of uterine myoma recurrence.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 4: 123-131 (in Russian)
https://dx.doi.org/10.18565/aig.2022.4.123-131



