Immune response in women with different morphological forms of chronic endometritis and impaired implantation
Objective: To investigate immune response in women with chronic endometritis and impaired implantation.Mikhnina E.A., Davydova N.I., Kazantsev V.A., Ellinidi V.N., Bezhenar V.F.
Materials and methods: The study included 59 women with chronic endometritis managed for infertility and miscarriage and 48 healthy fertile women, previously examined in a separate study of local and systemic immunities. We used standard histological and immunohistochemical analysis of endometrial biopsy specimens to determine the number of lymphocytes carrying markers of HLA-DR+ activation and the number of lymphocytes expressing the B-cell marker CD20+. All women underwent immunological examination, including flow cytometry, DNA flow cytometry, and enzyme immunoassay.
Results: There were local and systemic immunity changes in polypoid and lymphofollicular chronic endometritis, suggesting the autoimmune nature of inflammation in chronic lymphofollicular endometritis.
Conclusion: Characteristic features of the local B-cell response in each group of chronic endometritis determine the nature of the inflammatory process. An aberrant immune-inflammatory response contributes to the restructuring of the endometrium, which is characteristic of polypoid or lymphofollicular types of chronic endometritis, causing the development of an autoimmune component in the lymphofollicular variant of chronic endometritis, alters the microenvironment of the blastocyst, and leads to impaired implantation and reproductive losses.
Keywords
Continuing decline in the population of Russia highlights the importance of addressing issues of infertility.
Most reproductive losses occur in early pregnancy. The critical factor in impaired implantation is insufficient uterine lining invasion by an invading extravillous trophoblast [1]. During the window of implantation, the endometrial glands reach a peak of functional activity, and the structural composition of the stroma changes [2]. Superficial uterine epithelium (luminal epithelium, LE) makes the endometrium susceptible to blastocyst implantation and becomes the tissue of the first embryo-maternal interactions [3].
It was found that 14–67% of implantation failures [4–7] and 9.3–67.6% of early miscarriage [4, 7, 8] are associated with chronic endometritis (CE). CE prevalence is 2.8–56.8% in infertile patients and 9.3–67.6% in women with recurrent pregnancy loss [7].
Despite numerous studies, CE remains a poorly understood pathology. There is no systemic understanding of underlying CE mechanisms; diagnostic criteria and effective treatments have not been developed [9].
CE is a long-term, ongoing process simultaneously involving exudation, alteration, and repair in various combinations. Inflammation is manifested by developing focal or diffuse cellular infiltrate caused by edema and leukocytes migrating into the stroma. Progressive, productive inflammation leads to the development of new morphological structures facilitating the resolution of inflammation and preventing implantation. So, due to the hyperplasia of the functional layer, the surface epithelium changes resulting in micropolyps formation, while the LE structure type is preserved. Micropolypoid endometrium is a typical manifestation of chronic inflammation [10]. Micropolyps indicate the presence of an agent stimulating proliferation and characterize a more pronounced endometrial inflammation. Endometrial micropolyps are histologically recognized as subtle vascularized protrusive lesions in the edematous stroma, covered with a single-row epithelium [11, 12]. Upon the termination of inflammation, micropolyps disappear without a trace [13].
Micropolyposis can be identified by histological and hysteroscopic investigations [14]. They can be located in the endometrium as focal, diffuse, or in groups [15, 16]. Incidence rates of endometrial micropolyps found during hysteroscopy ranges from 2.1% [17] –11% [15] to 41.8% [18].
Unlike micropolyp, a true endometrial polyp is a benign nodular exophytic formation of the mucous membrane of the uterine body, consisting of endometrial glands and stroma, mainly fibrous, containing a tangle of thick-walled blood vessels [19].
Typically, a woman of reproductive age has lymphoid infiltrates in the endometrial basal layer from the late proliferative to the late secretory stage of the menstrual cycle, consisting of a zone of B cells surrounded by T cells and a halo of macrophages. The size of lymphoid infiltrates changes depending on the stage of the menstrual cycle, increasing in the secretory phase to 3000–4000 cells compared to 300–400 cells in the proliferative phase due to immune cells migration [20]. The number of B-lymphocytes in the basal layer of the endometrium increases from the proliferative phase, amounting to 0.3%, to the secretory phase of the cycle, reaching 1.6–2% of the lymphoid population [21, 22]. B-lymphocytes are detected in the stromal layer of the endometrium extremely rarely; they are absent in the superficial glandular epithelium or the lumen of the glands.
In CE, B cells are recruited in the functional layer, and single cells can be found between epithelial cells and within gland lumina. They can form intra-, periglandular, and perivascular lymphoid infiltrates, regardless of the phase of the menstrual cycle [21, 23]. B cell infiltration may be related to plasma cells in the stromal field of the functional layer [4, 7] that synthesize various antibodies.
B cells in the endometrium are identified by an immunophenotypic marker, a membrane-embedded surface molecule CD20. Its function is to enable optimal B-cell immune response, specifically against T-independent antigens, and it disappears at the stage of differentiation of activated B-lymphocytes into plasma cells.
An indicator of immune cell activation is the HLA-DR molecule, typically expressed by antigen-presenting cells, including B-lymphocytes. HLA-DR molecules are involved in presenting genetically foreign antigens (pathogens) or aberrantly expressed autoantigens (tumor cells) to effector cells of the immune system. The quantitative content of HLA-DR+ cells determines the degree of cell involvement in defense reactions [24].
Ellinidi V.N. et al. (2017), summarizing the data of histological and hysteroscopic studies, proposed to distinguish two clinical and morphological forms of CE as polypoid (CPE) and lymphofollicular (LFE) [11].
Today, the diagnosis of CE refers to various conditions [25]. The diagnosis of CE only by the presence of expression of the syndecan-1 (Sdc-1) CD138 molecule detected by immunohistochemical analysis in the absence of evidence of the expression of this molecule by plasma cells is incorrect [26], since CD138 is expressed by epithelial cells and pre-B cells [27], which may be the reason for overdiagnosis, inappropriate and ineffective empirical treatment. Plasma cells are normally present in the endometrium; identification is determined by the phenotype CD38+CD138+.
The present study aimed to investigate immune response in women with chronic endometritis and impaired implantation.
Materials and methods
The study was conducted at the Nikiforov’s All-Russian Center for Emergency and Radiation Medicine of the Emergencies Ministry of Russia and the Clinic of Obstetrics and Gynecology of the Pavlov First St. Petersburg SMU of Minzdrav of Russia. The study included women managed for infertility and miscarriage and had classical histological signs of CE in the endometrium [10, 28].
The study was reviewed and approved by the Local Ethics Committee of the Pavlov First St. Petersburg SMU.
The study group included 59 women of reproductive age with CE. The study group was divided into two clinical groups categorized by the presence of lymphoid follicular infiltrates in the interglandular and perivascular zones in the functional layer of the endometrium, consisting of B cells, or micropolypoid (in the form of villi, micropapillae) LE changes and with a diffuse arrangement of B cells. Group 1 included 26 women with CPE aged 30 (18–39) years; group 2 included 33 women aged 30 (20–43) with LFE. The exclusion criteria were uterine fibroids >35 mm, severe adenomyosis, antiphospholipid syndrome, infectious and severe somatic comorbidities.
The control group consisted of 48 somatically healthy fertile women, previously examined in a separate study of local and systemic immunities. The criteria for inclusion in the control group were the birth of one or more children. In this group, the local and systemic immunity parameters were analyzed in 30 and 18 women, respectively.
The mean age of the patients in the control group was 29 (18–40) years. The inclusion criteria were the absence of gynecologic and somatic pathology and acute and chronic inflammatory diseases.
The endometrial material was obtained in the follicular phase of the menstrual cycle.
We used a standard histological examination of the surgical specimens stained with hematoxylin and eosin. For endometrial biopsies, immunohistochemical analysis was used to determine lymphocytes carrying markers HLA-DR+ and CD20+. The results of the immunohistochemical reaction in the control and study groups were assessed with a positive expression of the markers under study (CD20, HLA-DR) by direct counting of immunocompetent cells in the endometrial stroma under a Leica DM2000 microscope with a microscope magnification of ×400 (eyepiece 10, objective 40), which was the standard diameter of the field of view for all samples, which was 250 μm. The analysis results of markers CD20 and HLA-DR are summarized in table 1.
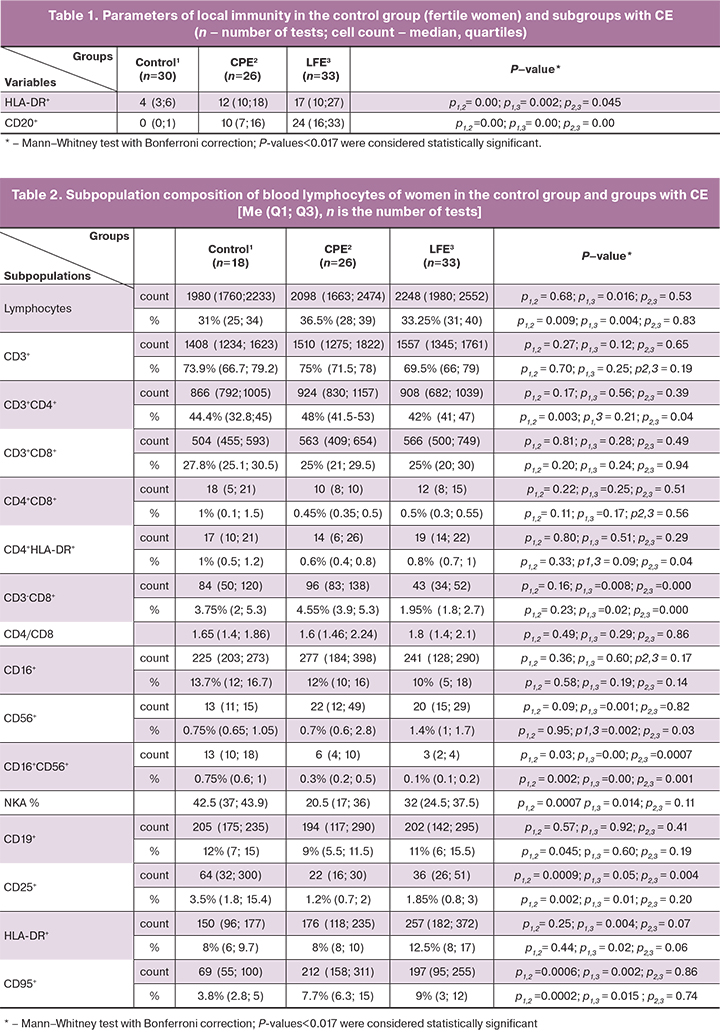
Expression of markers HLA-DR+ and CD20+ on lymphocytes (brown staining of membranes with diaminobenzidine), lymphoid accumulations in the functional layer, and micropolyps of the endometrial epithelium are shown in Figures 1–4.
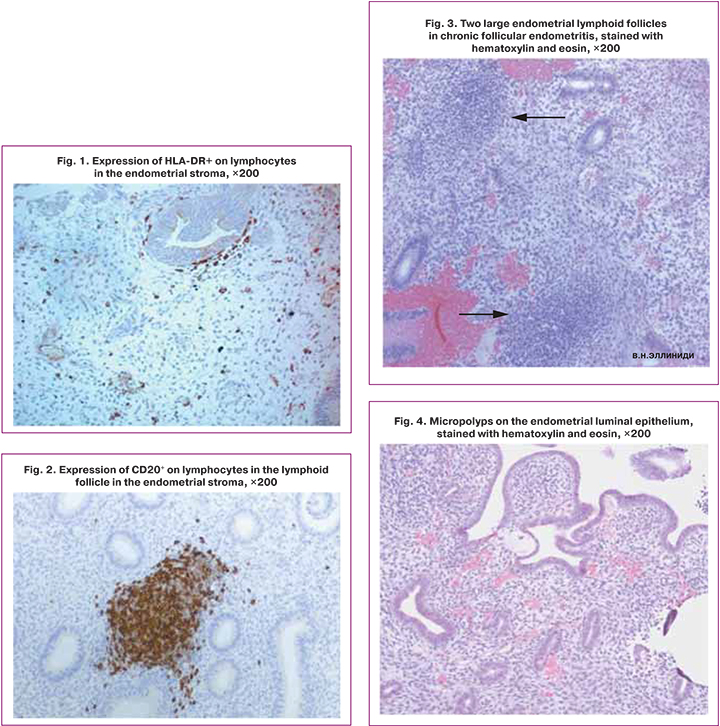
All patients underwent immunological testing, including flow cytometry, DNA flow cytometry using the method described by Dmitrieva I.B. et al. (2004) [29], and enzyme-linked immunosorbent assay (ELISA). The findings are presented in Tables 2–4. Monoclonal antibodies were used to visualize lymphocyte subpopulations: HLADR-FITC, CD4-PE, CD3-ECD, CD56-PC5.5, CD16-PE, CD25-PC7, CD8-APC, CD95-APC, CD19-APC-AF700, CD45-APC-AF750. Samples were analyzed on a Navios flow cytometer in a multicolor protocol (Beckman Coulter instrument and reagents). The lymphocyte population was assessed as CD45+bright SSdim cells. The analysis of the samples was carried out with a set of 5000 events in the lymphocytic region.
The levels of cytokines interleukin (IL)-2, IL-4, IL-6, tumor necrosis factor (TNF-α), interferon (INF)-γ, INF-α in culture media (spontaneous, induced production; to stimulate the production of IL- 2, IL-4, INF-γ used PHA, for IL-6, TNF-α – pyrogenal, for INF-α – Newcastle disease virus) and in serum was determined using reagent kits for ELISA (manufactured by JSC Vektor-Best, Novosibirsk, Russia). Serum levels of IgA, M, G were determined by the turbodimetric method, sIgA – by the ELISA using test systems manufactured by JSC Vektor-Best. According to the author's technique, serum levels of anti-endometrial antibodies (AEA) were determined by ELISA using test systems manufactured by the Laboratory of Biotechnology of RSCRST (RF patent No. 2303267). The cytotoxic activity of NK cells (NKA%) was determined by DNA flow cytometry (Table 2). The study took into account the different DNA content in NK cells and target cells. The erythromyeloblastoid line K-562 obtained from the Research Institute of Cytology bank, Russian Academy of Sciences, was used as target cells. The modal number of chromosomes of lymphocytes was 46, and the number of K-562 cells was 64 (according to the passport). Blood lymphocytes were isolated by centrifugation in a density gradient Fikoll-pak, and the K-562 line cells were mixed in a ratio of 20: 1. One sample of a heterogeneous suspension of cells was treated with a triton solution, allowing ethidium bromide to penetrate the nucleus and stain nucleic acids. An RNAse enzyme was used to ensure staining of DNA only. The sample was mixed and incubated for 30 minutes at 4+–8+. Another suspension sample was incubated for 4 hours in a CO2 incubator, after which it was mixed, stained, and incubated for 30 minutes at 4+–8+. Samples were then analyzed on a flow cytometer using the System II DNA protocol. When evaluating DNA histograms, two cell cycles with modal G1 values differing by 1.47 times were identified; DNA samples were analyzed using the MultiCycle AV software (Phocnix Flow System).
Statistical analysis
Statistical analysis was performed using the Stat Soft Statistica v. 7 and Excel 2003. Descriptive statistics included medians (Me), first and third quartiles (Q1; Q3), and frequencies and proportions for categorical variables. The differences in variables between groups were assessed using the nonparametric Mann–Whitney test with Bonferroni correction. When using the Bonferroni correction for pairwise comparison of three groups, statistical significance was set at p<0.017. Correlation analysis was conducted by calculating Spearman's rank correlation coefficients with confidence intervals of the correlation coefficients. When testing statistical hypotheses, the critical level of significance was considered at p<0.05.
Results and discussion
The results of analyzing the content of CD20+B cells and lymphocytes expressing HLA-DR+ in the endometrium of women with CE are presented in Table 1.
Patients with CPE and LFE had a statistically significantly higher number of HLA-DR+ cells than control subjects. The number of B-lymphocytes CD20+ in the endometrium in the LFE group was statistically significantly higher than that in the CPE group.
According to the data presented in table 2, the absolute count of lymphocytes in the group with LFE was statistically significantly higher than in the group of fertile women. The relative count of lymphocytes in both study groups was higher than in healthy women.
The relative and the absolute counts of T-cells CD3+, absolute count of T-helpers CD3+CD4+, T-cytotoxic cells CD3+CD8+, double-positive T-cells CD4+CD8+, B-cells CD19+, T-helpers CD4+HLADR+, and NK cells CD16+ in the control group and groups with CPE and LFE were comparable. However, the relative counts of lymphocytes with the CD3+CD4+ phenotype in the CPE group were statistically significantly higher than in the group of healthy women.
As shown in Table 2, patients with CPE had statistically significantly lower relative and absolute blood counts lymphocytes expressing CD25+ – the receptor for the growth factor IL-2 than control subjects. In patients LFE the absolute count of CD25+ lymphocytes was also statistically significantly reduced, and the relative tended to decrease. CD25+ is a marker of early activation. Probably, lymphocytes expressing CD25+ are eliminated during inflammation by apoptosis. There was a direct correlation between the relative and absolute number of lymphocytes expressing CD25+ and an increased relative and absolute number of cells expressing CD95+ in the LFE group (r=0.752 95% CI 0.439–0.902, p=0.0003; r=0.771 95% CI 0.47–0.910, p=0.0002; r=0.779 95% CI 0.491–0.914, p=0.0001; r=0.814 95% CI 0.560–0.928, p=0.00004).
The chronic inflammatory process was characterized by a statistically significant increase in the relative and the absolute number of lymphocytes with the marker of readiness for apoptosis CD95+ in the CPE and LFE groups compared to the fertile women. In the group of women with CPE, a direct correlation was established between the absolute number of CD4+ lymphocytes and the absolute and relative number of CD95+ lymphocytes (r=0.706 CI 0.371–0.878, p<0.05; and r=0.666 CI 0.304–0.860, p<0.05) , also a direct correlation was found between the absolute number of CD3+CD8+ lymphocytes and the absolute number of CD95+ lymphocytes (r=0.518 CI 0.030–0.807, p<0.05) and the absolute number of CD3-CD8+ lymphocytes and the relative and absolute number of CD95+ lymphocytes (r=0.610 CI 0.215–0.833, p<0.05, and r=0.593 CI 0.190–0.825, p<0.05).
In the group of women with LFE, a direct correlation was found between the absolute number of CD4+ lymphocytes and the absolute and relative number of lymphocytes expressing the marker of readiness for apoptosis CD95+ (r=0.475 CI 0.01–0.771, p<0.05; and r=0.493 CI 0.034–0.780, p<0.05).
This indicates the readiness for apoptosis of specific effector cells capable of initiating an immune response to both cells infected by pathogens and endometrial cells with a modified antigenic structure. In both cases, the immune response is inadequate.
A direct correlation was found between the relative and absolute numbers of CD56+ NK cells, lymphocytes with predominantly regulatory functions, and cells with a marker of readiness for apoptosis CD95+ (r=0.626 95% CI 0.224–0.846, p=0.004 and r=0.559 95% CI 0.125–0.814, p=0.02). There was a direct correlation between serum level of TNF-α in and the relative and absolute counts of CD95+ lymphocytes in the blood (r=0.726 95% CI 0.223–0.924, p=0.008 and r=0.695 95% CI 0.163–0.914, p=0.02).
In inflammation, cytokines affect antigen presentation, clonal expansion and differentiation of specific clones of lymphocytes, and antibody production. Findings of cytokine analysis are presented in Table 3.
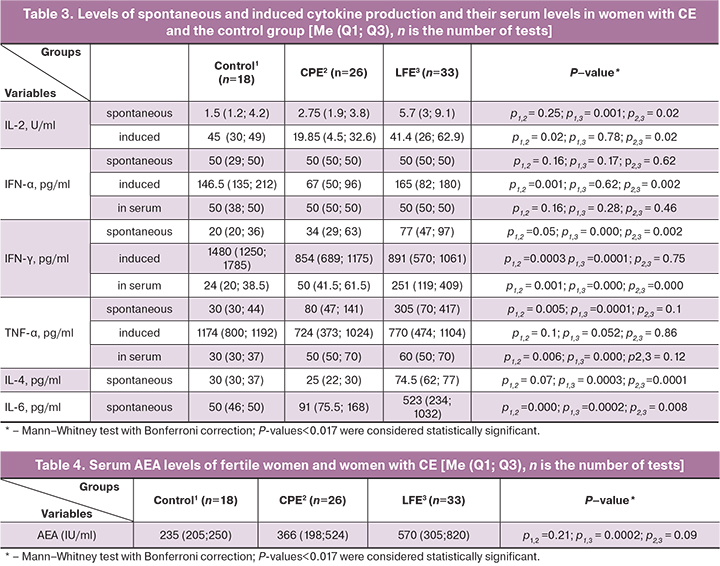
In the CPE group, a direct correlation was found between the number of HLA-DR+ lymphocytes in the endometrium and the levels of spontaneous, induced production and the serum IFN-γ level (r=0.561 95% CI 0.110–0.820, p=0.02; r=0.587 95% CI 0.129–0.839, p=0.02; r=0.636 95% CI 0.313–0.827, p=0.0008, respectively).
IFN-γ is known to stimulate HLA-DR expression not only by classical antigen-presenting cells of hematopoietic origin but also by non-traditional antigen-presenting cells, which include cells of the epithelium of the lungs, gastrointestinal tract, endometrium, and others [30–35].
In the CPE group, there was a direct correlation between the number of B cells in the endometrium and Th-1 type cytokines: spontaneous production (r=0.662 95% CI 0.353-0.841, p=0.0003) and serum content (r=0.633 95% CI 0.154–0.871, p=0.015) IFN-γ and spontaneous production of TNF-α (r=0.658 95% CI 0.220–0.875, p=0.003).
In the LFE group, levels of spontaneous production of IL-4, spontaneous production, and serum IFN-γ were above the population levels. These changes indicate the possible presentation of both exoantigens and antigens of the endometrial epithelium and the switching of the immune response to the Th-2 type (humoral).
Serum levels of AEA in the study participants are presented in Table 4.
The number of AEA was statistically significantly higher in patients with LFE than in the group of fertile women. The levels of immunoglobulins A, M, G, and sIgA were comparable in all study groups (data not shown).
In the LFE group, an inverse correlation was found between the number of CD20+ lymphocytes in the endometrium, which mainly formed lymphoid infiltrates, and the relative, absolute number of lymphocytes expressing the marker of readiness for apoptosis CD95+ (r= -0.621 95% CI -0.217– -0.843, p=0.006; r= -0.604 95% CI -0.206– -0.830, p=0.008), which may indicate that CD20+ endometrial cells do not undergo apoptosis during LFE.
In both groups with CE, a direct correlation was established between the increased number of B-endometrial cells and antibodies to endometrial antigens (r=0.484 95% CI 0.110–0.738, p=0.014 and r=0.549 95% CI 0.110–0.809, p=0.012), which confirms the autoimmune component of CE. There was a direct correlation between the AEA level and the number of endometrial cells expressing HLA-DR in both groups with CE (r=0.616 95% CI 0.263–0.824, p=0.002 and r=0.572 95% CI 0.159–0.815, p=0.008).
In CE, the immune response is characterized by several features:
* in CPE, the presence of antigen and migration of immunocompetent cells causes a local response in the form of changes in the surface epithelium, which are accompanied by the formation of micropolyps; in the functional layer of the endometrial stroma, B-lymphocytes accumulate diffusely, and the number of cells expressing the HLA-DR+ activation marker increases.
Systemic changes are characterized by a decrease in the cytotoxic activity of NK lymphocytes, the number of lymphocytes expressing CD25+, and an increase in the number of CD95+ lymphocytes. There is an increased spontaneous production of TNF-α and IL-6;
* in LFE, B cells migrate into the endometrium and form lymphoid clusters; the content of CD20+ cells and activated HLA-DR+ lymphocytes in the endometrium increases. In peripheral blood, there was a decrease in effector cells CD3-CD8+ and CD16+CD56+, lymphocytes expressing CD25+, the low cytotoxic activity of NK cells, an increase in the number of activated HLA-DR+ and CD95+ cells.
The chronic inflammatory process in the endometrium is characterized by increased spontaneous production of proinflammatory cytokines IL-2, IFN-γ, TNF-α, and increased IL-4, IL-6 – cytokines involved in the proliferation and differentiation of B cells.
There was an increased serum level of antibodies to endometrial antigens. A characteristic feature of LFE is the autoimmune nature of inflammation.
Conclusion
A chronic inflammatory process in the endometrium contributes to morphological changes in the endometrium in the form of endometrial micropolyps or lymphoid accumulations in the functional layer of the endometrium. These changes alter the microenvironment of the blastocyst and lead to impaired implantation and reproductive losses.
Our findings on immunological and immunohistochemical parameters of LFE suggest an autoimmune nature of inflammation in the endometrium.
References
- Alecsandru D., García-Velasco J.A. Why natural killer cells are not enough: a further understanding of killer immunoglobulin-like receptor and human leukocyte antigen. Fertil. Steril. 2017; 107(6): 1273-8. https: //dx.doi.org/10.1016/j. fertnstert. 2017.04.018.
- Ashary N., Tiwari A., Modi D. Embryo implantation: war in times of love. Endocrinology. 2018; 159(2): 1188-98. https://dx.doi.org/10.1210/en.2017-03082.
- Ye X. Uterine luminal epithelium as the transient gateway for embryo implantation. Trends Endocrinol. Metab. 2020; 31(2):165-80. https://dx.doi.org/10.1016/j.tem.2019.11.008.
- Buzzaccarini G., Vitagliano A., Andrisani A., Santarsiero C.M., Cicinelli R., Nardelli C. et al. Chronic endometritis and altered embryo implantation: a unified pathophysiological theory from a literature systematic review. J. Assist. Reprod. Genet. 2020; 37(12): 2897-911. https://dx.doi.org/10.1007/s10815-020-01955-8.
- Sato T., Sugiura-Ogasawara M., Ozawa F., Yamamoto T., Kato T., Ku-rahashi H. et al. Preimplantation genetic testing for aneuploidy: a comparison of live birth rates in patients with recurrent pregnancy loss due to embryonic aneuploidy or recurrent implantation failure. Hum. Reprod. 2019; 34(12): 2340-8. https://dx.doi.org/10.1093/humrep/dez 229.
- Феоктистов А.А., Ниаури Д.А., Эллиниди В.Н., Обидняк Д.М. Влияние хронического эндометрита на эффективность программ вспомогательных репродуктивных технологий. В кн.: Материалы XXV Юбилейной международной конференции Российской Ассоциации Репродукции Человека. Сентябрь 9-12 2015, Сочи. 2015: 49. [Feoktistov A.A., Niauri D.A., Ellinidi V.N., Obidnyak D.M. Influence of chronic endometritis on the effectiveness of programs of assisted reproductive technologies / Materials of the XXV Anniversary International Conference of the Russian Association for Human Reproduction, September 9–12, 2015, Sochi. (in Russian)].
- Kimura F., Takebayashi A., Ishida M., Nakamura A., Kitazawa J., Morimune A. et al. Review: Chronic endometritis and its effect on reproduction. J. Obstet. Gynaecol. Res. 2019; 45(5): 951-60. https://dx.doi.org/10.1111/jog.13937.
- Таболова В.К., Корнеева И.Е. Влияние хронического эндометрита на исходы программ вспомогательных репродуктивных технологий: морфофункциональные и молекулярно-генетические особенности. Акушерство и гинекология. 2013; 10: 17-22. [Tabolova V.K., Korneeva I.E. Impact of chronic endometritis on the outcomes of assisted reproductive technology programs: morphofunctional and molecular genetic features. Obstetrics and Gynecology. 2013; 10: 17-22. (in Russian)].
- Puente E., Alonso L., Lagana A.S., Ghezzi F., Casarin J., Carugno J. Chronic endometritis: old problem, novel insights and future challenges. Int. J. Fertil. Steril. 2020; 13(4): 250-6. https://dx.doi.org/10.22074/ijfs. 2020.5779.
- Хмельницкий О.К. Цитологическая и гистологическая диагностика заболеваний шейки и тела матки. Руководство. СПб.; 2000. 332с. [Khmelnitsky O.K. Cytological and histological diagnosis of diseases of the cervix and the body of the uterus: a guide. SPb; 2000. 332p. (in Russian)].
- Эллиниди В.Н., Феоктистов А.А., Обидняк Д.М., Лямина А.В., Суворова И.Ю. Хронический полипоидный и лимфофолликулярный эндометрит: гистероскопическая и гистологическая диагностика. Журнал акушерства и женских болезней. 2017; 66(6): 59-65. [Ellinidi V.N., Feoktistov A.A., Obidnyak D.M., Lyamina A.B., Suvorova I.U. Chronic polypoid and lymphofollicular endometritis: hysteroscopic and histological diagnostics. Journal of Obstetrics and Women's Diseases. 2017; 66(6): 59-65. (in Russian)]. https://dx.doi.org/10.17816 / JOWD66659-65.
- Kitaya K., Matsubayashi H., Yamaguchi K., Nishiyama R., Takaya Y., Ishikawa T. et al. Chronic endometritis: potential cause of infertility and obstetric and neonatal complications. Am. J. Reprod. Immunol. 2016; 75(1): 13-22. https://dx.doi.org/10.1111/aji.12438.
- Cicinelli E., Tinelli R., Lepera A., Pinto V., Fucci M., Resta L. Correspond-ence between hysteroscopic and histologic findings in women with chronic endometritis. Acta Obstet. Gynecol. Scand. 2010; 89(8):1061-5. https://dx.doi.org/10.3109/00016349.2010.498496.
- Cicinelli E., Vitagliano A., Kumar A., Lasmar R.B., Bettocchi S., Haimovich S. International working group for standardization of chronic endometritis diagnosis. Unified diagnostic criteria for chronic endometritis at fluid hysteroscopy: proposal and reliability evaluation through an international randomized-controlled observer study. Fetil. Steril. 2019; 112(1): 162-73.e2. https://dx.doi.org/10.1016/j.fertnstert.2019.03.004.
- Cicinelli E., Resta L., Nicoletti R., Zappimbulso V., Tartagni M., Saliani N. Endometrial micropolypes during liquid hysteroscopy indicate the presence of chronic endometritis. Hum. Reprod. 2005; 20(5): 1386-9. https://dx.doi.org/10.1093/humrep/deh779.
- Kitaya K., Tada Y., Taguchi S., Funabiki M., Hayashi T., Nakamura Y. Local mononuclear cell infiltrates in infertile patients with endometrial macropolypes versus micropolypes. Hum. Reprod. 2012; 27(12): 3474-80. https://dx.doi.org/10.1093/humrep/des323.
- Song D., Li T.C., Zhang Y., Feng X., Xia E., Huang X. et al. Correlation between hysteroscopy findings and chronic endometritis. Fertil. Steril. 2019; 11(4):772-9. https://dx.doi.org/10.1016/j.fertnstert.2018.12.007.
- Савельева Г.М., Сухих Г.Т., Серов В.Н., Радзинский В.Е., Манухин И.Б., ред. Гинекология. Национальное руководство. 2-е изд. М.: ГЭОТАР-Медиа; 2020. 1008с. [Savelyeva G.M., Sukhikh G.T., Serov V.N., Radzinsky V.E., Manukhin I.B., ed. Gynecology: national guidelines. 2nd ed., rev. and add. M.: GEOTAR-Media; 2020. 1008 p. (in Russian)]. ISBN 978-5-9704-5707-8.
- Кондриков Н.И. Патология матки. М.: Практическая медицина; 2008. 334с. [Kondrikov N.I. Pathology of the uterus. M.: Practical medicine; 2008. 334p. (in Russian)].
- Wira C.R., Fahey J.V., Rodriguez-Garcia M., Shen Z., Patel M.V. Regulation of mucosal immunity in the female reproductive tract: the role of sex hormones in immune protection against sexually transmitted pathogens. Am. J. Reprod. Immunol. 2014; 72(2): 236-58. https://dx.doi.org/10.1111/aji.12252.
- Kitaya K., Takeuchi T., Mizuta S., Matsubayashi H., Ishikawa T. Endometritis: new time, new concepts. Fertil. Steril. 2018; 110(3): 344-50. https://dx.doi.org/10.1016/j.fertnstert.2018.04.012.
- Radović Janošević D.R., Trandafilović M., Krtinić D., Čolović H., Milošević Stevanović J., Pop-Trajković Dinić S. Endometrial immunocompetent cells in proliferative and secretory phase of normal menstrual cycle. Folia Morphol. (Warsz). 2020; 79(2): 296-302. https://dx.doi.org/10.5603/FM.a2019.0095.
- Kosei N., Zakharenko N., Herman D. Endometrial polios in women of reproductive age: clinical and patogenetic variatios. Georgian Med. News. 2017; 273: 16-22.
- Green M.R., Kihira S., Liu C.L., Nair R.V., Salari R., Gentles A.J. et al. Mutations in early follicular lymphoma progenitors are associated with sup-pressed antigen presentation. Proc. Natl. Acad. Sci. USA. 2015; 112(10): E1116-25. https://dx.doi.org/10.1073/pnas.1501199112.
- Groth J.V. Chronic endometritis and the plasma cell, fact versus fiction. Fertil. Steril. 2018; 109(5): 788. https://dx.doi.org/10.1016/j.fertnstert.2018.02.116.
- O'Connell F.P., Pinkus J.L., Pinkus G.S. CD138 (syndecan-1), a plasma cell marker immunohistochemical profile in hematopoietic and nonhematopoietic neoplasms. Am. J. Clin. Pathol. 2004; 121(2): 254-63. https://dx.doi.org/10.1309/617D-WB5G-NFWX-HW4L.
- Луговская С.А., Почтарь М.Е., Тупицин Н.Н. Иммунофенотипирование в диагностике гемобластозов. М.; 2005. 166c. [Lugovskaya S.A., Pochtar M.E., Tupitsin N.N. Immunophenotization in the diagnosis of hemoblastoses. M.; 2005. 166 p. (in Russian)].
- Рунец У.Ф., Акулич Н.С., Юдина О.А. Хронический эндометрит, как этиологический фактор женского бесплодия. УО «Белорусский государственный медицинский университет»; 2016. [Runets U.F., Akulich N.S., Yudina O.A. Chronic endometritis as an etiological factor of female infertility. Belarusian State Medical University. 2016. (in Russian)]. bsmu.by> files/2017040613500812.pdf
- Бычкова Н.В. Анализ содержания ДНК методом проточной цитометрии. Возможности применения в клинической практике. Тверь: Триада; 2015. 103 с. [Bychkova N.V. Analysis of DNA content by flow cytometry. Possibilities of application in clinical practice. Tver: Triad; 2015. 103 p. (in Russian)]. ISBN 978-5-94789-686-2.
- Gauthier T., Filloux M., Guillaudeau A., Essig M., Bibes R., Pacha A.F., Piver P., Aubard Y., Marquet P., Drouet M. Uterus human leucocyte antigen expression in the perspective of transplantation. J. Obstet. Gynaecol. Res. 2016; 42(12): 1789-95. https://dx.doi.org/10.1111/jog.13107.
- Koumantakis E.E., Panayiotides J.G., Goumenou A.G., Ziogos E.Ch., Margariti A., Kalapothaki V., Matalliotakis I.M. Different HLA-DR expression in endometriotic and adenomyotic lesions: correlation with transvaginal ultrasonography findings. Arch. Gynecol. Obstet. 2010; 281(5): 851-6. https://dx.doi.org/10.1007/s00404-009-1168-z.
- Tabibzadeh S. Induction of HLA-DR expression in endometrial epithelial cells by endometrial T-cells: potential regulatory role of endometrial T-cells in vivo. J. Clin. Endocrinol. Metab. 1991; 73(6): 1352-9. 10.1210/jcem-73-6-1352.
- Tabibzadeh S.S., Satyaswaroop P.G., Rao P.N. Antiproliferative effect of interferon-gamma in human endometrial epithelial cells in vitro: potential local growth modulatory role in endometrium. J. Clin. Endocrinol. Metab. 1988; 67(1): 131-8. https://dx.doi.org/10.1210/jcem-67-1-131.
- Wosen J.E., Mukhopadhyay D., Macaubas C., Mellins E.D. Epithelial MHC class II expression and its role in antigen presentation in the gastrointestinal and respiratory tracts. Front. Immunol. 2018; 9: 2144. https://dx.doi.org/10.3389/fimmu.2018.02144.
- Heuberger C., Pott J., Maloy K.J. Why do intestinal epithelial cells express MHC class II? Immunology. 2021; 162(4): 357-67. https://dx.doi.org/10.1111/imm.13270.
Received 11.06.2021
Accepted 29.11.2021
About the Authors
Elena A. Mikhnina, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynaecology and Reproductology, Pavlov First Saint Petersburg State Medical University, Ministry of Health of Russia, emikhnina@yandex.ru, https://orcid.org/0000-0002-0460-9804, 197022, Russia, Saint Petersburg, Lev Tolstoy str., 6-8.Natalia I. Davydova, MD, PhD, Senior Researcher, Head of the Laboratory of Clinical Immunology, Nikiforov's All-Russian Center for Emergency and Radiation Medicine
of the Emergencies Ministry of Russia, 194044, Russia, St. Petersburg, Acad. Lebedev str., 4/2.
Vladimir A. Kazantsev, MD, PhD Student at the Department of Obstetrics and Gynaecology and Reproductology, Pavlov First Saint Petersburg State Medical University, Ministry of Health of Russia, 197022, Russia, Saint Petersburg, Lev Tolstoy str., 6-8.
Vera N. Ellinidi, MD, PhD, Senior Researcher, Associate Professor, Head of the Laboratory of Morphological Research, Nikiforov's All-Russian Center for Emergency and Radiation Medicine of the Emergencies Ministry of Russia, 194044, Russia, St. Petersburg, Acad. Lebedev str., 4/2.
Vitaly F. Bezhenar, Dr. Med. Sci., Professor, Head of the Departments of Obstetrics, Gynecology and Neonatology/Obstetrics, Gynecology and Reproductology,
Pavlov First Saint Petersburg State Medical University, Ministry of Health of Russia, 197022, Russia, Saint Petersburg, Lev Tolstoy str., 6-8.
Corresponding author: Elena A. Mikhnina, emikhnina@yandex.ru
Authors' contributions: All authors contributed significantly to this study and approved it for publication. Mikhnina E.A., Davydova N.I., Ellinidi V.N., Bezhenar V.F. – conception and design of the study; E.A. Mikhnina, V.A. Kazantsev – data collection and analysis, statistical analysis; Mikhnina E.A., Davydova N.I., Ellinidi V.N. – manuscript drafting;
Davydova N.I., Bezhenar V.F. – manuscript editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Mikhnina E.A., Davydova N.I., Kazantsev V.A., Ellinidi V.N.,
Bezhenar V.F. Immune response in women with different morphological forms
of chronic endometritis and impaired implantation.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 12: 110-118 (in Russian)
https://dx.doi.org/10.18565/aig.2021.12.110-118



