Surgical anatomy of the extraorganic anastomoses of the uterine artery
Objective. To determine the topography variants and quantity of the extraorganic anastomoses of the uterine artery (UA) in females with different somatotypes.Kuzmenko A.V., Shkvarko M.G.
Materials and methods. The study was carried out on 113 unfixed female corpses (aged from 32 to 93 years). The method of somatotyping, intravascular injection, dissection method and statistical processing of the obtained data were used to achieve the aim of the study.
Results. UA anastomoses were formed with other branches of the internal iliac artery in 21.2% of cases on the right and in 23.0% of cases on the left. It was found that two thirds of all UA anastomoses were located in its proximal third and one third was localized in its middle third. Extraorganic anastomoses of the UA were more frequently noted in the group of females of the dolichomorphic somatotype, and less commonly in females of the brachymorphic somatotype.
Conclusions. The study showed that extraorganic anastomoses of the UA have a certain pattern of its origin.
Keywords
In the surgical treatment of uterine bleeding of different etiologies, the choice of a certain tactic aimed at preserving the organ remains one of the topical issues of modern medicine [1, 2]. It should be noted that bilateral uterine artery (UA, a. uterina) ligation provides complete hemostasis in this situation only in 71–75% of cases [1]. In other cases, secondary bleeding occurs due to the presence of a well-developed system of collaterals in the female pelvic cavity [1]. For this reason, iatrogenic injury of a. uterina is also dangerous [3]. In some cases performing bilateral endovascular occlusion of the UA in the treatment of uterine tumors cannot provide a complete hemostatic effect due to the presence of extraorganic anastomoses of this artery [2, 4].
It should be noted that new studies concerning the UA and its branches provide fragmentary information on the anatomy of extraorganic and other localizations of anastomoses of a. uterina [5–8]. The topography and anastomoses of these extraorganic collaterals are not described in the studies either. The data on the number of UA anastomoses and their frequency depending on the type of female somatotype are not presented.
Therefore, the surgical anatomy of extraorganic anastomoses of a. uterina remains a relevant issue and requires a further study.
The aim of the study was to determine the topography variants and quantity of the extraorganic anastomoses of the UA in females with different somatotypes.
Materials and Methods
The study was carried out on 113 unfixed female corpses (aged from 32 to 93 years) on both sides of the pelvic cavity. When measuring the length of isolated arterial vessels, caliper 0-150-0.02 was used, and a micrometer MK-63 was applied to determine the diameter of the arterial vessels. When measuring the length of the body (from the extreme point of the crown to heel) ATLAS TAPE MEASURE was used, and the shoulder width (the distance between the extreme points of acromions) was measured using caliper 0-500-0.1. These tools passed a specialized metrological verification in the Republican Unitary Enterprise ‘Vitebsk Center for Standardization, Metrology and Certification’ before the measurement. The research results were obtained in accordance with the Law of the Republic of Belarus and approved by the Ethics Committee.
In order to obtain the information on the number and frequency of extraorganic anastomoses of the UA depending on the type of female somatotype, the method of somatotyping developed by B.A. Nikityuk and A.I. Kozlova was used [9].
Before calculating the border intervals for each group of somatotypes, the value of the relative shoulder width for each woman was calculated using the formula: shoulder width×100÷ length of the body. Then the arithmetic mean of the relative shoulder width (M) and its standard deviation (SD) were calculated. The interval borders of relative shoulder width of the dolichomorphic somatotype were calculated using the formulas M-3×SD and M-0.67×SD, mesomorphic somatotype M-0.67×SD and M+0.67×SD, brachymorphic somatotype M+0.67×SD and M+3×SD. Then the value of the relative shoulder width of each woman was correlated with the numerical values of the borders of the calculated intervals and it was determined what group of the three somatotypes the woman belonged to. When performing an operative access to the branches of the internal iliac artery (IIA), total median laparotomy was performed from the level of the xiphoid process of the sternum to the pubic symphysis. In case of excessive subcutaneous adipose tissue, operative access was performed from the xiphoid process of the sternum in an oblique direction to the lowest points of the right and left tenth ribs. After that, the incision was made symmetrically to the right and left iliac crests. The final part of the operative access was performed from the anterior superior iliac spine parallel to the inguinal fold of the skin to the outer edge of the abdominal rectus muscle. When incising the anterior wall of the abdomen, it was possible to cut out the skin flap and deeper located soft tissues; this way the difficulty in dealing with a thick layer of subcutaneous fat through a linear incision was eliminated. After a layer-by-layer dissection of the anterior abdominal wall, the peritoneal cavity organs were displaced towards the diaphragm. Then, the posterior leaf of the parietal peritoneum was dissected; the aortic bifurcation was identified and common, external and internal iliac arteries were subsequently found. Kocher clamps were applied on the common and external iliac arteries near the place of their branching. After that, the common iliac artery was punctured with a syringe and 50 ml of red ink solution was injected into it. Contrast substance injected into the pelvic arteries significantly increased the degree of visualization of the IIA branches and their anastomoses.
Before dissecting the UA, the posterior leaf of the parietal peritoneum was moved to the medial side and connective tissue was removed from the IIA and the umbilical artery (UmbA) in one half of the pelvic cavity using anatomic tweezers and vascular scissors. In order to establish anastomotic connections of a. uterina with visceral branches of the IIA, the following arteries were gradually identified: superior vesical artery (SVA), inferior vesical artery (IVA), middle rectal artery (MRA), and the intrapelvic part of the internal pudendal artery (IIPA). To identify the variants of anastomosing the UA with parietal branches of the IIA, the following arteries were exposed: obturator artery (OA), inferior gluteal artery (IGA), superior gluteal artery (SGA), iliolumbar artery (ILA) and lateral sacral artery (LSA). During the dissection of a. uterina all its extraorganic anastomoses were identified. The variants of their localization were evaluated.
Statistical processing of the data obtained during the study was performed using MedStat program (license version #3, serial number: MS 000050). When determining the type of distribution (following the normal law or differing from it) of the obtained numerical variation series, the Shapiro-Wilk W test was calculated. On the basis of the obtained calculations, it was established that all variation series follow the normal distribution law. Then the values of the average length and diameter of the UA and its extraorganic anastomoses were calculated, confidence intervals (CI) for them were determined. In order to make a comparative assessment between the diameter of a. uterina and its anastomotic branches, Student’s t-test was calculated for two independent samples. The Pearson correlation coefficient (R) was calculated to determine the correlation between the increase in the diameter of the UA and the increase in the diameter of its extraorganic anastomoses.
Results and Discussion
When performing the calculations with the descriptive statistics of the obtained numerical variational series, it was established that the average length of the UA in the right half of the pelvis was equal to 5.0 cm, CI: 4.5–5.4, and the average diameter of this artery was 4.4 mm, CI: 3.7-5.1. At the same time, the average length of extraorganic anastomoses of a. uterina was 1.6 cm, CI: 1.3–1.8, and the average diameter of these arterial anastomoses was 2.0 mm, CI: 1.7–2.3.
The analysis of the results showed that UA extraorganic anastomoses were formed in the right half of the pelvis in 21.2% of cases (24 specimens). It should be noted that due to the presence of two arterial anastomoses in two specimens taken from the right half, the total number of a. uterina anastomoses was 26 vessels.
When comparing the diameters of the UA and its extraorganic anastomoses on the right, it was found that their average values differed with the significance level of p˂0.001 (Student’s t-criterion is 6.47). When calculating the Pearson’s correlation coefficient (R=0.22, p=0.278), it was found that there was no linear correlation between the diameters of a. uterina and its extraorganic anastomoses. Thus, as the diameter of the UA increases, the diameter of its extraorganic anastomoses branches does not increase proportionally. In general, 61.5% of all isolated extraorganic anastomoses (16 vessels) were found in the right half of the pelvis in the proximal third of the UA, and 38.5% of the total number of isolated arterial anastomoses were found in the middle third of this artery. In the distal third of the a. uterina, its extraorganic anastomotic branches were absent.
According to the data of the study, the average length of the UA in the left half of the pelvis was 5.4 cm, CI: 4.9–5.9; the average diameter of this artery was 4.1 mm, CI: 3.6–4.5. The average length of extraorganic anastomoses of a. uterina was 1.5 cm, CI: 1.3–1.8; the average diameter of these arterial anastomoses was 2.1 mm, CI: 1.8–2.3.
The UA anastomoses in the left half of the pelvis was noted in 23.0% of cases (26 specimens). However, due to the presence of two arterial anastomoses in one specimen, the total number of a. uterina anastomoses was 27 vessels.
When comparing the diameter of the UA and its extraorganic anastomoses in the left half of the pelvis, it was found that their average values differed with significance level of p˂0.001 (Student’s t-criterion is 7.45). When calculating the Pearson’s correlation coefficient (R=0.076, p=0.707), it was found that there was no linear correlation between the diameter of a. uterina and its extraorganic anastomoses. Therefore, an increase in the diameter of the UA does not result in the linear increase in the diameter of its extraorganic anastomoses.
It should be noted that the difference between the average diameter of extraorganic anastomoses of a. uterina in the right and left halves of the pelvis was not statistically significant (Student’s t-criterion is 0.81, p=0.422).
It was found that 81.5% of all its isolated extraorganic anastomoses (22 vessels) were detected in the left half of the pelvis in the proximal third of a. uterina, and 18.5% of the total number of isolated arterial anastomoses were detected in the middle third of this artery. In the distal third of the UA, its extraorganic anastomotic branches were absent.
The data on the frequency of UA anastomosis with IIA branches are presented in the Table.
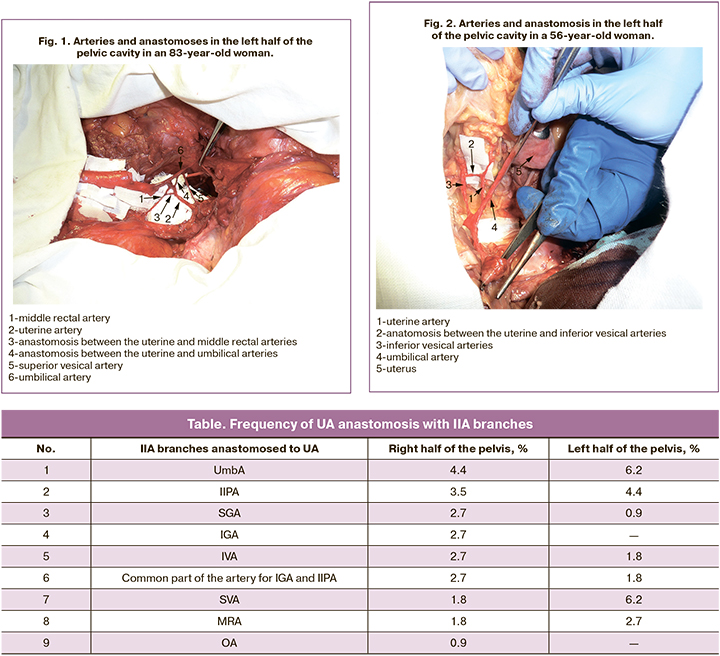
The illustration of anastomosis of UA and UmbA is shown in Fig. 1, and anastomosis of a. uterina and IVA is presented in Fig. 2.
The analysis of the results of somatotyping revealed that the group with dolichomorphic somatotype consisted of 25 women, the group with mesomorphic somatotype included 59 women and the group with brachymorphic somatotype included 29 women.
In women of dolichomorphic somatotype, a. uterina was anastomosed in the right half of the pelvis in 32.0% of cases (8 specimens), and in the left half of the pelvis in 24.0% of cases (6 specimens). In women of mesomorphic somatotype, extraorganic anastomoses of the UA were formed in the right and left halves in 20.3% of cases (12 specimens). However, due to the presence of two anastomoses in the right half of the pelvis in two specimens, the total number of extraorganic anastomotic branches on the right was 14 vessels. Anastomosis of a. uterina with other IIA branches in women of brachymorphic somatotype was detected in 13.8% of cases on the right (4 specimens) and in 24.1% of cases on the left (7 specimens). It should be noted that due to the presence of two arterial anastomoses in the left half of the pelvis in the same specimen, the total number of anastomoses on the left was 8 vessels. In general, women of the dolichomorphic somatotype were significantly more likely to have extraorganic anastomotic branches of the UA; this factor may lead to the conclusion that it will be more difficult to achieve effective hemostasis in endovascular occlusion of a. uterina in women of dolichomorphic somatotype than in women of mesomorphic or brachymorphic somatotypes.
It is worth noting that the comparison of the results of our study and the data presented in the literature [5, 6, 7, 10] revealed that other scientists mainly described different variants of anastomoses of a. uterina and the frequency of the arterial collaterals. The researchers described the formation of anastomotic branches of the UA with SGA, IVA, MRA, IIPA, UmbA and SVA [5, 6, 7, 10], and these findings are consistent with the results of our study. A distinctive feature of our research is the description of the topography and quantitative assessment of UA extraorganic anastomoses, that have not been available in the previous studies on this issue.
Bilateral laparoscopic UA clipping is one of the recommended modern organ-preserving methods for the treatment of uterine fibroids [2]. Clips are placed on a. uterina near its origin from the IIA or UmbA. This operation is performed under local anesthesia and must include coagulation of the peripheral branches of the UA and ovarian artery, which can anastomose with each other. The need to perform such a coagulation is due to the fact that branches of a. uterina anastomose with branches of a. ovarica in 10–30% of cases [2]. According to some authors, bilateral laparoscopic UA clipping is as effective as endovascular embolization of this artery and significantly reduces the time of hospitalization [2]. However, it should be noted that when applying clips at the point of origin of the right and left a. uterina, extraorganic arterial anastomoses of the UA remain functionally active and can neutralize the hemostatic effect of this operation. According to the data of our study, extraorganic anastomoses of a. uterina occur in every fifth woman, therefore, the percentage of unsuccessful outcomes of bilateral laparoscopic UA clipping can be quite high.
It is worth mentioning that the situation when it is not possible to achieve complete hemostasis inside this artery after endovascular embolization of the UA using to the standard scheme occurs only in a small number of cases (1 case for 19–20 endovascular occlusions) [4]. Taking into account that 71.7% of all extaorganic anastomotic branches of a. uterina are located in its proximal third, and 28.3% of arterial anastomoses are located in its middle third, it is necessary to recommend placing additional emboli in the areas of the most likely localization of the anastomoses during intravascular occlusion of this artery. In this case, it will be possible to achieve the highest possible hemostatic effect.
It should be emphasized that when performing an uncomplicated cesarean section, certain specific types of extraorganic anastomoses of the UA do not affect the technique and tactics of this operation. In cases when the fetus implanted into the uterine scar that was formed after the previous cesarean section, specialists recommend performing bilateral embolization of a. uterina with additional devascularization of the sections of this artery where there are anastomoses with IIPA [7]. At the second stage of the operation, uterine curettage should be performed.
When performing an operation for uterine fibromyoma, after the formation of the main endovascular embolus inside the UA, some authors recommend to exclude those areas of the a. uterina where anastomoses with MRA may be located from the bloodstream [10]. According to these experts, it is the arterial collaterals between the UA and MRA that can lead to the failure of occlusion of a. uterina.
Conclusion
Extraorganic anastomoses of the UA were found to be located along the proximal, middle and distal thirds of this artery with various frequencies. This suggests that there are areas of a. uterina where arterial anastomoses with other branches of the IIA are much more frequent. Two-thirds of extraorganic anastomotic branches of the UA are located in its proximal third, and one third can be found in the middle third of this artery. The data on the topography of the most likely location of a. uterina anastomoses will allow the surgeons to achieve a high hemostatic effect in case of an injury to the artery, control of postpartum bleeding or in the treatment of uterine tumors.
References
- Lindquist J.D., Vogelzang R.L. Pelvic artery embolization for treatment of postpartum hemorrhage. Semin. Interv. Radiol. 2018; 35(1): 41-7. https://dx.doi.org/10.1055/s-0038-1636520.
- Szkodziak P., Szkodziak F., Trzeciak K., Czuczwar P. Minimally invasive procedures in the management of uterine fibroids. Menopause Rev. 2017; 16(4): 122-5. https://dx.doi.org/10.5114/pm.2017.72756.
- Seki T., Yoshinobu H., Ichikawa T., Onota S., Nakota M., Takakura S. Uterine artery pseudoaneurysm caused by a uterine manipulator. Gynecol. Minim. Invasive Ther. 2017; 6(1): 25-7. https://dx.doi.org/10.1016/j.gmit.2016.04.002.
- Kim T., Shin J.H., Kim I., Yoon H., Ko G., Cwon D. Management of bleeding uterine arteriovenous malformation with bilateral uterine artery embolization. Yonsei Med. J. 2014; 55(2): 367-73. https://dx.doi.org/10.3349/ymj.2014.55.2.367.
- Selcuk I., Yassa M., Huri E. Anatomic structure of the internal iliac artery and its educative dissection for peripartum and pelvic hemorrhage. Turk. J. Obstet. Gynecol. 2018; 15(2): 126-9. https://dx.doi.org/10.4274/tjod.23245.
- Lipshutz B. A composite study of the hypogastric artery and its branches. Ann. Surg. 2018; 67(5): 584-608. https://dx.doi.org/10.1097/00000658-191805000-00012.
- Zhang G., Li J., Tang J., Wang D., Sun Z. Role of collateral embolization in addition to uterine artery embolization followed by hysteroscopic curettage for the management of cesarean scar pregnancy. BMC Pregnancy Childbirth. 2019; 19(1): 502. https://dx.doi.org/10.1186/s12884-019-2590-2.
- Хачатрян А.С., Гришин И.И., Капранов С.А., Доброхотова Ю.Э. Ретроспективный анализ осложнений при эмболизации маточных артерий. Акушерство и гинекология. 2012; 8: 15-9. [Khachatryan A.S., Grishin I.I., Kapranov S.A., Dobrokhotova Yu.E. Retrospective analysis of uterine artery embolization. Akusherstvo i ginekologiya / Obstetrics and Gynecology. 2012; 8: 15-9. (in Russian)].
- Никитюк Б.А., Козлов А.И. Новая техника соматотипирования. Новости спортивной и медицинской антропологии. 1990; 3: 121-41. [Nikityuk B.A., Kozlov A.I. New technique of somatotyping. Novosti sportivnoi i meditsinskoi antropologii / News of sport and medical anthropology. 1990; 3: 121-41. (in Russian)].
- Ольшанский М.С., Коротких Н.Н., Казарезов О.В. Клиническое значение анастомозов средних прямокишечных и маточных артерий при эндоваскулярных вмешательствах. Медицинский вестник Юга России. 2015; 4: 68-71. [Olshansky M.S., Korotkikh N.N., Kazarezov O.V. Clinical value of anastomosis between middle rectal and uterine arteries in endovascular interventions. Meidicinskij vestnik Uga Rossii / Medical Herald of the South of Russia. 2015; 4: 68-71. (in Russian)]. https://dx.doi.org/10.21886/2219-8075-2015-4-68-71.
Received 27.02.2020
Accepted 24.06.2020
About the Authors
Alexander V. Kuzmenko, PhD, Ass. Professor, Department of human anatomy, Gomel State Medical University. Tel.: +375 33 3417868. E-mail: alexxx3800@mail.ru.ORCID: 0000-0002-0116-7481.246000, Republic of Belarus, Gomel, Lange Str., 5.
Michail G. Shkvarko, PhD, Ass. Professor, Department of human anatomy, Gomel State Medical University. Tel.: +375 29 2126274. E-mail: miskv@mail.ru.
ORCID: 0000-0003-3264-2510.246000, Republic of Belarus, Gomel, Lange Str., 5.
For citation: Kuzmenko A.V., Shkvarko M. G. Surgical Anatomy of the Extraorganic Anastomoses of the Uterine Artery.
Akusherstvo i Ginekologiya / Obstetrics and Gynecology. 2020; 7: 117-121 (in Russian)
https://dx.doi.org/10.18565/aig.2020.7.117-121



