Бесплодие – это неспособность сексуально активной, не использующей контрацепцию пары добиться беременности в течение одного года [1]. Согласно сводным данным литературы, частота бесплодия в мире колеблется от 8 до 29% [2]. В Европе бесплодными являются около 10% супружеских пар, в США – 8–15%, в Канаде – около 17% [3]. Доля бесплодных браков на территории России колеблется от 8 до 21% и в настоящее время не имеет тенденции к снижению [2].
По данным службы государственной статистики в 1995 году был зафиксирован минимальный показатель (52 случая бесплодия на 100 тыс. населения) [4].
К 2012 году этот показатель увеличился в 4,4 раза, составив 227 женщин на 100 тыс. населения [5], в 2016 году – 278 женщин на 100 тыс. населения [6]. В ответ на рост количества бесплодных пар быстрый прогресс в репродуктивной медицине и многочисленные исследования в данной сфере позволили значительно развить новейшие технологии в решении данной проблемы по всему миру.
Вспомогательные репродуктивные технологии (ВРТ) оказывают существенное влияние на демографические, социальные и экономические показатели за счет потенциального увеличения объема трудовых ресурсов в экономике.
Указом Президента РФ была утверждена концепция демографической политики на период до 2025 г. [7]. Одним из пунктов концепции значилась разработка и реализация системы мер по медицинской профилактике и лечению бесплодия. В 2007 г. были бесплатно проведены первые 500 процедур экстракорпорального оплодотворения (ЭКО). Количество проведенных процедур увеличивалось с каждым годом, составив в 2016 г. 47,5 тыс. В 2017 г. Министерство здравоохранения РФ профинансировало 65 тыс. циклов ЭКО в рамках обязательного медицинского страхования [8]. Всего по данным регистра на территории РФ в период с 1995 по 2015 г. проведено более 740 тыс. циклов ВРТ, из которых 160 тыс. закончились рождением ребенка [9].
В целом, протоколы отличаются друг от друга длительностью стимуляции и разными лекарственными средствами (ЛС), применяемыми для стимуляции суперовуляции. Важную роль в достижении результата ЭКО играет рациональный выбор ЛС. В настоящее время используются рекомбинантные и менопаузальные гонадотропины, так как они зарекомендовали себя как наиболее эффективные средства.
Существует особая подгруппа женщин, у которых лечение методами ВРТ зачастую не позволяет добиться наступления желанной беременности, в частности, у пациенток с так называемым «бедным ответом». Эксперты ESHRE пришли к соглашению, что к группе потенциально «бедного ответа» могут быть отнесены женщины, у которых имеются как минимум 2 из 3 критериев: 1. возраст ≥40 лет либо любой другой фактор риска «бедного ответа» (резецированные яичники и др.); 2. «бедный ответ» на стандартную (обычную) стимуляцию яичников в анамнезе (≤3 ооцитов при использовании стандартного протокола стимуляции); 3. снижение показателей маркеров овариального резерва, таких как AFC 5–7 фолликулов или AMH 0,5–1,1 нг/мл [10].
В настоящее время обсуждается расширение определения понятия «бедный ответ», а именно «недостаточный ответ», с тем, чтобы охватить большую группу пациентов молодого возраста (<35 лет) с показателями AFC 5–9 [11]. Чтобы добиться полноценного роста фолликулов у данных пациенток, традиционно используют длинные протоколы стимуляции с высокой суммарной дозой ФСГ >3000 МЕ на цикл, однако существует и альтернативная точка зрения [12]. Основным решением в таком случае является добавление ЛГ, который может повысить чувствительность яичников к ФСГ; способствовать секреции эстрадиола предовуляторным фолликулом, улучшая тем самым рост эндометрия; защитить клетки кумулюса от апоптоза, в результате чего они поддерживают развитие ооцита вплоть до овуляции и обеспечивают ранние этапы оплодотворения; стимулировать позднюю лютеинизацию фолликула и выработку достаточного количества прогестерона [13]. Доказательство того факта, что вышеизложенный подход обладает большей эффективностью, нашло подтверждение в систематическом мета-анализе Lehert и соавт. [14].
Целью настоящего исследования является определение преимущественного с точки зрения фармакоэкономического анализа лекарственного препарата для лечения бесплодия у больных с недостаточным ответом яичников.

Материал и методы исследования
Для проведения фармакоэкономического исследования в соответствии с вышеизложенной целью был произведен информационный поиск по наличию публикаций, соответствующих теме настоящего исследования, по базам данных PubMed, Medlink, Cochrane Library, в результате которого было установлено, что в настоящее время доступно ограниченное количество исследований, посвященных лечению бесплодия у женщин с субоптимальным ответом яичников, проведенных на большой выборке пациентов.
Всего было найдено 134 статьи и тезиса. Для дальнейшего анализа были отобраны 2 публикации: открытое проспективное рандомизированное контролируемое исследование Ferraretti и соавт. [15] и открытое рандомизированное исследование Carone и соавт. [16]. Статьи, на основании которых проводится анализ, являются уникальными по содержанию и дизайну исследования. Эти работы рассматривали отдельно.

Результаты исследования и обсуждение
В данных статьях группы пациентов были сопоставимы по возрасту, числу ранее неудачных циклов, способу стимуляции, средней длительности терапии, что позволило провести непрямое наивное сравнение. Критериями эффективности в исследовании были: частота имплантации, частота наступления клинической беременности и частота живорождения.
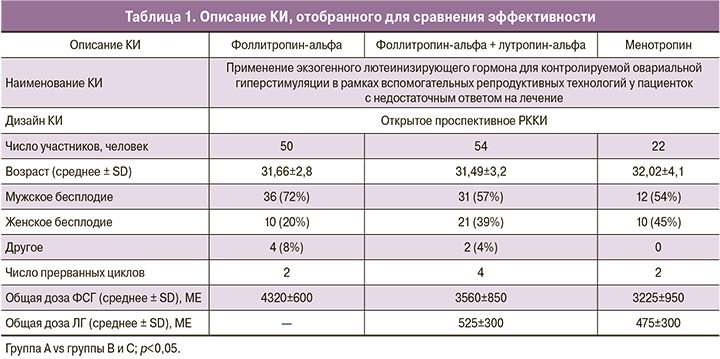
Описание клинического исследования (КИ) изложено в табл. 1.
Во втором исследовании впервые были проанализированы результаты 3 последовательных стимуляций у женщин с первичной гипоталамо-гипофизарной недостаточностью (первичный гипогонадизм) [16].
 Описание КИ изложено в табл. 2.
Описание КИ изложено в табл. 2.
Авторами была разработана математическая фармакоэкономическая модель. Горизонт моделирования был равен 1 году на анализируемых схемах лечения. Графическое представление математического аппарата представлено на рис. 1.
Ввиду разнящихся данных о распространенности бесплодия на территории Российской Федерации авторами было сделано допущение о количестве бесплодных женщин репродуктивного возраста, составляющем 10% женщин репродуктивного возраста. Численность группы на основании распространенности бесплодия среди женщин репродуктивного возраста со сниженным овариальным резервом на территории РФ составила 807 168 человека [17–19]. Полученное число является относительным, поскольку рассчитано исходя из процентного соотношения бесплодных женщин репродуктивного возраста с субоптимальным ответом проживающих на территории РФ. Более точные сведения о распространенности субоптимального ответа можно получить из отчетов Российской ассоциации репродукции человека (РАРЧ). Регистр РАРЧ ведется с 1995 года, и к 2015 году имеет сведения о 747 396 проведенных циклов ВРТ [9]. Вводными данными в исследовании использовано количество свежих циклов, включающих: циклы ЭКО, ИКСИ и свежие циклы ЭКО и ИКСИ с преимплантационной генетической диагностикой (ПГД). Суммарно количество свежих циклов за 2015 год составило 77 469 циклов [9]. Настоящая модель предусматривает возможность выбора данных для расчета: по среднестатистическим показателям или по данным отчета РАРЧ за 2015 г. (табл. 3).
Анализ эффективности
При определении критериев эффективности необходимо использовать конечные точки, поскольку они обладают наивысшей степенью убедительности в фармакоэкономическом анализе. К таковым относятся следующие критерии: добавленные годы жизни (LYG), год качественной жизни (QALY), медиана выживаемости (MS) и др. [20]. Однако ни в одном исследовании по данной теме не были использованы такие критерии. Поэтому критериями эффективности в настоящем исследовании были выбраны наиболее весомые аргументы, свидетельствующие об эффективности ВРТ: частота имплантации, частота наступления клинической беременности и частота живорождения [9, 12]. Источниками данных об эффективности в проведенном исследовании стали РККИ Ferraretti и соавт. [15] и Carone и соавт. [16]. Результаты исследований приведены в табл. 4, 5.
Анализ данных выявил статистически значимую разницу по трем исследуемым критериям в пользу группы, получавшей терапию рФСГ+рЛГ.
Анализ выявил, что при стимуляции рекомбинантной терапией частота наступления беременности в пересчете на цикл составляет 55,55%, а при стимуляции чМГ – 23,25% (p<0,05). Несмотря на сравнительно небольшое количество пациентов, вошедших в исследование, полученные результаты статистически значимы.
 Анализ затрат
Анализ затрат
В ходе проведения исследования было выявлено, что протоколы лечения, способы и кратность введения лекарственного препарата (ЛП), лабораторные исследования, необходимые для контроля за состоянием пациента, не отличаются при использовании сравниваемых альтернатив. Затраты на купирование побочных явлений, вызванных приемом препаратов, стимулирующих яичники, не учитывали. Итоговое значение анализа затрат складывалось из стоимости ЛП, необходимого на один курс стимуляции.
В качестве источника информации о ценах на препараты для лечения бесплодия были использованы данные, предоставленные компанией IMS Health Россия (IMS), представляющие собой усредненные стоимости по ценам тендеров за три квартала 2017 года. Результаты анализа затрат на закупку ЛП представлены в рис. 2.
Анализ «затраты-эффективность»
Анализ «затраты-эффективность» проводился с целью получения данных по удельной цене единицы эффективности применения анализируемых режимов лечения. При этом использовались результаты предыдущих блоков исследования (анализ затрат и анализ эффективности).
В проведенном анализе были получены следующие результаты анализа «затраты-эффективность» (табл. 6).
Так как ЛП перговерис характеризуется лучшей эффективностью и меньшим значением коэффициента «затраты-эффективность», то по результатам анализа затраты-эффективность ЛП перговерис является доминантным (строго предпочтительным).
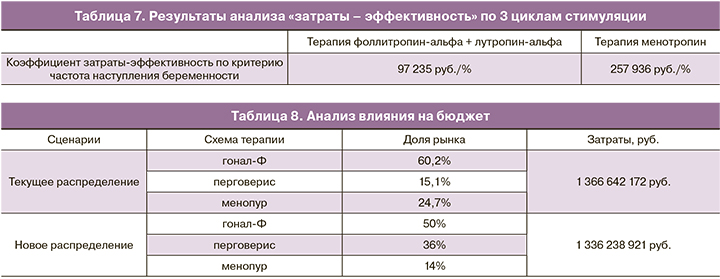
Исходя из данных, полученных авторами статьи Carone и соавт., были получены результаты анализа «затраты-эффективность» 3 циклов стимуляции (табл. 7).
Анализ «влияния на бюджет»
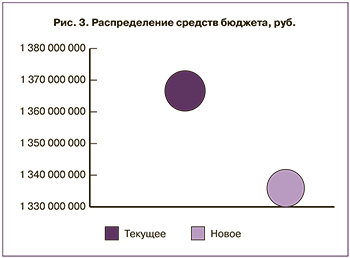
Был проведен анализ влияния на бюджет с точки зрения системы здравоохранения для лечения бесплодия по двум сценариям – текущее и новое распределение средств. Данные сценарии предусматривают возможность регулировать долю пациентов на той или иной схеме терапии, а также в модели задать число больных. Расчеты приведены на количество циклов с субоптимальным ответом по данным регистра РАРЧ за 2015 г. Временной горизонт анализа влияния на бюджет составил 1 год. Источником данных о текущем распределении средств служили данные, предоставленные компанией IMS Health Россия. Результаты проведенного анализа представлены в табл. 8.
Распределение средств при текущем и новом распределении отражено на рис. 3.
Анализ влияния на бюджет продемонстрировал, что при перераспределении доли закупок ЛП перговерис с 15,1 до 36% для лечения бесплодия в РФ, будет достигнута экономия в размере 30 403 251 руб. за счет более низкой стоимости терапии.
Анализ чувствительности
В рамках данного фармакоэкономического исследования был проведен анализ чувствительности с целью определить степень устойчивости полученных результатов при изменении исходных параметров.
Однофакторный анализ чувствительности проводили по трем критериям эффективности: частота имплантации, частота наступления беременности, частота живорождения оценивая изменения исходных параметров на величину от -10% до 10%. Проведенный анализ чувствительности продемонстрировал стабильность полученных в ходе фармакоэкономического анализа данных.
Заключение
По результатам фармакоэкономического анализа фоллитропин-альфа + лутропин-альфа доминирует с точки зрения анализа «затраты-эффективность» и приводит к экономии денежных средств при лечении бесплодия у пациентов с субоптимальным ответом яичников.



