Effectiveness of IVF in treating infertility in patients with internal endometriosis (adenomyosis)
Objective. To evaluate the effectiveness of IVF treatment in patients with various stages of adenomyosis.Aksenenko A.A., Ibragimova M.Kh., Gavisova A.A., Mishieva N.G.
Materials and methods. The study included 128 infertile patients after IVF failures; the patients were diagnosed with adenomyosis which was considered one of possible causes of implantation failure in previous IVF attempts. The patients were randomly divided into two groups. The patients of the first group underwent ovarian stimulation with GnRH-antagonists. The patients of the second group received IVF treatment using GnRH-agonist long protocol.
Results. The presence of adenomyosis and its stage do not affect the functional activity of the ovaries. Indicators of the ovarian reserve, parameters of folliculogenesis and embryogenesis in IVF programs are influenced by the state of the ovarian reserve and the woman’s age, but not by the presence of adenomyosis. The use of GnRH-antagonist protocols and GnRH-agonist long protocols in ovarian stimulation showed the same effectiveness in patients with internal endometriosis.
Conclusion. Pregnancy rate in IVF programs and reproductive losses can be affected by the stage of adenomyosis, which makes it possible to consider moderate-severe and severe stages of adenomyosis as the uterine factor infertility characterized by impaired implantation in the endometrium.
Keywords
The term adenomyosis currently refers to a disease which is morphologically manifested by the invasion (introduction) of the glandular and stromal components of the basal layer of the endometrium into the underlying myometrium [1]. In the 10th revision of the International Statistical Classification of Diseases (ICD-10), the section Endometriosis (code N80) contains a separate part Endometriosis of uterus (code N80.0), which can be considered completely analogous to adenomyosis. In some classifications of endometriosis of the Russian scientists, the term internal endometriosis is also used to refer to its localization in the body of the uterus [2]. Since adenomyosis and other forms of endometriosis were included in the ICD-10 in one nosological group, it is important to emphasize their common histological features, namely, the presence of endometrioid-like glands and the cytogenic basis in the pathological foci.
Nowadays, many researchers consider adenomyosis as an independent disease, separating it from other forms of endometriosis [3].
Adenomyosis is not only one of the main causes of algodismenorrhea, but it is also often associated with unexplained infertility and habitual miscarriage [4]. The decrease in the likelihood of successful conception in patients with adenomyosis is due to a lag in endometrial maturation in the follicular phase and a decrease in its receptivity, which, according to many researchers, is associated with defects in estrogen receptors of the endometrium [5, 6]. Habitual miscarriage in patients with adenomyosis is caused by increased rigidity of the uterine walls and vascular disorders that prevent from providing adequate endometrial trophicity in the gestational period and contribute to the development of placental insufficiency [7].
It should be noted that to date, the diagnostic criteria for adenomyosis and the stages of the pathological process are not completely identified, and some experts believe that an accurate diagnosis of the process is possible only with a histological examination of the removed uterus [8, 9]. However, according to other authors [10-13] who specialize in the field of ultrasound diagnostics, the use of modern ultrasound equipment makes it possible to identify quite successfully the patients with various stages of adenomyosis among the entire population of women with this disease (including the patients with stage I of the disease).
The attempts to treat infertility in patients with adenomyosis using anti-estrogen therapy and subsequent natural conception, in combination with ovarian stimulation or without it, seldom produce the desired result [14–16]. Therefore, infertile patients with adenomyosis have to be treated for infertility using IVF. But it is worth noting that the literature reports on the degree of negative impact of adenomyosis on the effectiveness of IVF are quite contradictory and do not take into account the dependence of treatment results on the severity of the disease [17–20]. The methods for preparing patients with various forms of adenomyosis for IVF programs have not been defined; there are no clear recommendations on the type of preparation and its duration; the effectiveness of drug treatment has not been evaluated either [21, 22].
Thus, despite the numerous theories of the origin and development of adenomyosis, the issues of the pathogenesis of the disease and its impact on the reproductive function remain understudied. The question about the influence of various methods of the treatment and rehabilitation on the restoration of generative function and the effectiveness of IVF programs continues to be relevant.
The aim of the study was to evaluate the effectiveness of IVF treatment in patients with various stages of adenomyosis.
Materials and Methods
The study included 128 infertile patients diagnosed with adenomyosis which was considered to be one of possible causes of implantation failure in previous IVF attempts. The age of women ranged from 22 to 38 years; the median was 34.5 (28; 35) years. All patients had a history of IVF attempts, ranging from one to three; pregnancy did not occur in 94% of women, and missed miscarriage was found in 6% of women.
The assumption was based on the following facts: the formation from 5 to 18 oocytes and from 2 to 13 embryos of good and excellent quality at the blastocyst stage in all patients; the absence of autoimmune, infectious and endocrine diseases in the partners, which could negatively affect the results of the treatment. The ultrasound examination was performed twice and indicated the signs of adenomyosis: the thickness of the uterus was from 4.7 to 7.2 cm; differences in the thickness of the uterine walls ranged from 0.3 to 2.2 cm; there was an increased hyperechogenicity of the myometrium of different thickness and the presence of anechogenic inclusions in the myometrium.
The signs of different stages of severity were present in all patients.
The criteria for inclusion in the study were reproductive age of patients (from 22 to 38 years), the recorded parameters of the ovarian reserve, the signs of adenomyosis revealed by ultrasound assessment, IVF failures in the history, and the possibility of IVF or ICSI fertilization after the proper results of the semen analysis.
The exclusion criteria were contraindications and limitations to the use of assisted reproductive technologies, regulated by Order No. 107n of the Ministry of Health of the Russian Federation dated 2012; late reproductive age and extremely low indicators of ovarian reserve; severe forms of pathozoospermia in men.
According to the method of ovarian stimulation in the IVF program, the patients were randomly divided into two groups: group I included 68 women who underwent ovarian stimulation with gonadotropin releasing hormone antagonists (GnRH-antagonists); group II consisted of 60 patients who received IVF treatment using gonadotropin releasing hormone agonists long protocol (GnRH – agonists).
In each of the groups, there was approximately the same number of women with different stages of adenomyosis: stage I (mild form) included 44 and 42 patients (64.7% and 70%, respectively); stage II (moderate form of diffuse endometriosis) consisted of 20 and 16 patients (29.4% and 26.6%, respectively); stage III (severe stage of diffuse nodular adenomyosis) included 4 and 2 patients (5.9% and 3.4%, respectively). The stage of adenomyosis was diagnosed using the signs of ultrasound assessment and classification of adenomyosis presented in the work of V.N. Demidov, A.K. Khachatryan, and A.I. Gus (1997) [23].
Diffuse adenomysis stage I:
- the thickness of the endometrium presenting thin transverse anechoic bands extending from the basal layer to the middle of the M-echo, as well as anechoic cysts in the myometrium and at the border of the endometrium and myometrium;
- increased hyperechogenicity of the myometrium of various thicknesses; the presence of anechoic cysts in the myometrium;
- uneven thickness of the basal layer of the endometrium and uneven contours of the endometrium along the basal plate, which looks like a hypoechoic defect of the endometrium; the myometrium behind the basal plate is not changed;
- the uterus is usually of normal size, but different thicknesses of the anterior and posterior walls of the uterus may be detected;
- the appearance of zones of decreased echogenicity in the endometrium and small hyperechoic formations in the thickness of the myometrium near the endometrium.
Diffuse adenomyosis stage II:
- increase in the thickness of the uterus that exceeds the upper normal level;
- thickening of one of the uterine walls compared to the other by 4 mm or more;
- the appearance of zones with areas of heterogeneous (increased and decreased) echogenicity of various sizes in the myometrium located closer to the uterine cavity;
- the presence of a small rounded anechoic formation with a diameter of 2–5 mm inside the zone of increased echogenicity;
- the appearance of anechoic cavities of various shapes and sizes, containing fine particles (blood), and sometimes dense inclusions of low echogenicity (blood clots);
- the thickness of the endometrium is usually reduced and does not correspond to the day of the menstrual cycle (due to the compression of the endometrium by the altered myometrium);
- the appearance of frequent transverse striation with moderate and low echogenicity at the place of pathology;
- the effect of ultrasonic wave attenuation behind the pathology.
Diffuse adenomyosis stage III:
- a significant increase in the uterus, mainly its anterior-posterior size;
- thickening of one of the uterine walls compared to the other by 10 mm or more;
- the presence of a zone of increased heterogeneous echogenicity in the myometrium, which occupies more than half of the thickness of the uterine wall;
- the detection of a zone of increased echogenicity in the area of the leading edge of the scan and detection of an anechoic zone in the area of the far front.
The ovarian reserve of the patients was assessed taking into account the levels of follicle-stimulating hormone (FSH) and Anti-Müllerian hormone (AMH) in the blood plasma, performing an ultrasound assessment and counting the number of antral follicles in each ovary. Before starting the IVF treatment, all patients were examined in accordance with the Order of the Ministry of Health of the Russian Federation, No.107n, dated 2012. Clinical, laboratory and instrumental methods of examination were used in the study. IVF programs with GnRH-agonists and GnRH-antagonists were carried out according to the general rules; the dose of gonadotropins was determined depending on the parameters of the ovarian reserve. Human chorionic gonadotropin at a dose of 10,000 units was used as a trigger for the final maturation of oocytes. The number of mature oocytes, fertilization, and the number of obtained blastocysts were evaluated.
Statistical analysis
Statistical processing of the data was performed using Statistica 10 software package (StatSoft Inc., USA). The median and quartile range of Me (Q1; Q3) were used to describe the quantitative variables (since it was not possible to do the normality test due to the small sample size), and the qualitative absolute values and percentages were used. The Pearson χ2 test for twofold or fourfold contingency tables was used for the data processing in the study (the prevalence of variables in the analyzed groups was compared). The comparative analysis of the variables was performed using the nonparametric Kruskal–Wallis and Mann–Whitney tests for independent combinations. The differences were considered statistically significant at the level of p<0.05.
Results and Discussion
One of the aims of the present study was to assess the ovarian reserve in the patients of the study groups. These data are presented in Tables 1 and 2.
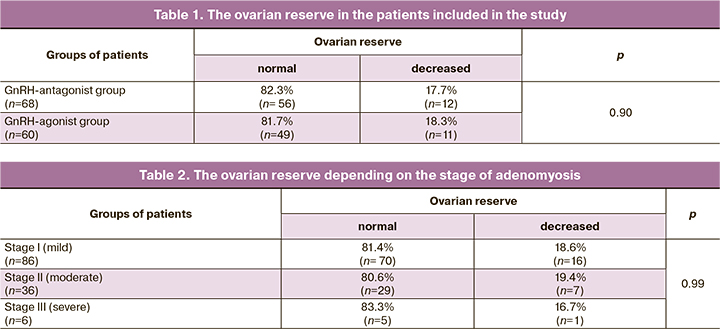
The presented data in Table 1 demonstrate the absence of differences in the ovarian reserve in the patients of the study groups. Most of them had the parameters of the ovarian reserve corresponding to the standard indicators of the patients at the age of 31 (26; 35) years. Women with the indicators of decreased ovarian reserve were older than 37 (37; 38) years, which is quite natural for the situation. At the same time, a number of researchers note that endometriosis, not only endometrioid cysts that occupy a certain area of the ovaries, but also small forms of external genital endometriosis, have a negative impact on the ovarian reserve, contributing to its decrease [24–27].
To assess the impact of the severity of the pathological process on the function of the ovaries, we analyzed the parameters of the ovarian reserve at different stages of the disease. These data are presented in Table 2.
The obtained data confirmed the absence of the influence of adenomyosis stage on the ovarian reserve and allowed us to conclude that the function of the ovaries is not affected in adenomyosis, unlike other forms of endometriosis. Perhaps pathogenesis of adenomyosis is different from that of external genital endometriosis. Moreover, it can be assumed that in severe forms of adenomyosis, infertility and IVF failures can be associated with the absence of implantation, which in turn makes it possible to classify the severe stages of adenomyosis as uterine forms of infertility.
Some clinicians believe that the use of GnRH-agonist long protocol should be prioritized during ovarian stimulation in IVF programs. We have tried to compare GnRH-antagonist protocols and GnRH-agonist long protocols. One should remember that the ovarian reserve was not different in the patients of the study groups. Table 3 shows the characteristics of the stimulation protocols.
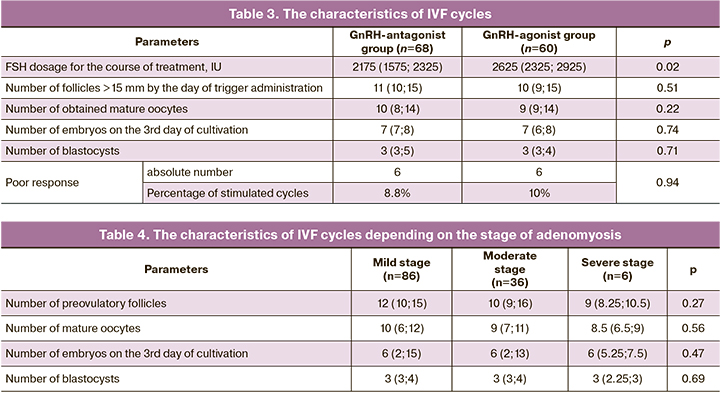
According to the data presented, the dose of FSH for the course of treatment was different, all other indicators were not different. However, the use of large dose of gonadotropins is typical when using GnRH-agonist long protocol. Therefore, the results of the study contradict the opinion that it is necessary to use GnRH-agonist long protocol in patients with endometriosis. In this case, the stimulation protocol should be chosen based on the individual characteristics of the patient and the doctor’s decision.
It was interesting to analyze the parameters of ovarian stimulation depending on the stage of adenomyosis. These data are presented in Table 4.
The obtained data did not reveal any differences in the parameters of the stimulated cycle in patients with different stages of adenomyosis, which proves the absence of the influence of adenomyosis on the functional activity of the ovaries.
Pregnancy rate and reproductive losses in the groups did not depend on the ovarian stimulation protocol, and it was 33.5% when using GnRH-antagonists and 34.2% when using GnRH-agonist protocols. The rate of biochemical pregnancies and missed miscarriages in the early stages (up to 7 weeks) was 13.6% in group I, and it was 12.9% in group II; these results did not differ between the groups and corresponded to the average statistical data presented in the records of RAHR for 2018 [28].
However, the analysis of the data on the rate of progressive pregnancies differed depending on the stage of the endometrioid process. Thus, at stage I (mild), pregnancy occurred in 35 out of 86 women, which was 40.7%, only two of them resulted in missed miscarriages. At stage II (moderate), pregnancy occurred in 11 patients, which was 30.6%, while the proportion of missed miscarriages increased to 14.5% in women who became pregnant. Patients with severe adenomyosis did not become pregnant in any case.
The presented data clearly demonstrate the role of the stage of adenomyosis in the frequency of implantation and rate of progressive pregnancy. When the number of the obtained embryos was the same, the frequency of implantation and reproductive losses was dramatically different. The high rate of pregnancies including progressive ones in the study groups can be due to the fact that the majority of the patients were women with the first, mild stage of adenomyosis, which does not affect the rate of pregnancy and gestation.
Conclusion
All the obtained data allows us to draw the following conclusions:
1. Adenomyosis and its stage do not affect the functional activity of the ovaries and the indicators of the ovarian reserve, which can be suggestive of a different genesis of the pathological process with different localization of endometriosis.
2. The use of GnRH-antagonist protocols and GnRH-agonist long protocols in ovarian stimulation showed the same effectiveness in patients, namely in the number of obtained mature oocytes and blastocysts of good quality.
3. The stage of adenomyosis does not affect the parameters of folliculogenesis and embryogenesis, which are determined by the ovarian reserve and the age of the woman.
4. Pregnancy rate and reproductive losses can be affected by the stage of adenomyosis, which makes it possible to consider moderate and severe stages of adenomyosis as the uterine factor infertility characterized by impaired implantation in the endometrium, whereas mild stage adenomyosis does not affect the effectiveness of IVF programs.
References
- Адамян Л.В., Кулаков В.И., Андреева Е.Н. Эндометриозы. Руководство для врачей. М.: Медицина; 2006. 410с. [Adamyan L.V., Kulakov V.I., Andreeva E.N. Endometriosis: A Guide for Physicians. M.: Medicine; 2006. 410 р. (in Russian)].
- Кулаков В.И., Манухин И.Б., Савельева Г.М., ред. Гинекология. Национальное руководство. М.: ГЭОТАР-Медиа; 2011. 1088с. [Kulakov V.I., Manukhin I.B., Savelyeva G.M., ed. Gynecology. National leadership. M.: GEOTAR-Media; 2011. 1088 р. (in Russian)].
- Баскаков В.П., Цвелев Ю.В., Кира Е.Ф. Эндометриоидная болезнь. СПб.: Изд-во Н-Л; 2002. 448с. [Baskakov V.P., Tsvelev Yu.V., Kira E.F. Endometrioid disease. SPb: Publishing house N-L. 2002; 448 р. (in Russian)].
- Линде В.А., Татарова Н.А. Эндометриозы: патогенез, клиническая картина, диагностика и лечение. М.: ГЭОТАР-Медиа; 2010. 189с. [Linde V.A., Tatarova N.A. Endometriosis. Pathogenesis, clinical picture, diagnosis and treatment. M.: GEOTAR-Media; 2010. 189 p. (in Russian)].
- Carroll J.S., Brown M. Estrogen receptor target gene: an evolving concept. Mol. Endocrinol. 2006; 20(8): 1707-14. https://dx.doi.org/10.1210/me.2005-0334.
- Ellmann S., Sticht H., Thiel F., Beckmann M.W., Strick R., Strissel P.L. Estrogen and progesterone receptor: from molecular structures to clinical targets. Cell. Mol. Life Sci. 2009; 66(15): 2405-26. https://dx.doi.org/10.1007/s00018-009-0017-3.
- Абрамченко В.В. Беременность и роды высокого риска. М.: МИА; 2004. 400с. [Abramchenko V.V. High-Risk Pregnancy and Childbirth: A Guide for Physicians. M.: Medical Information Agency; 2004. 400 p. (in Russian)].
- Дамиров М.М. Генитальный эндометриоз – болезнь активных и деловых женщин. М.: БИНОМ; 2010. 192с. [Damirov M.M. Genital endometriosis is a disease of active and business women. M.: Publishing house BINOM-Press; 2010. 192 р. (in Russian)].
- Рухляда Н.Н. Диагностика и лечение манифестного аденомиоза. СПб.: Медкнига «ЭЛБИ»; 2004. 204с. [Rukhlyada N.N. Diagnostics and treatment of manifest adenomyosis. SPb: ELBI-SPb; 2004. 204 р. (in Russian)].
- Демидов В.Н., Адамян Л.В., Хачатрян А.К. Ультразвуковая диагностика эндометриоза. II. Внутренний эндометриоз. Ультразвуковая диагностика в акушерстве, гинекологии и педиатрии. 1996; 1: 32-42. [Demidov V.N., Adamyan L.V., Khachatryan A.K. Ultrasound diagnostics of endometriosis. II Internal endometriosis. Ultrasound diagnostics. 1996; 1: 32-42.(in Russian)].
- Митьков В.В., ред. Клиническое руководство по ультразвуковой диагностике. Том 3. М.: Видар-М; 2005: 105-10. [Mitkov V.V., ed. Clinical guidelines for ultrasound diagnostics. Vol. 3. M.: Publishing house Vidar-M; 2005: 105-10. (in Russian)].
- Труфанов Г.Е., Рязанов В.В., Багненко С.С. Ультразвуковая диагностика. Руководство для врачей. СПб.: ФОЛИАНТ; 2009. 800с. [Trufanov G.E., Ryazanov V.V. Ultrasound Diagnostics: A Guide for Physicians. SPb: FOLIANT Publishing House; 2009. 800 р. (in Russian)].
- Абдуллаев Р.Я., Головко Т.С. Ультрасонография. Харьков: Новое слово; 2009. 180с. [Abdullaev R.Ya., Golovko T.S. Ultrasonography. Kharkiv: New Word; 2009.180 p. (in Russian)].
- McWilliams G.D., Frattarelli J.L. Changes in measured endometrial thickness predict in vitro fertilization success. Fertil. Steril. 2007; 88(1): 74-81. https://dx.doi.org/10.1016/j.fertnstert.2006.11.089.
- Ng E.H., Chan C.C., Tang O.S., Yeung W.S., Ho P.C. Changes in endometrial and subendometrial blood flow in IVF. Reprod. Biomed. Online. 2009; 18(2): 269-75. https://dx.doi.org/10.1016/s1472-6483(10)60265-9.
- Бурлев А.В., Кузьмичев Л.Н., Онищенко А.С., Ильясова Н.А., Щетинина Н.С. Функциональная активность эндометрия влияет на результаты ЭКО и перенос эмбрионов: молекулярные механизмы регуляции фертильности. Проблемы репродукции. 2010; 16(2): 41-52. [Burlev A.V., Kuzmichev L.N., Onishchenko A.S. et al. The functional activity of the endometrium affects the results of IVF and embryo transfer: molecular mechanisms of the regulation of fertility. Problems of reproduction. 2010; 16(2): 41-52.(in Russian)].
- Batt R., Yeh J. Mullerianosis: four developmental (embryonic) müllerian diseases. Reprod. Sci. 2013; 20(9): 1030-7. https://dx.doi.org/10.1177/1933719112472736.
- Carmona F., Martínez-Zamora A., Bassols M.L., Balasch J. Environmental influences on the development of endometriosis. J Endometriosis and Pelvic Pain Disorders (JEPPD). Endometriosis. 2013; 5(2): 49-61. https://dx.doi.org/10.5301/je.5000153.
- Dhesi A., Morelli S. Endometriosis: a role for stem cells. Womens Health (Lond). 2015; 11(1): 35-49. https://dx.doi.org/10.2217/whe.14.57.
- Morelli S., Rameshwar P., Goldsmith L.T. Experimental evidence for bone marrow as a source of nonhematopoietic endometrial stromal and epithelial compartment cells in a murine model. Biol. Reprod. 2013; 89(1): 7. https://dx.doi.org/ 10.1095/biolreprod.113.107987.
- Khan K.N., Kitajima M., Hiraki K., Fujishita A., Sekine I., Ishimaru T. et al. Changes in tissue inflammation, angiogenesis and apoptosis in endometriosis, adenomyosis and uterine myoma after GnRH agonist therapy. Hum. Reprod. 2010; 25(3): 642-53. https://dx.doi.org/10.1093/humrep/dep437.
- Lagana'A.S., Condemi I., Retto G., Muscatello M.R., Bruno A., Zoccali R.A. et al. Analysis of psychopathological comorbidity behind the common symptoms and signs of endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015: 194: 30-3. https://dx.doi.org/10.1016/j.ejogrb.2015.08.015.
- Демидов В.Н., Гус А.И., Адамян Л.В., Хачатрян А.К. Эхография органов малого таза. Эндометриоз: Практическое пособие. М.: ООО «Форза»; 2010. 64 с. [Demidov V.N., Gus A.I., Adamyan L.V., Khachatryan A.K. Ultrasound diagnostics of the pelvic organs. Endometriosis: A Guide for Physicians. M.; LLS "Forza"; 2010. 64 p. (in Russian)].
- Salamansen L.A., Hannan N.J., Dimitriadis E. Cytokines and chemokines during human embryo implantation: roles in implantation and early placentation. Semin. Reprod. Med. 2007; 25(6): 437-44. https://dx.doi.org/10.1055/s-2007-991041.
- Muzii L., Bianchi A., Croce C., Manci N., Panici P.B. Laparoscopic excision of ovarian cysts: is the stripping technique a tissue-sparing procedure? Fertil. Steril. 2002; 77(3): 609-14. https://dx.doi.org/10.1016/s0015-0282(01)03203-4.
- Kitajima M., Defrere S., Dolmans M.M., Colette S., Squifflet J., Van Langendonckt A. et al. Endometriomas as a possible cause of reduced ovarian reserve in women with endometriosis. Fertil. Steril. 2011; 96(3): 685-91. https://dx.doi.org/10.1016/j.fertnstert.2011.06.064.
- Uncu G., Kasapoglu I., Ozerkan K., Seyhan A., Yilmaztepe A.O., Ata B. Prospective assessment of the impact of endometriomas and their removal on ovarian reserve and determinants of the rate of decline in ovarian reserve. Hum. Reprod. 2013; 28(8): 2140-5. https://dx.doi.org/10.1093/humrep/det123.
- Корсак В.С., ред. Регистр ВРТ РАРЧ. Отчет за 2018 год. СПб.; 2020. 55с. [Korsak V.S., ed. Register of ART RARCH. Report for 2018. St. Petersburg; 2020. 55 р. (in Russian)].
Received 09.11.2020
Accepted 10.12.2020
About the Authors
Artem A. Aksenenko, gynecologist of the 1st Gynecology Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. E-mail: a_axenenko@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.Muminat Kh. Ibragimova, Ph.D., gynecologist of the 1st Gynecology Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. E-mail: m_ibragimova@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Alla A. Gavisova, Ph.D., Senior Researcher of the 1st Gynecology Department, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. E-mail: gavialla@yandex.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Nona G. Mishieva, PhD, Senior researcher of 1st gynecology department, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. Tel.: +7(495)438-26-22. E-mail: nondoc555@mail.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
For citation: Aksenenko A.A., Ibragimova M.Kh., Gavisova A.A., Mishieva N.G. Effectiveness of IVF in treating infertility in patients with internal endometriosis (adenomyosis).
Akusherstvo i Ginekologiya / Obstetrics and gynecology. 2021; 1: 120-125 (in Russian)
https://dx.doi.org/10.18565/aig.2021.1.120-125



