Привычная потеря беременности ранних сроков (привычный выкидыш) представляет собой актуальную проблему современного акушерства. Установлено, что женщины с привычным выкидышем составляют группу риска, в которой существенно повышена вероятность развития плацентарной недостаточности, наступления ранних преждевременных родов, задержки роста плода, излития околоплодных вод при недоношенном сроке беременности, отслойки плаценты во втором и третьем триместрах беременности [1, 2].
Поэтому в настоящее время наличие даже двух последовательных выкидышей от одного партнера рассматривается как основание для постановки диагноза «привычный выкидыш», последующего обязательного обследования, в том числе и иммунологического, и оптимизации подготовки к беременности [3].
Особое место занимает идиопатический привычный выкидыш (от 5 до 20% в структуре невынашивания), этиологию которого на настоящий момент связывают с нарушениями функционирования материнской иммунной системы, развивающей неадекватный ответ на отцовские антигены плода, то есть с аллоиммунными причинами [4]. По данным зарубежных авторов, шанс доносить беременность без терапии после трех выкидышей, обусловленных аллоиммунными механизмами, составляет 30%, после четырех – 25%, после пяти – 5% [5, 6]. Своевременная диагностика и назначение иммунокорригирующей терапии составляет основу предгестационной подготовки пациенток с идиопатическим привычным выкидышем.
Одним из вариантов иммуномодулирующей терапии является иммуноцитотерапия (ИЦТ) – иммунизации женщин с привычным выкидышем аллогенными лимфоцитами партнеров. Показано, что ИЦТ в предгестационной подготовке способствует формированию состояния иммунной системы, необходимого для успешной имплантации, а во время беременности – для стимуляции развития плаценты и обеспечения нормального развития эмбриона [7, 8]. ИЦТ широко используется в лечении привычного выкидыша как в России [9, 10], так и за рубежом [11, 12], однако механизмы влияния аллоиммунизации на имплантацию и последующую гестацию остаются неизученными.
Много внимания в мире уделяется изучению иммуномодулирующего действия прогестерона на развитие и течение беременности. Эффекты иммуномодуляции прогестерона связывают с индукцией образования активированными лимфоцитами прогестрон-индуцированного блокирующего фактора (ПИБФ). ПИБФ – важный иммуномодуляторный белок, способствующий формированию иммунологического фона, поддерживающего внутриутробное развитие полуаллогенного плода. Среди биологических эффектов ПИБФ – снижение цитолитической активности НК-клеток через блокирование экзоцитоза перфорина и ингибирование интерферона-γ, фактора некроза опухоли-α и интерлейкин-2-опосредованной трансформации НК-клеток в активированные киллерные клетки и активация Jak1 и STAT6 сигнального пути, поддерживающего Th2-тип цитокиновой продукции [13]. Представленные данные указывают на значимость прогестерон-зависимой иммуномодуляции в формировании толерантности иммунной системы матери к антигенам плода [14].
Эндогенным стимулом для увеличения секреции ПИБФ служат события, происходящие сразу после имплантации [15]. ПИБФ необходим для нормального протекания беременности, являясь медиатором с антиабортивной активностью. Низкий уровень сывороточного ПИБФ ассоциирован с потерями беременности в первом триместре или с преждевременными родами до 34 недель гестации [16, 17].
Экзогенным стимулом для синтеза ПИБФ является стресс и аллогенный стимул. Например, переливание крови или пересадка печени также увеличивают экспрессию рецепторов к прогестерону [18]. Данный факт явился обоснованием создания третьего типа иммунотерапии привычного выкидыша неясного генеза – сочетание ИЦТ и приема прогестероновых препаратов, например, дидрогестерона [19].
Оценка клеточных субпопуляций периферической крови – необходимая составляющая анализа эффектов иммунокорригирующей терапии, поскольку главной целью такой терапии рассматривается подавление агрессивных эффекторных реакций, связанных, в первую очередь, с увеличенным содержанием в периферической крови пациенток с привычным выкидышем субпопуляций клеток с естественной киллерной активностью.
Киллерные клетки с фенотипом CD56+, CD16+, CD3-CD56,16+, CD3-CD8+ являются клетками врожденного иммунитета. Цитотоксические клетки, появляющиеся в эффекторной фазе иммунного ответа и имеющие фенотип CD3+CD8+ и CD3+CD56,16+, относят к клеткам адаптивного иммунитета. Предполагается, что в генезе привычного выкидыша большую роль играет нарушение взаимодействий НК-клеток (CD56+, CD16+) и клеток адаптивного иммунитета. Однако, несмотря на довольно продолжительный период изучения НК-клеток периферической крови в генезе привычного выкидыша, значимость их оценки для прогноза ранней беременности остается невыясненной [20].
Особую значимость в последнем десятилетии приобрели исследования субпопуляции Т-регуляторных клеток с фенотипом CD4+CD25+ и конститутивной экспрессией фактора Foхр3 (Т-рег). Считается, что осуществляемая Т-рег специфическая иммуносупрессия способствует формированию состояния толерантности, необходимого для реализации гестационных процессов в первом триместре беременности [21]. В связи с этим обсуждается точка зрения, что целью иммунотерапии должна быть не иммуносупрессия, а стимуляция толерантности к аллоантигенам плода отцовского происхождения [22].
Показано, что важная роль в иммунорегуляции принадлежит толерогенной молекуле СD200. В результате взаимодействия CD200 с рецепторами как дендритных клеток, так и клеток с естественной киллерной активностью, индуцируется секреция индоламиндиоксигеназы, и они приобретают способность посредством продукции трансформирующего фактора роста-β стимулировать генерацию Трег, соответственно, индуцировать формирование специфической периферической толерантности [23–26].
Исследований, посвященных одновременной оценке динамики субпопуляций лимфоцитов периферической крови, включая субпопуляции с киллерной активностью, Трег и CD200+-клеток, в течение предгестационной иммунотерапии с использованием ИЦТ или дидрогестерона как монотерапии, а также сочетания дидрогестерона с ИЦТ, не проводилось.
Целью работы было исследование динамики субпопуляционного состава лимфоцитов периферической крови после различных вариантов иммуномодулирующей терапии в процессе предгестационной подготовки женщин с привычным выкидышем аллоиммунного генеза.
Материал и методы исследования
Основная группа исследования состояла из 51 женщины с двумя и более потерями беременности от одного партнера в анамнезе (если в каждом случае был подтвержден нормальный кариотип плода), произошедшими в первом триместре беременности, с установленным диагнозом привычного выкидыша неясного (аллоиммунного) генеза.
Критериями включения женщин в исследование явились: подписание формы информированного согласия на участие в исследовании, возраст женщины от 20 до 40 лет, самопроизвольное наступление беременностей, нормальный кариотип обоих партнеров, нормозооспермия у партнера, отсутствие анатомических, генетически обусловленных, аутоиммунных, гормональных нарушений, препятствующих наступлению и вынашиванию беременности, отсутствие тяжелых экстрагенитальных заболеваний.
Предгестационная подготовка женщинам проводилась по трем схемам. Первой группе была назначена процедура ИЦТ – иммунизация женщин аллогенными клетками партнеров (1-я основная группа, n=24). Второй группе была назначена процедура ИЦТ совместно с дидрогестероном (2-я основная группа, n=17). Третьей группе пациенток был назначен дидрогестерон (3-я основная группа, n=10). Пациентки были разделены по группам случайным образом.
ИЦТ проводили дважды с интервалом в один месяц на 5–9-й день менструального цикла. При этом пациенткам подкожно вводили 50 млн клеток лейкоцитарной взвеси партнера в ладонную поверхность предплечья в 10–12 точек.
В контрольной группе было обследовано вне беременности 15 фертильных женщин без соматических заболеваний с неотягощенным акушерско-гинекологическим анамнезом, имеющих одного ребенка не старше 2 лет или более одного ребенка, последний из которых не старше 2 лет.
Дидрогестерон назначался в дозе по 10 мг 2 раза в день с 14-го по 25-й день менструального цикла, как минимум за 2 месяца до зачатия.
Кровь у женщин 1-й и 2-й групп для анализа забирали натощак из локтевой вены до иммунизации и после каждого введения клеток на 18–22-й день менструального цикла; у женщин 3-й группы – до назначения дидрогестерона и после 2 курсов дидрогестерона также на 18–22-й день менструального цикла.
Поверхностный фенотип клеток периферической крови определяли с помощью стандартного набора моноклональных антител (мАт), меченных флуоресцеин-изотиоцианатом (FITC) или фикоэритрином (PE), против антигенов CD3, CD4, CD5, CD8, CD16, CD19, CD16, CD56, CD200 (Becton Dickinson и eBioscience, США). Оценивали содержание основных субпопуляций иммунокомпетентных T-клеток (СD3+, CD4+, CD8+), В-клеток (CD19+), B1-клеток (CD19+CD5+), NK-клеток (СD56+, СD16+), а также содержание Т-рег (СD4+CD25highCD127low/-). Лимфоцитарный гейт, позволяющий исключить из анализа другие клетки крови, выявляли с помощью мАт к СD45, меченных перидинин-хлорофилл протеином (Per-CP) (Dako, Дания). Для оценки процентного содержания Т-рег использовали набор, содержащий моноклональные антитела к антигенам CD4, меченые Per-CP (eBioscience, США), CD25, меченые FITC (Becton Dickinson, США) и СD127, меченые PE (eBioscience, США). Моноклональные антитела добавляли непосредственно к цельной крови, затем лизировали с помощью раствора FACS Lysing Solution (Becton Dickinson, США). Анализ проводили с использованием проточного цитофлуориметра FACSСalibur (Becton Dickinson, США).
Статистическую обработку данных производили общепринятыми методами вариационной статистики. Данные представлены как среднее±ошибка среднего. Соответствие расчетных выборок показателей нормальному распределению оценивали с помощью критерия Колмогорова–Смирнова с использованием пакета Statistica 6 для Windows XP. Значимость наблюдаемых отклонений средних значений измеренных параметров оценивали с помощью двухвыборочного t-критерия Стьюдента с различными дисперсиями для средних значений с использованием пакета статистического анализа для Microsoft Office Excel 2007.
Результаты исследования
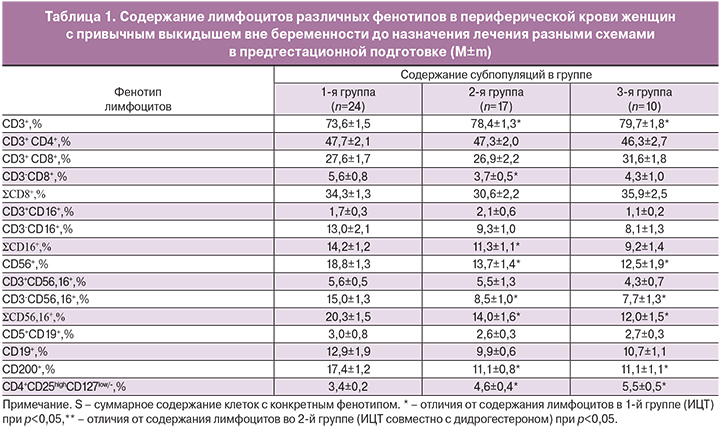
Было проанализировано содержание лимфоцитов различных фенотипов в периферической крови женщин с привычным выкидышем трех основных групп до назначения соответствующего лечения. Результаты исследования представлены в табл. 1.
Как видно из представленных данных, содержание исследованных субпопуляций во 2-й и 3-й группах до проведения предгестационной подготовки не отличалось, все отмеченные различия были от содержания субпопуляций в 1-й группе. Обращает на себя внимание меньшее, чем в первой группе, содержание субпопуляций с киллерной активностью (CD56+, SCD16+, CD3-CD56,16+, SCD56,16+, CD3-CD8+), меньшее содержание CD200+-клеток, большее содержание Трег и общей популяции CD3+.
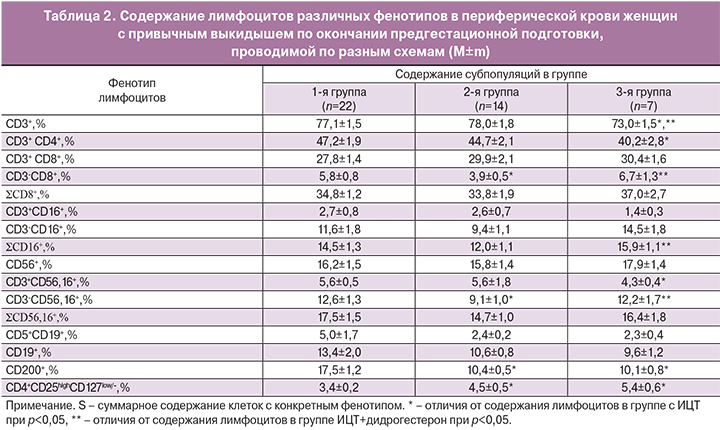
Содержание лимфоцитов различных фенотипов в периферической крови женщин с привычным выкидышем после проведения предгестационной подготовки представлены в табл. 2.
После проведенной предгестационной подготовки различия во 2-й и 3-й группах по сравнению с 1-й группой и между собой по содержанию Трег и CD200+-клеток остались без изменений. Во 2-й группе минимальным по сравнению с другими группами оказалось содержание субпопуляций с киллерной активностью и фенотипом CD3-CD8+ и CD3-CD56,16+ и максимальным – общее содержание клеток фенотипом CD3+. Содержание лимфоцитов с киллерной активностью и фенотипом CD3+CD56,16+ стало одинаковым в группах с использованием ИЦТ (и 1-й, и 2-й) и минимальным – в группе с дидрогестероном.
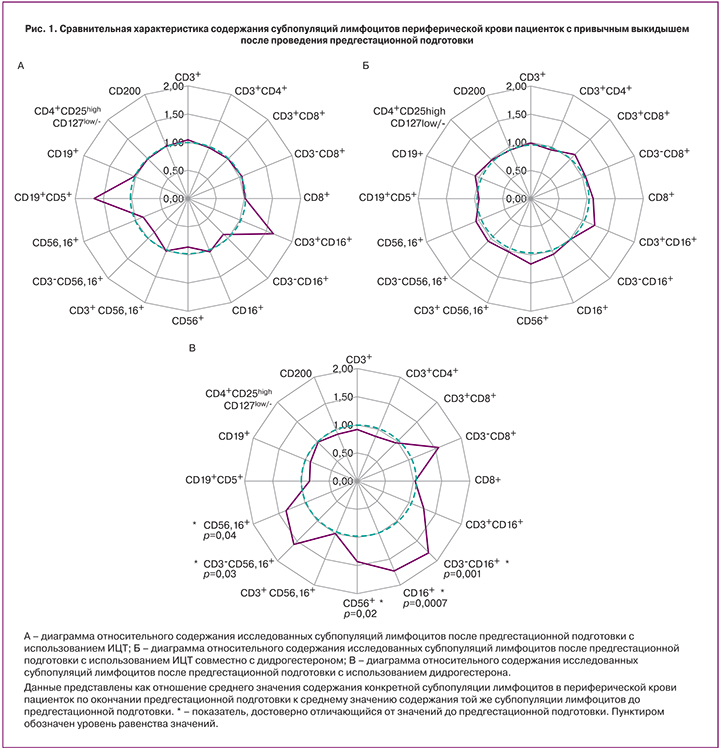
На диаграммах (рис. 1) представлены результаты анализа содержания субпопуляций лимфоцитов после предгестационной подготовки в каждой группе по отношению к исходному содержанию субпопуляций.
Из представленных данных видно, что в 1-й и 2-й группах значимых отличий от исходного уровня не зарегистрировано, а в 3-й группе зарегистрировано значимое увеличение содержания субпопуляций с киллерной активностью (CD3-CD16+, CD16+, CD56+, CD3-CD56,16+, CD56,16+).
На диаграммах (рис. 2) представлены результаты анализа в каждой группе до предгестационной терапии (цифра 1 на рисунке) и после нее (цифра 2 на рисунке) по отношению к содержанию тех же субпопуляций в контрольной группе фертильных женщин.
Из представленных данных видно, что в 1-й группе пациенток (диаграммы А-1 и А-2) после иммунотерапии произошло значимое увеличение субпопуляции лимфоцитов с фенотипом CD5+CD19+ по отношению к контролю, изменений в содержании остальных субпопуляций не было.
Во 2-й группе (диаграммы Б-1 и Б-2) после предгестационной подготовки превышение содержания субпопуляций с фенотипом CD56,16+, CD56+ и CD3+CD56,16+ над содержанием в контроле стало значимым, а содержание Трег осталось без изменений.

В 3-й группе (диаграммы В-1 и В-2) исходный уровень субпопуляций до начала иммунотерапии не отличался от содержания в контроле. После 2 курсов дидрогестерона без изменений по сравнению с контролем осталось содержание Т-рег, CD200+, CD19+, CD5+19+, CD3+8+, CD3+CD56,16+ -клеток, но значимо увеличилось содержание субпопуляций с фенотипом CD3-CD8+, CD3-CD16+, CD16+, CD56+, CD3-CD56,16+, CD56,16+ и снизилось – CD3+, CD3+CD4+.
Обсуждение
Использование иммуномодулирующей терапии для лечения привычного выкидыша аллоиммунного генеза не вызывает сомнений, и различные варианты ее использования насчитывают более чем 30-летнюю историю. Основным мотивом ее назначения служат теоретические предпосылки, рассматривающие плод как аллогенный трансплантат и объясняющие иммунные взаимоотношения материнской иммунной системы и плода с позиций достаточности или недостаточности снижения агрессивности реакций со стороны материнского организма. В этой связи эффективность иммуномодулирующей терапии связывают с формированием противовоспалительной направленности материнского иммунного ответа на отцовские антигены плода. Следовательно, успех иммунокоррекции выражается, прежде всего, в снижении содержания субпопуляций с естественной киллерной активностью, а также в продукции активированными клетками цитокинов Th2-типа, способствующими развитию гуморального, антительного иммунного ответа на аллоантигены.
В данной работе оценивалось соотношение субпопуляций лимфоцитов в периферической крови пациенток с привычным выкидышем аллоиммунного генеза после трех схем предгестационной подготовки с использованием различных вариантов иммунотерапии – ИЦТ, дидрогестерона и их сочетания. Обращает на себя внимание, что изначально, до назначения иммунотерапии, исследуемые группы пациентов (1-я, 2-я, 3-я) отличались по субпопуляционному составу лимфоцитов периферической крови, причем в группе с ИЦТ у пациентов были большие значения в содержании киллерных субпопуляций и CD200+-клеток, но меньшие – в содержании Трег. После проведения иммунотерапии выявленные закономерности практически не изменились. Изменения выявлены по отношению к исходным значениям и по отношению к содержанию субпопуляций в контрольной группе фертильных женщин.
Оказалось, что после ИЦТ изменений в содержании субпопуляций не выявлено, кроме увеличения субпопуляции лимфоцитов с фенотипом CD5+CD19+ по отношению к контролю, что может свидетельствовать о влиянии иммунизации на состояние гуморального иммунитета. Известно, что большинство антител, секретируемых CD5+CD19+-лимфоцитами, специфично к собственным белкам организма (ДНК, антигенам групп крови и др.) и многие из них полиреактивны. В норме эти антитела имеют низкое сродство к антигенам, включая аутоантигены, и не способны вызывать повреждение тканей. Признаком активации В-клеточного звена иммунитета является также появление антиотцовских антилейкоцитарных антител в крови пациенток после иммунизации [22, 27].
После предгестационной подготовки с использованием ИЦТ в сочетании с дидрогестероном по отношению к контролю увеличилось содержание только субпопуляций с фенотипом CD56,16+, CD56+ и CD3+CD56,16+, без изменений в содержании субпопуляции B1-лимфоцитов, что может отражать разное состояние иммунной системы пациенток после ИЦТ и после ИЦТ в сочетании с дидрогестероном.
Иммуномодулирующий эффект дидрогестерона в качестве монотерапии был выражен значительнее, чем ИЦТ и сочетания ИЦТ с дидрогестероном, поскольку после предгестационной подготовки с использованием дидрогестерона изменения коснулись всего спектра основных субпопуляций лимфоцитов. По отношению к исходному уровню наблюдалось увеличение содержания киллеров с фенотипом CD3-CD16+, CD16+, CD56+, CD3-CD56,16+, CD56,16+ без драматических изменений в содержании Трег и CD200+-клеток. По отношению к контролю выявлено увеличение, в дополнение к перечисленным киллерным субпопуляциям, клеток с фенотипом CD3-CD8+, снижение содержания общей популяции CD3+Т-лимфоцитов, популяции хелперных клеток CD3+CD4+ и отсутствие изменений в содержании Т-рег, CD200+, CD19+, CD5+19+, CD3+8+, CD3+CD56,16+ -клеток.
Резонно отметить, что активационное состояние лимфоцитов периферической крови пациенток с привычным выкидышем вне беременности отличается от такового при беременности, поскольку ранние сигналы от оплодотворенной яйцеклетки, продвигающейся к месту имплантации, служат эндогенным стимулом для увеличения рецепторов к прогестерону на лимфоцитах и к продукции ПИБФ. Именно с ПИБФ связывают иммуномодулирующие эффекты гестагенных препаратов. Иммунизация клетками полового партнера является тем аллогенным стимулом, который должен влиять на активационное состояние лимфоцитов, и, следовательно, на состояние рецепторов к прогестерону. Однако отсутствие изменений в субпопуляционном составе лимфоцитов в группах с ИЦТ (и 1-й, и 2-й) по отношению к исходным значениям позволяет предположить, что такое влияние не имеет такого биологического эффекта, какой наблюдается во время беременности. При наступлении беременности терапия привычного выкидыша обоснованно включает сочетание ИЦТ и гестагенных препаратов. В результате вне зависимости от типа предгестационной подготовки все пациенты в I триместре получают два курса ИЦТ в сочетании с лечением дидрогестероном.
Заключение
Все вышеизложенное позволяет оценить влияние различных видов предгестационной подготовки на субпопуляционный состав лимфоцитов периферической крови у женщин с привычным выкидышем аллоиммунного генеза. Показано, что каждый из видов терапии обладает определенным иммуномодулирующим эффектом, клиническую значимость которого предстоит оценить в последующих исследованиях, проводимых во время беременности.



