COVID-19 in obstetrics and neonatology: regional experience
Objective: Analysis of COVID-19 clinical course based on mild and moderate symptoms of coronavirus infection in infants born to SARS-CoV-2 positive women, and evaluation of their adaptation in postnatal period.Esedova A.E., Ragimov R.M., Gatagazheva Z.M., Abdullaeva N.M., Idrisova M.A., Gatagazheva M.M., Daurova Z.A.
Materials and methods: A retrospective analysis of medical records of 280 pregnant women, who had confirmed clinical diagnosis of coronavirus infection with mild and moderate symptoms of COVID-19 and medical records of 267 infants born to women with SARS-CoV-2 infection, who are residents of the North Caucasian Federal District (the Republic of Ingushetia, Dagestan and North Ossetia–Alania).
Results: The analysis of the course of the disease and major laboratory test results did not show significant differences between pregnant women with mild and moderate COVID-19 depending on the gestational age at the time of infection. High temperature, continuous cough, a loss or change to the sense of smell and taste, chest pain with breathing were the most common symptoms during three trimesters of pregnancy. Assessment of clinical laboratory results in pregnant women with COVID-10 and in infants born to patients with COVID-19 showed that biochemical blood test and a complete blood count play an important role in assessment of severity and prognosis of the disease. The data on the clinical manifestations of COVID-19 in pregnant women showed that immune responses induced by COVID-19 do not correlate with the severity of the disease. The analysis of infants’ health records showed that 12/267 (4.5%) newborns required admission to NICU due to low oxygen saturation levels, shortness of breath and tachypnea; 7/267 (2.6%) newborns required mechanical ventilation.
Conclusion: Most likely, the severity of COVID-19 in pregnant women depends not only and not so much on gestational age, but also on the presence of concomitant extragenital pathology and aggravated obstetric and gynecological anamnesis. Apparently, it is important that approach to management of pregnant women based on obstetric indications and maternal and fetal condition should individualized. Perinatal complications in newborns are likely due to impaired placental perfusion and/or thrombotic changes in mother, decreased barrier function and placental inflammatory changes.
The issues discussed in this article confirm the high relevance of the problem of infants’ health status who were born to mother, who underwent COVID-19 at different terms of pregnancy, and this poses new challenges for identification of important features in monitoring, diagnosis, therapy and prevention of pathological conditions in newborns.
Keywords
The outbreak of COVID-19 has caused challenges facing healthcare specialists related with prompt diagnosis and delivery of health care to patients. In turn, complications of COVID-19 (SARS-CoV-2) pose growing public health threat due to high mortality rates in the virtual absence of unified antiretroviral therapy. Currently, intensive study of clinical and epidemiological features of the disease and development of new preventive measures and treatment is being continued.
Undoubtedly, the most vulnerable and unprotected group of patients are pregnant women with COVID-19 due to maternal physiological changes associated with fetal development. Due to ethical and legal issues in pregnancy, as well as health concerns for the baby during pregnancy, harmacokinetic/pharmacodynamic and clinical trials are nearly impossible to carry out. As a result, healthcare practitioners bear all responsibility for assessment of risks and benefits of treatment with particular drugs in the particular clinical situation. The greatest concern is teratogenesis, that may occur at any term of pregnancy.
The results of research papers presented in international scientific publications on the effects of COVID-19 on pregnancy outcomes vary significantly. Some authors suggest that pregnancy and childbirth do not exacerbate the course of novel coronavirus infection, and severe acute respiratory syndrome in pregnant women with COVID-19 is not associated with a high risk of miscarriage and preterm birth [1]. However, there are risks of complications in pregnancy at the stages of prenatal development, and long-term complications especially after severe COVID-19 [2, 3]. The third opinion is the fact that due to specific hormones (progesterone and human chorionic gonadotropin), pregnancy inhibits the development of cytokine storm – the main cause of mortality and patient’s serious condition [4].
Recently, researchers in Spain and Canada made an attempt to detect a key factor for risk identification of lethal outcomes. They found that the level of specific antibodies depends on the total number of antigens and viral RNA fragments in blood plasma. They evaluated the role of three hemogram parameters and found that a low level of specific antibodies against the spike protein (S protein) of COVID-19 produced after vaccination correlates with a high risk of death, and the other two parameters are associated with the first one, and RNA load is inversely proportional to the amount of antibodies [5].
The clinical laboratory test of angiotensin converting enzyme 2 (ACE2) used by the virus as a receptor binding at cell adhesion sites, was performed. This enzyme was expressed predominantly by epithelial cells of the upper and lower respiratory tract. Perhaps, that is why respiratory failure is a common condition in clinical presentation. The surfactant is destroyed and collapse of alveoli occurs as a result of the cytolytic effect of SARS-CoV-2 on alveolar epithelial cells type II. This can lead to complications in pregnant women, especially when resuscitation measures are taken. [6].
The SARS-CoV-2 glycoprotein has a tropic effect on endothelial cells, where the angiotensin-converting enzyme 2 (ACE2) receptors are present. Active reproduction of pathogens leads to panvasculitis, impaired tissue perfusion, disseminated intravascular coagulation, development of the diffuse alveolar hemorrhage (DAH) syndrome, myocardial infarction, renal failure, cerebrovascular disease and other conditions [7]. A number of researches showed that severe hemodynamic disorders in pregnant women with coronavirus infection influence not only the severity of the disease course, but also a high incidence of adverse pregnancy outcomes, such as spontaneous abortion, preterm birth, etc. [8–10].
Most infants born to SARS-CoV-2 positive women, have negative PCR tests after birth, and this indicates the presence of the blood-placental barrier [11]. Perinatal and neonatal outcomes continue to worsen during the ongoing COVID-19 pandemic, increasing maternal and neonatal mortality [12–17], although some studies report that transplacental transmission of SARS-CoV-2 infection is possible in patients with multiple chronic conditions. According to some systematic reviews, the newborns and children below 1 year of age are more vulnerable to SARS-CoV-2 infection; they are more likely to have a severe course of the disease compared to older children [18, 19].
Protection of a newborn against infection primarily depends on the innate immunity and maternal antibodies [20]. Assessment of the level of maternal antibodies that are produced in response to infection caused by coronavirus during pregnancy and transported across placenta is important for understanding the protection of newborns against COVID-19. It is important to note that passive immunity in breastfeeding provides protection against coronavirus. An observational study in Standford University showed that 147 infants born to 145 women, who had COVID-19 infection during pregnancy, IgG levels in the samples of maternal blood and umbical cord blood obtained during childbirth significantly correlated. High rates of transplacental transfer of IgG were observed in cases when infection occurred less than 60 days prior to delivery or in the second trimester of pregnancy. Maintenance of maternal IgG in newborns correlated with the initial level of antibodies in umbical cord blood [21]. At the same time, a decline in antibody titers was observed in all infants during the subsequent test, while the values of maternal antibodies titers were the same or even increased [22, 23].
Thus, we analyzed the data in three republics of the North-Caucasian Federal District (NCFD): Ingushetia, Dagestan and North Ossetia–Alania to detect the features of COVID-19 clinical course, pregnancy outcomes and health assessment of newborns, as well as the effectiveness of therapeutic strategy.
The purpose of research was to evaluate the course of pregnancy on the background of mild and moderate COVID-19 and adaptation of infants born to SARS-CoV-2 positive women in the early neonatal period.
Materials and methods
A retrospective analysis of medical records of 280 pregnant, parturient and puerperant women was performed. These women are residents of the North Caucasian Federal District (the Republics of Ingushetia, Dagestan and North Ossetia–Alania). They received inpatient medical care (No. 096/u-20) in 2021, and had confirmed clinical diagnosis of coronavirus infection with mild and moderate symptoms of COVID-19.
Among analyzed 280 medical records, 114 were from Central district hospital in Sunzha (the Republic of Ingushetia), 69 – from Republican Center for Infectious Diseases of the Ministry of Health of the Republic of Dagestan and City hospital No. 1, and 97 records were from maternity hospital – a division Pravoberezhny Municipal Hospital (the Republic of North Ossetia–Alania).
Along with assessment of medical records of 280 infected pregnant women, a retrospective analysis of medical records of 267 infants (No. 097/u) born to women with confirmed mild and moderate SARS-CoV-2 infection in three regions of the NCFD in I–IV quarters of the year 2021was performed.
According to the Order. No 198n of March 19, 2020 “In-hospital routing of patients”, all infected pregnant women with COVID-19 stayed in red zones in clinical hospitals.
Prior to starting the study, the approval of the Ethics Committee was obtained, and ethical principles for conducting the research were observed.
All parturient and puerperant women have signed a written consent for processing the medical records of newborns.
Statistical analysis
Software package BioStat (Version 5.9.8.5) was used for statistical data processing. To calculate descriptive statistics, the median value (M) for the quantitative parameters in three trimesters of pregnancy, and standard deviation (SD) – M (SD) were used.
The Shapiro-Wilk test was used for normality of distribution. In this study, the quantitative parameters had a normal distribution. The qualitative parameters were presented both as absolute and relative (%) values.
Analysis of variance was used to identify the differences in quantitative parameters in the study groups (in 3 trimesters). The Student's t-test with the Bonferroni correction was used for pairwise comparisons at p<0.05. Also, the Student’s t-test was used for comparison of quantitative parameters in 3 trimesters (in 3 groups). The variables were statistically significant at р<0.017. Fisher’s exact test was used for pairwise comparison of clinical manifestations in the groups (in 3 trimesters of pregnancy). Statistically significant differences were at р<0.05.
Results and discussion
The course and outcomes of pregnancy and infants’ functional status, born to women with confirmed diagnosis of novel coronavirus infection was analyzed in three republics of the North-Caucasian Federal District: Ingushetia, Dagestan and North Ossetia–Alania.
All hospitalized pregnant women were stratified by age into 3 groups (16–25, 26–35, 36–45 years). The largest group comprised 102/280 (36.4%) patients aged 26–35 years.
The PCR test for detection of SARS-CoV-2 RNA in nasopharyngeal swabs confirmed the diagnosis of COVID-19 in 265/280 (94.6%) pregnant women, negative PCR test result was in 15/280 (5.4%) women, but by the time of diagnosis, IgM was detected in their blood. The pregnant women were hospitalized on day 3.5 (0.9) day after the onset on the disease. 120/280 (42.9%) pregnant women infected with COVID-19 did not receive treatment at the stage of outpatient care.
All women with coronavirus infection were divided into groups considering the trimester of pregnancy: group1, group 2 and group 3. The assessment of gestational age showed that most infected women were admitted to hospital in the third trimester of pregnancy – 131/280 (46.8%).
Generally, coronavirus infection manifested with non-specific symptoms: in most cases febrile symptoms were in 188/280 (67.1%) pregnant women, subfebrile symptoms were in 63/280 (22.5%); and asymptomatic course of COVID-was in 29/280 (10.4%) patients. Either a dry cough or a severe and mild cough with a small amount of sputum was observed in 254/280 (90.7%) women. Loss of smell was in 189/280 (67.5%), and loss of taste was in 93/280 (33.2%) patients. Tightness in the chest and a feeling of pressure was in 137/280 (48.9%) pregnant women, and all patients felt extreme fatigue on the background of the normal course of pregnancy. In addition to the above mentioned symptoms, it should be noted that the COVID-19 pandemic caused a psychological stress and anxiety in all pregnant women, and this could have an adverse effect on the course of pregnancy and lead to complications.
The most common somatic pathology in pregnant women with COVID-19 was obesity of different stages in 62/280 (22.1%) women, anemia of different severity in 51/280 (18.2%) women, gastrointestinal disorders in 29/280 (10.4%) women, hypertensive disease in 17/280 (6.1%) women, and varicose veins in 13/280 (4.6%) women.
We analyzed clinical laboratory test results in pregnant women with COVID-19. Biochemical blood test and the complete blood count play an important role in assessment of severity and prognosis of the disease and help to improve treatment scheme. The figure below shows the major laboratory parameters (haemogram test, biochemical blood test) at the time when the patients were admitted to hospital, on the background of treatment (days 4–8) and before hospital discharge (days 9–15).
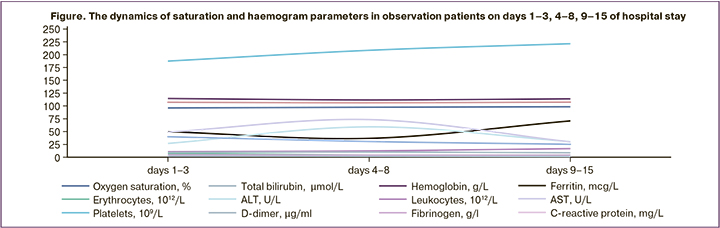
We analyzed laboratory test results in patients with COVID-19 and found that hemoglobin level was initially low in 51/280 (18.2%) pregnant women, and the average level was 102.02 (3.61) g/L. Most likely, this was due to anemia in pregnant women before they were infected with SARS-CoV-2 infection (most patients were in the third trimester of pregnancy). In dynamics, the average hemoglobin level increased to 113.40 (2.75) g/L on days 9–15, possibly due to antianemic therapy.
The median value for platelet counts was 187.38 (6,07)×109/l on days 1–3. In dynamics, it increased to 221.24 (5.83)×109/l. The median value for white blood cells was 10.93 (0.82)×109/l on days 1–3. Before hospital discharge their number increased to12.50 (0.96)×109/l. Median D-dimer level was 2.416 (027) μg/ml on days 1–3, subsequently it decreased to 1.93 (0,21) μg/ml.
Procalcitonin (PCT) concentrations also increased in dynamics: 0.26 (0.07) ng/mL on days 1–3 and 0.4 (0.08) ng/mL upon hospital discharge. Median ferritin concentration was 49.75 (3.67) μg/L on days 1–3, and it decreased toдо 36.77 (4.03) μg/L on days 4–8 with a subsequent increase to70.79 (3.77) μg/L in the second and the third trimesters. This situation was likely due to the fact that a secondary bacterial infection occurred in patients with COVID-19 and pneumonia. Body's response to hypoxia and increased blood viscosity lead to high concentration of the protein complex.
One of the most common complications in pregnant women with COVID-19 infection was liver damage. At the same time, the level of transaminases tended to increase: the average alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were 6.98 (3.51) and 49.14 (5.75) U/L on days 1–3, 59.25 (3.82), 73.47 (5.11) on days 4–8 and 30.44 (3.51), and 30.08 (2.97) on days 9–15 before hospital discharge, respectively. This was possible due to hepatotoxicity of the drugs used by pregnant women during the specific treatment of COVID-19. The median value of total protein was 61.43 (3.74) g/L, then it tended to decrease to 57.12 (2.99) g/l on days 9–15. Other biochemical parameters (creatinine, urea, cholesterol, glucose) did not change significantly on the background of treatment.
The analysis of blood clotting parameters showed that the median value of prothrombin time index (PTI) was 118.20 (4.33)% per day on days 1–3, but in dynamics it decreased to105.17 (4.89)%; fibrinogen concentration was 4.81 (0.32) g/L on days 1–3, and 4.19 (0.41) g/L on days 9–15; the median value of activated partial thromboplastin time (aPTT) was 34.8 (3.04) sec. on days 1–3, and in dynamics it was 40.14 (4.08) sec. It is most likely, that these changes in aPTT values were on the background of anticoagulant therapy.
The infected patients underwent treatment in hospital in accordance with clinical recommendations including “Organization of healthcare to pregnant, parturient and puerperant women with novel coronavirus infection (COVID-19). Version 4” (approved by the Ministry of Health of Russia on July 05, 2021).
The analysis of antimicrobial therapy showed that cefepime and ceftazidime were most often used for treatment of pregnant women in the Republic of Ingushetia and the Republic of North Ossetia–Alania, and ceftriaxone from the cephalosporin family of antibiotics (when necessary in combination with azithromycin) was used in the Republic of Dagestan. Therefore, the antibiotic treatment regimen included cefepime for 70/211 (33.2%) patients, ceftazidime for 65/211 (30.8%), ceftriaxone for 41/69 (59.4%) and azithromycin for 19/69 (27,5%) patients. 9/280 (3.2%) patients with complicated course of the disease received antimicrobial therapy with meropenem. In addition to antimicrobial therapy for pregnant women with COVID-19, treatment regimen included dexamethasone for 98/280 (35%) patients, enoxaparin sodium for 75/280 (26.6%) and 5% ascorbic acid for 85/280 (30.3%) patients. Also, 9/280 (3.2%) patients were treated with human recombinant interferon alfa-2b, 23/280 (8.2%) received acetylcysteine, 13/280 (4.7%) received fluimucil and 22/280 (7.9%) patients received omeprazole. After consultation with hepatologist, hepatoprotectors were used for treatment in 182/280 (65%) cases and chophytol was used in 62/280 (22.1%) cases. A part of pregnant women with COVID-19 had anemia of different severity, due to this iron supplements were prescribed to treat anemia in 51/280 (18.2%) patients.
Considering the above clinical manifestations of COVID-19 in pregnant women, it can be concluded that the degree of immune response in patients with COVID-19 did not correlate with the severity of the disease. Thus, high levels of inflammatory markers (C-reactive protein, D-dimer) in the blood serum at early stages of COVID-19 could reflect the occurrence of acute inflammation.
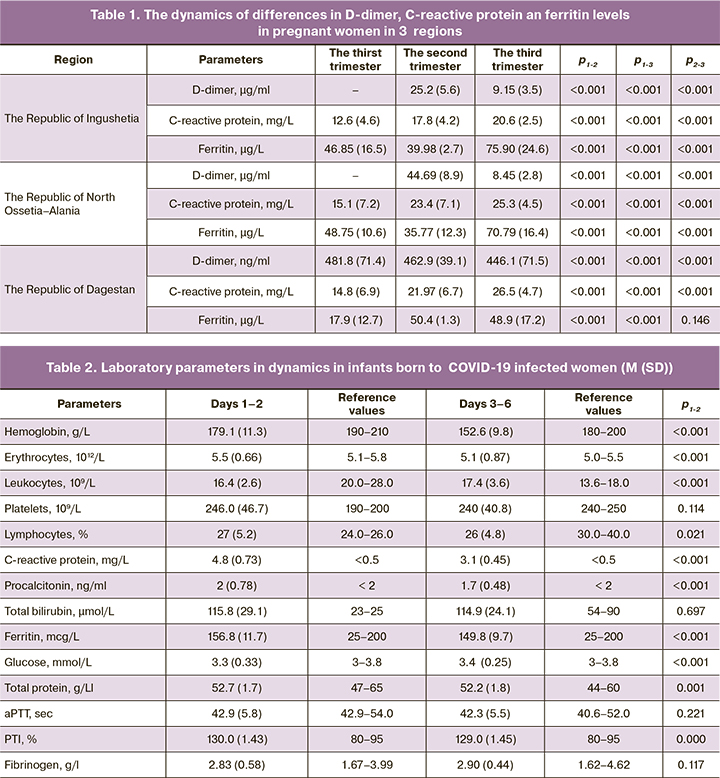
The study showed that the median value of hemoglobin increases in dynamics due to antianemic therapy. Despite the fact that ferritin level in pregnant women was high, it never reached the critical threshold level, which could lead to lethal outcome due to cytokine storm (Table 1.).
In parallel to assessment of medical records of pregnant women, we made a detailed retrospective analysis of development of 267 infants born to women infected with SARS-CoV-2 in the Republics of the North-Caucasian Federal District (the Republics of Ingushetia, Dagestan and North Ossetia–Alania).
The analysis showed that 12/267 (4.5%) newborns required admission to NICU due to low oxygen saturation levels, shortness of breath and tachypnea; 7/267 (2.6%) newborns required mechanical ventilation.
Development records of 70 infants born in the Central district hospital in Sunzha (the Republic of Ingushetia) were analyzed. 61/70 (87.1%) newborns were not infected, and 9/70 (12.9%) newborns had confirmed COVID-19 by positive PCR test results. Among infected infants, 5/9 (55.6%) were born through the vaginal canal, and 4/9 (44,4%) by cesarean section. Multisystem inflammatory syndrome temporally related to COVID-19 was in 3/9 (33.3%) infected newborns. Moreover, pneumonia was diagnosed in 6/61 (9.8%) infants with negative PCR test results for CОVID-19. Among infected infants, 3/9 (33.3%) required mechanical ventilation on the first day of life.
Assessment of blood oxygen (O2) level showed that oxygen saturation level at birth was 93% in 8/70 (11.4%) newborns, 86% in 3/70 (4.3%) newborns, and 97–99% in 59/70 (84.3%) newborns. There were no cases of neonatal death in infants born to mothers with confirmed diagnosis of SARS-CoV-2 infection in this region.
97 records on development of infants born to mothers with SARS-CoV-2 infection in maternity hospital in Beslan – a division of City Hospital in Pravoberezhny District of North Ossetia–Alania were analyzed. 76/97 (78.4%) newborns were not infected, and 21/97 (21.7%) newborns had confirmed COVID-19 infection by positive PCR test results. Of 21 newborns with COVID-19 infection, 19 infants (90.5%) were born through the vaginal canal, and 2/21 (9.5%) by cesarean section. Assessment of blood oxygen (O2) level showed that oxygen saturation level was 96% in 2/21 (9.5%) newborns, 98% in 8/21 (38%) newborns and 99% in 10/21 (47.6%) newborns, respectively. None of newborns required mechanical ventilation. No lethal outcomes among infected newborns was registered in this region.
Development records of 100 infants born to infected mothers in Maternity hospital of “Central City hospital” (Kaspiysk, the Republic of Dagestan) were analyzed. Of them, 76/100 (76%) newborns were not infected, and 24/100 (24%) newborns had confirmed COVID-19 infection by positive PCR test results. In the group of infected newborns, 14/24 (58.3%) were born through the vaginal canal and 10/24 (41.7%) by cesarean section. Within one day after the birth 4/24 (16,7%) newborns required mechanical ventilation due to low blood oxygen level (88%). Before 7 days of life, O2 saturation level in 16/24 (66,7%) newborns was 97–99%, in 4/24 (16,7%) babies 95–96%. No lethal outcomes among newborns were registered in this region.
Based on laboratory and instrumental test results, the patients were divided into 2 groups (before and after treatment). Test results are shown in Table 2.
Several laboratory parameters in newborns with COVID-19 differed significantly in the regions. Statistically significant decrease in hemoglobin level to179.1 (11.3) g/L in infected newborns in the Republic of Dagestan versus 189 (9) g/L and 196 (3.9) g/L in the Republics of Ingushetia and North Ossetia–Alania, respectively. Statistically significant increase in the level of C-reactive protein to 4.8 (0.73) mg/L was found in infected newborns in the Republic of Dagestan. In the Republic of Ingushetia the level of C-reactive protein was 2.3 (0.9) mg/L, and in North Ossetia–Alania – 2.6 (0.98) mg/L.
Assessment of gestational age of newborns and the number of births in each region showed that the highest number of births was in the third trimester (Table 3).
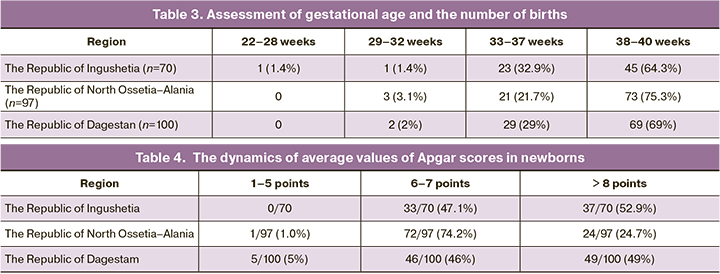
The dynamics of average values of Apgar scores in newborns was 5.47 points at 1 minute and 8.97 points at five minutes of life (Table 4).
Treatment of newborns infected with novel coronavirus infection COVID-19 was performed in accordance with clinical recommendations “Organization of healthcare to pregnant, parturient and puerperant women and newborns with novel coronavirus infection (COVID-19). Version 2” (approved by the Ministry of Health of Russia on July 03, 2020).
Conclusion
Thus, the analyses of the course of the disease and major laboratory test results in pregnant women with mild and moderate COVID-19 did not find significant differences between the gestational age in the infection period. In three trimesters of pregnancies the following clinical symptoms were diagnosed: high temperature, cough, a loss or change to the sense of smell and taste, as well as chest pain with breathing. However, the frequency of clinical symptoms, including major laboratory test results had not significant differences in the first, second and third trimesters (р>0.05). It is most likely that the severity of COVID-19 course in pregnant women depends not only on gestational age, but also on comorbid extragenital pathology with aggravated obstetric and gynecological anamnesis (previous pregnancy pathology and birth), since these pathologies occurred in pregnant women with severe course of COVID-19.
Apparently, it is important that approach to management of pregnant women based on obstetric indications and maternal and fetal condition should be individualized. Careful attention should be paid when prescribing antimicrobial therapy for pregnant women. It is especially important to take into account gestational age, the severity of the condition and to control hepatotoxic effects of the drugs.
In our study, perinatal complications in newborns were likely due to impaired placental perfusion and/or thrombotic changes in mother, decreased barrier function and placental inflammatory changes.
The issues related to the general health status and reproductive health of parturient women were of interest to us. But currently, the category of children born to women who had coronavirus infection at different stages of pregnancy is of particular interest. For this reason the studies will be continued.
Only after the end of pandemic it will be possible to make a final conclusion about specific features of the course of COVID-19 in pregnant women. The issues discussed in this article confirm a high relevance of the problem of infants’ health status who were born to mothers with COVID-19 at different stages of pregnancy, and this poses new challenges for identification of important features in monitoring, diagnosis, therapy and prevention of pathological conditions in newborns.
References
- Yan J., Guo J., Fan C., Juan J., Yu X., Li J. et al. Coronavirus disease 2019 in pregnant women: a report based on 116 cases. Am. J. Obstet. Gynecol. 2020; 223(1): 111.e1-111.e14. https://dx.doi.org/10.1016/j.ajog.2020.04.014.
- Liu H., Wang Li.L., Zhao Si.J., Kwak-Kim J., Mor G., Liao Ai.H. Why are pregnant women susceptible to COVID-19? An immunological viewpoint. J. Reprod. Immunol. 2020; 139: 103122. https://dx.doi.org/10.1016/j.jri.2020.103122.
- Shanes E.D., Mithal L.B., Otero S., Azad H.A., Miller E.S., Goldstein J.A. Pathology in COVID-19. Am. J. Clin. Pathol. 2020; 154(1): 23-32. https://dx.doi.org/10.1093/ajcp/aqaa089.
- Elshafeey F., Magdi R., Hindi N., Elshebiny M., Farrag N., Mahdy S. et al. A systematic scoping review of COVID-19 during pregnancy and childbirth. Int.. J Gynaecol. Obstet. 2020; 150(1): 47-52. https://dx.doi.org/10.1093/ajcp/aqaa08910.1002/ijgo.13182.
- Martin-Vicente M., Almansa R., Martínez I., Tedim A.P., Bustamante E., Tamayo L. et al. Low anti-SARS-CoV-2 S antibody levels predict increased mortality and dissemination of viral components in the blood of critical COVID-19 patients. J. Intern. Med. 2022; 291(2): 232-40. https://dx.doi.org/10.1111/joim.13386.
- Zhao Y., Zhao Z., Wang Y., Zhou Y., Ma Y., Zuo W. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. bioRxiv. 2020; 01: 26.919985. https://dx.doi.org/10.1101/2020.01.26.919985.
- Sevajol M., Subissi L., Decroly E., Canard B., Imbert I. Review insights into RNA synthesis, capping, and proofreading mechanisms of SARS-coronavirus. Virus Res. 2014; 194: 90-9. https://dx.doi.org/10.1016/j.virusres.2014.10.008.
- Di Mascio D., Khalil A., Saccone G., Rizzo G., Buca D., Liberati M. et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM. 2020; 2(2): 100107. https://dx.doi.org/10.1016/j.ajogmf.2020.100107.
- Schwartz D.A., Graham A.L. Potential maternal and infant outcomes from (Wuhan) coronavirus 2019-nCoV infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses. 2020; 12(2): 194. https://dx.doi.org/10.3390/v12020194.
- Baud D., Greub G., Favre G., Gengler C., Jaton K., Dubruc E., Pomar L. Second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infection. JAMA. 2020; 323(21): 2198-200. https://dx.doi.org/10.1001/jama.2020.7233.
- Sánchez-Luna M., Colomer B.F., de Alba Romero C., Allen A.A., Souto A.B., Camba Longueira F. et al. Neonates born to mothers with COVID-19: data from the Spanish society of neonatology registry. Pediatrics. 2021: 147(2): e2020015065. https://dx.doi.org/10.1542/peds.2020-015065.
- Shanes E.D., Mithal L.B., Otero S., Azad H.A., Miller E.S., Goldstein J.A. Placental pathology in COVID-19. Am. J. Clin. Pathol. 2020; 154(1): 23-32. https://dx.doi.org/10.1093/ajcp/aqaa089.
- Simões E Silva A.C., Leal C.R.V. Is SARS-CoV-2 vertically transmitted? Front. Pediatr. 2020; 8: 276. https://dx.doi.org/10.3389/fped.2020.00276.
- Suy A., Garcia-Ruiz I., Carbonell M., Garcia-Manau P., Rodo C., Maiz N. et al. Gestation and COVID-19: clinical and microbiological observational study (Gesta-COVID19). BMC Pregnancy Childbirth. 2021; 21(1): 78. https://dx.doi.org/10.1186/s12884-021-03572-4.
- Verma S., Bradshaw C., Auyeung N.S.F., Lumba R., Farkas J.S., Sweeney N.B. et al. Outcomes of maternal-newborn dyads after maternal SARS-CoV-2. Pediatrics. 2020; 146(4): e2020005637. https://dx.doi.org/10.1542/peds.2020-005637.
- Vivanti A.J., Vauloup-Fellous C., Prevot S., Zupan V., Suffee C., Do Cao J. et al. Transplacental transmission of SARS-CoV-2 infection. Nat. Commun. 2020; 11(1): 3572. https://dx.doi.org/10.1038/s41467-020-17436-6.
- Chamseddine R.S., Wahbeh F., Chervenak F., Salomon L.J., Ahmed B., Rafii A. Pregnancy and neonatal outcomes in SARS-CoV-2 infection: a systematic review. J. Pregnancy. 2020; 2020: 45924. https://dx.doi.org/10.1155/2020/4592450.
- Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020; 324(8): 782-93. https://dx.doi.org/10.1001/jama.2020.12839.
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020: 323(13): 1239-42. https://dx.doi.org/10.1001/jama.2020.2648.
- Абатуров А.Е., Агафонова Е.А., Кривуша Е.Л., Никулина А.А. Патогенез COVID-19. Здоровье ребенка. 2020; 15(2): 133-44. [Abaturov A.E., Agafonova E.A., Krivusha E.L., Nikulina A.A. Pathogenesis of COVID-19. Child's Health. 2020; 15(2): 133-44. (in Russian)].
- Белокриницкая Т.Е., Артымук Н.В., Филиппов О.С., Фролова Н.И. Клиническое течение, материнские и перинатальные исходы новой коронавирусной инфекции COVID-19 у беременных Сибири и Дальнего Востока. Акушерство и гинекология. 2021; 2: 48-54. [Belokrinitskaya T.E., Artymuk N.V., Filippov O.S., Frolova N.I. Clinical course, maternal and perinatal outcomes of the new COVID-19 coronavirus infection in pregnant women of Siberia and the Far East. Obstetrics and Gynecology. 2021; 2: 48-54. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.2.48-54.
- Коммуникационный центр правительства Российской Федерации. Отчет о текущей ситуации по борьбе с коронавирусом. 01.08.2021. [Communication Center of the Government of the Russian Federation. Report on the current situation in the fight against coronavirus. 01.08.2021. (in Russian)].
- Щеголев А.И., Туманова У.Н., Серов В.Н. Поражения плаценты у беременных с SARS-CoV-2-инфекцией. Акушерство и гинекология. 2020; 12: 44-52. [Shchegolev A.I., Tumanova U.N., Serov V.N. Placental lesions in pregnant women with SARS-CoV-2 infection. Obstetrics and Gynecology. 2020; 12: 44-52. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.12.44-52.
Received 21.02.2022
Accepted 17.03.2022
About the Authors
Asiyat E. Esedova, Dr. Med. Sci., Professor, Head of the Department of Obstetrics and Gynecology of the Pediatric, Dental and Preventive Medicine Faculties,Dagestan State Medical University, Ministry of Health of Russia, +7(928)297-41-92, asiyat.idrisova@bk.ru, https://orcid.org/0000-0002-9311-1791,
367000, Russia, Respublic of Dagestav, Makhachkala, Lenin sqr., 1.
Razin M. Ragimov, Dr. Med. Sci., Professor, Head of the Department of Normal Physiology, Dean of the Medical Faculty, Dagestan State Medical University,
Ministry of Health of Russia, +7(928)945-38-19, razinragimov@mail.ru, https://orcid.org/0000-0002-5442-5528,
367000, Russia, Respublic of Dagestav, Makhachkala, Lenin sqr., 1.
Zareta M. Gatagazheva, Dr. Med. Sci., Associate Professor, Head of the Department of Obstetrics and Gynecology, Faculty of Medicine, Ingush State University,
Ministry of Health of Russia, +7(8732)22-38-54, +7(8734)55-42-22, +7(928)727-75-97, ing_gu@mail.ru, zareta1@list.ru, https://orcid.org/0000-0001-8067-378X,
386001, Russia, Republic of Ingushetia, Magas, I.B. Zyazikov str., 7.
Naida M. Abdullaeva, PhD (Bio), Associate Professor of the Department of Normal Physiology, Head of the Department of Grants and Innovations,
Dagestan State Medical University, Ministry of Health of Russia, +7(989)661-75-73, +7(903)429-16-23, caca1@yandex.ru, https://orcid.org/0000-0002-9616-9606, ResearcherID: 5576-2016, 367000, Russia, Republic of Dagestan, Makhachkala, Lenin sqr., 1.
Muminat A. Idrisova, PhD, Assistant of the Department of Obstetrics and Gynecology of the Pediatric, Dental and Preventive Medicine Faculties,
Dagestan State Medical University, Ministry of Health of Russia, +7(922)280-46-99, muminat.idrisova.88@mail.ru, https://orcid.org/0000-0002-3251-541,
367000, Russia, Republic of Dagestan, Makhachkala, Lenin sqr., 1.
Malika M. Gatagazheva, PhD, Associate Professor, Head of the Department of Obstetrics and Gynecology, Faculty of Medicine, Ingush State University,
Ministry of Health of Russia, +7(8732)22-38-54, +7(8734)55-42-22, ing_gu@mail.ru; https://orcid.org/0000-0002-6010-0287,
386001, Russia, Republic of Ingushetia, Magas, I.B. Zyazikov str., 7.
Zarina A. Daurova, postgraduate student of the Department of Obstetrics and Gynecology No. 2, North Ossetian State Medical Academy, Ministry of Health of Russia, +7(8672)53-03-97, daurova.zarina@mail.ru, 362019, Russia, RSO–Alania, Vladikavkaz, Pushkinskaya str., 40.
Corresponding author: Naida M. Abdullaeva, caca1@yandex.ru
Authors' contributions: Esedova A.E., Ragimov R.M., Gatagazheva Z.M. – the concept and design of the study, data preparation and analysis, establishment of database, writing the article, correction of the article after review; Gatagazheva Z.M., Daurova Z.A. – the concept and design of the study, analysis of the obtained results, writing the article; Abdullaeva N.M. – review of publications on the topic of the article; writing the article; Idrisova M.A., Ragimov R.M. – database correction, statistical data processing and analysis of the obtained results, writing the article.
Conflicts of interest: The authors declare that they have no conflict of interests.
Funding: The study was conducted without any sponsorship.
Ethical Approval: The study was approved by the local Ethics Committee of DSMU, Ministry of Health of Russia (Protocol No. 3 of February 26, 2021).
Patients’ Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding authors Abdullaeva N.M., Gatagazheva Z.M. after approval from the principal investigator.
For citation: Esedova A.E., Ragimov R.M., Gatagazheva Z.M., Abdullaeva N.M., Idrisova M.A., Gatagazheva M.M., Daurova Z.A. COVID-10 in obstetrics and neonatology: regional experience.
Akusherstvo i Gynecologia/Obstetrics and Gynecology. 2022; 4: 55-63 (in Russian)
https://dx.doi.org/10.18565/aig.2022.4.55-63



