Association of blood vitamin K level and polymorphism of detoxification genes with the outcomes of an assisted reproductive technology program
Objective. To search for biochemical and molecular genetic predictors of pregnancy in an IVF/ICSI program.Khechumyan L.R., Kalinina E.A., Donnikov A.E., Ivanets T.Yu., Kulakova E.V.
Subjects and methods. The investigation enrolled 100 women who were eligible for inclusion. In accordance with the results of infertility treatment, the investigators formed two study groups: 1) 28 patients with the onset of clinical pregnancy; 2) 72 patients with a negative treatment result. All the women underwent determination of the serum level of vitamin K and the polymorphism of the VKORC1 gene and the genes involved in xenobiotic metabolism.
Results. In patients who had class C embryos (low- or poor-quality embryos) according to the classification proposed by D. Gardner, the serum level of vitamin K was lower than in those with good-quality embryos. The probability of obtaining immature oocytes for female carriers of slow CYP2C9*2 and CYP2C9*3 haplotypes was 61 and 46%, respectively, whereas immature oocytes were obtained for patients with normal genotype (*1/*1) in 72% of cases. A correlation was also found between the probability of pregnancy in the IVF/ICSI program and the EPHX1 337 T>C (Tyr113His) rs1051740 genotype.
Conclusion. Serum vitamin K levels in women receiving infertility treatment in the IVF/ICSI program was shown to be of prognostic value for the quality of embryos. The polymorphism of the detoxification genes (CYP2C9 and EPHX1) was found to have a significant impact on the quality of oocytes and the chance of getting pregnant, which can be a prognostic criterion for assessing the quality of embryos.
Keywords
Approximately 70 million couples in the world are annually diagnosed with infertility [1]. Assisted reproductive technologies (ART) are the most effective among all currently available treatment methods. However, according to the European Society for Human Reproduction and Embryology (ESHRE), the rate of clinical pregnancy in Europe in 2013 was 29.6% per oocyte collection and 34.5% per embryo transfer [2]. Considering reproductive losses, only about a quarter of in vitro fertilization (IVF) cycles ends with the childbirth. The ART effectiveness is unlikely to increase in future without a deep study of the mechanisms regulating implantation in humans.
Embryo implantation is a complex of molecular and cellular interactions regulated by paracrine and autocrine factors [3]. The occurrence of pregnancy depends greatly on two components: a functionally complete embryo and an endometrial receptivity [4]. Currently, the quality assessment of embryos is primarily based on the morphological criteria, however, the accuracy of such method of selecting embryos remains insufficiently high, although its implementation has led to a significant increase in the IVF programs effectiveness [5].
In recent years, there has been a growing interest in the development of the new non-invasive methods of the analysis of oocytes quality, embryos and embryo implantation. A number of factors involved in the latter process include endometrial integrins, extracellular matrix molecules, adhesion molecules, growth factors and ion channels. In order to improve the understanding of the implantation failure mechanisms, an in vitro model of embryo implantation is being developed. Understanding the exact molecular pathways associated with implantation failures will allow to develop new prognostic and diagnostic biomarkers and subsequently identify molecular targets for therapeutic action [6].
Among the wide range of biochemical markers defined in various biological materials, a matricellular protein periostin has recently attracted attention of researchers. It can be considered as one of the future biomarkers characterizing the process of interaction between the endometrium and the embryo during implantation [7] and it can play a specific role in improving future results of IVF programs. The evidence from the literature indicates that there is a correlation between the serum level of periostin in women undergoing infertility treatment in the IVF/ICSI program and the quality of the obtained oocytes and blastocysts [8].
Periostin (POSTN) also known as osteoblast-specific factor 2 belongs to the family of extracellular matrix proteins and functions as an integrin ligand (intercellular adhesion molecule), and it is involved in cell adhesion and migration. According to the literature, periostin is a vitamin K-dependent protein [8]. Vitamin K provides carboxylation of glutamic acid residues that form some proteins. As a result, they are converted into gamma-carboxylglutamic acid residues (Gla-radicals) and acquire the ability to bind calcium. Proteins that require such carboxylation for activation are called “vitamin K-dependent” [9]. It is well known that the degree of vitamin K epoxide reductase (VKOR) activity is genetically determined, and the mutation of VKORC1 gene encoding subunit 1 of the vitamin K epoxide reductase complex is one of the reasons for the activated vitamin K deficiency, that leads to a decrease in the synthesis of this enzyme [10, 11].
Researchers from Japan have demonstrated that vitamin K also serves as a ligand for the steroid and xenobiotics receptor (SXR) [12]. SXR belongs to the superfamily of nuclear receptors. Nuclear receptors (NRs) are a class of intracellular proteins that regulate the transcription of specific genes in certain DNA genome sequencing, activating the synthesis of enzymes and various proteins that control embryonic development, cell differentiation, apoptosis, immune response, homeostasis and metabolic processes. As a result, NRs play a key role in the embryonic development, in stem cell growth and differentiation, apoptosis, homeostasis, and metabolism of exogenous and endogenous lipophilic compounds [13].
Therefore, the objective of this study was to search for biochemical and molecular genetic predictors of pregnancy in the IVF/ICSI program.
Materials and Methods
The study included couples who presented to the Department of Assisted Reproductive Technologies in Infertility Treatment, National Medical Research Center of Obstetrics, Gynecology, and Perinatology named after Academician V.I. Kulakov, Ministry of Health of Russia, Moscow.
The study enrolled 100 patients who were eligible for inclusion according to the following criteria: tubal factor infertility, male factor infertility with unexpressed pathozoospermia, patient’s age (not more than 37 years old), regular menstrual cycle, absence of systemic autoimmune diseases). The exclusion criteria were contraindications for IVF, namely extragenital pathology, cancer, external and internal endometriosis of stage III and IV, large uterine fibroid, pathozoospermia of grades III and IV, malignant neoplasms of any localization, malformation of internal genital organs.
As a result of infertility treatment using IVF/ ICSI method, two main groups were formed in accordance with the results of infertility treatment: group I including patients with the clinical pregnancy (n = 28); group II comprising patients with a negative outcome of the treatment (n = 72).
To stimulate ovarian function, a standard ovarian stimulation protocol with gonadotropin – releasing hormone antagonist and recombinant follicle-stimulating hormone (FSH) and/or human menopausal gonadotropin was used. The ovulation trigger was introduced when there were follicles with a diameter of ≥17 mm in the ovaries. The human chorionic gonadotropin at a dose of 10,000 IU was used as an ovulation trigger. The ovarian puncture was made 36 hours after the ovulation trigger injection. Oocyte fertilization was performed by IVF/ICSI method. The luteal phase of the cycle in all patients was supported with progesterone drugs starting from the day of the follicular puncture.
The follicular fluid was collected from the first follicle of each ovary, with a needle flushing after each follicle, into the individual labeled tubes without heparin. Each portion of follicular fluid was centrifuged for 20 minutes. The study included those samples of the follicular fluid in which the oocyte was found (n = 87). Since the periostin level in the follicular fluid obtained from the different ovaries in one patient was comparable, the average periostin level in the follicular fluid obtained from the different follicles was analyzed. Oocytes from these follicles were fertilized using IVF or ICSI method. Embryos cultivation was carried out in the separate drops.
Estimation of periostin concentration (POSTN) in the follicular fluid was performed by the ELISA method using the commercial Human periostin /OSF – 2 ELISA kit (Cat. No. SK00072-08, USA) in the Clinical Diagnostic Laboratory of National Medical Research Center of Obstetrics, Gynecology, and Perinatology named after Academician V.I. Kulakov, Ministry of Health of Russia.
The morphological quality assessment of embryos was carried out on the 5th day, according to the classification approved by the Istanbul consensus workshop on embryo assessment (ESHRE, 2011) (“modified” classification of D. Gardner). Only good-quality embryos were transferred to all patients. The number of transferred embryos did not exceed two.
Genotyping was performed by polymerase chain reaction with melting curve analysis using a modified method of “adjacent probes” (kissing probes) with the help of commercial test systems “NPO DNK-Tekhnologiya” LLC, Russia. When studying CYP2C9 genotype, the presence of one of the three most common haplotypes in the European population *1, *2, *3 was determined on the basis of the NCBI Reference Sequence M61857.1 using the locus investigation of the 430 C > T (Arg144Cys) rs 1799853 and 1075 A > C loci (Ile359Leu ) rs1057910.
DNA for genotyping was isolated from peripheral blood samples with EDTA as an anticoagulant using the “Proba – GS – genetica” reagent kit by the “NPO DNK – Tekhnologiya” LLC, Russia. DNA concentration was determined by the DNA minifluorimeter (Nofer, USA) and amounted to 50–100 µg/ml on average.
Blood serum vitamin K level was determined by the ELISA method using the Vitamin K1 (VK1) commercial kit (Cat. No. CEA926Ge, USA) in the Clinical Diagnostic Laboratory of National Medical Research Center of Obstetrics, Gynecology, and Perinatology named after Academician V.I. Kulakov, Ministry of Health of Russia. The serum samples obtained from patients on the day of the transvaginal puncture were used for the study.
The study was approved by the Ethics Committee of National Medical Research Center of Obstetrics, Gynecology, and Perinatology named after Academician V.I. Kulakov, Ministry of Health of Russia. Statistical data processing was performed using the SPSS Statistics 21.0 application package. The correspondence of the analyzed parameters to the Gaussian distribution law was evaluated using the values of the Kolmogorov – Smirnov, Lilliefors tests and the Shapiro – Wilk W-test. To assess the normally distributed quantitative data, the mean value (M), the standard deviation (δ) and the mean error (m) were determined. Results were presented as M ± m. For data whose distribution differed from the normal one, the median (Me) was chosen as the measure of the central tendency, and the upper (H) and lower quartiles (L) - as the interval estimation. The results are presented as Me (L – H).
To assess the significance of intergroup differences, the Mann–Whitney U test was used for unrelated populations. The significance of differences in the frequency of qualitative traits occurrence was determined by the likelihood ratio χ2 criterion.
Spearman’s rank correlation coefficient was calculated to identify the correlation between quantitative traits.
Differences were considered statistically significant with p < 0.05 (95th level of significance) and with P < 0.01 (99th level of significance). The odds ratio is provided with a 95% confidence interval (CI).
Results
When evaluating clinical and anamnestic data, the parameters of folliculogenesis, oogenesis and early embryogenesis in patients of the studied groups, there were no statistically significant differences, the groups were homogeneous and comparable in the analyzed parameters (Table).
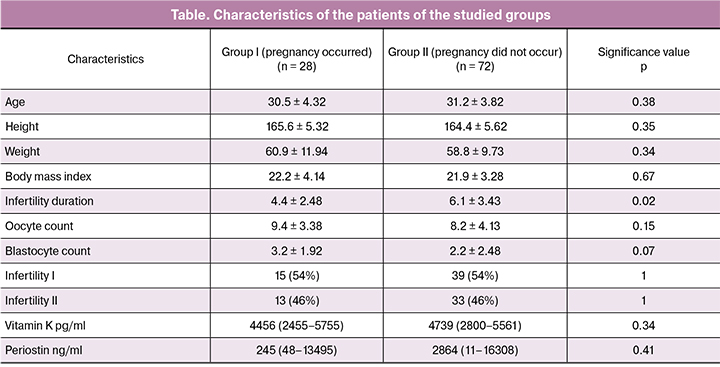
The study revealed that the serum vitamin K level in patients undergoing fertility treatment in the IVF/ICSI program and the VKORC1 gene polymorphism do not have a significant correlation with the pregnancy occurrence. However, it is worth mentioning that the serum vitamin K level is significantly lower in patients with class C embryos (i.e. low- or poor-quality embryos) according to D. Gardner classification. A critical level of vitamin K was determined using ROC-analysis and amounted to 1190 pg/ml. Among women with a vitamin K level below the threshold, the proportion of patients from whom class C embryos were obtained was 57% (4 of 7), while among women with high vitamin K levels there were only 16% (13 of 81) of such patients. The odds ratio was 0.14 (95% CI 0.03 - 0.65), p = 0.024.
No significant correlation between the quality of the obtained embryos and the VKORC1 gene polymorphism was revealed during the study. There was no association of vitamin K blood levels in women in stimulated cycles with the VKORC1 gene polymorphism. There was no significant correlation either between the periostin level in the follicular fluid and the vitamin K level.
 In probability analysis of obtaining immature oocytes in women undergoing treatment in the IVF/ICSI program, an association with the CYP2C9 genotype was detected (Fig. 1). For carriers of “slow” haplotypes (*2 and *3), the probability of obtaining immature oocytes was 61% and 46%, respectively, whereas immature oocytes were obtained for patients with a normal genotype (*1/*1) in 72% of cases. The revealed dependence had marginal statistical significance (p = 0.055). Whereas, there was no statistically significant association of CYP2C9 genotype with vitamin K and periostin levels in this study, although in the carriers of the haplotype *3 vitamin K levels were still slightly lower: 3540 (2495–5022) pg/ml compared to the other genotypes: 4540 (2730–5747) pg/ml.
In probability analysis of obtaining immature oocytes in women undergoing treatment in the IVF/ICSI program, an association with the CYP2C9 genotype was detected (Fig. 1). For carriers of “slow” haplotypes (*2 and *3), the probability of obtaining immature oocytes was 61% and 46%, respectively, whereas immature oocytes were obtained for patients with a normal genotype (*1/*1) in 72% of cases. The revealed dependence had marginal statistical significance (p = 0.055). Whereas, there was no statistically significant association of CYP2C9 genotype with vitamin K and periostin levels in this study, although in the carriers of the haplotype *3 vitamin K levels were still slightly lower: 3540 (2495–5022) pg/ml compared to the other genotypes: 4540 (2730–5747) pg/ml.
According to the study results a correlation between the probability of pregnancy occurrence in the IVF/ICSI program and the EPHX1: 337 T> C (Tyr113His) rs1051740 (Fig. 2) genotype was also revealed, although the differences did not reach statistical significance.
Discussion
The study revealed that serum vitamin K levels were significantly lower in patients with class C embryos (low- or poor-quality embryos) according to D. Gardner classification; however, there were no statistically significant differences in pregnancy occurrence rates. Apparently, this may be associated with a small set and requires further research, since the oocyte quality is a significant predictor of the IVF program success [4].
 Since vitamin K serves as a ligand for the steroid receptor and xenobiotics, its biological effects during ART programs might be mediated through a change in the detoxification system. This may explain its influence on the quality of oocytes and embryos. Polymorphism of other xenobiotic detoxification system genes in our study demonstrated the similar effects.
Since vitamin K serves as a ligand for the steroid receptor and xenobiotics, its biological effects during ART programs might be mediated through a change in the detoxification system. This may explain its influence on the quality of oocytes and embryos. Polymorphism of other xenobiotic detoxification system genes in our study demonstrated the similar effects.
Thus, the effect of the CYP2C9 genotype on the oocyte maturation process remains understudied. The CYP2C9 gene encodes the amino acid sequence of the cytochrome P450 enzyme (family 2, subfamily C, polypeptide 9). CYP2C9 is one of the enzymes detoxifying the body from xenobiotics. If the carriers of “wild-type” CYP2C9*1 have the standard xenobiotic metabolism rate, the enzyme activity can be reduced to 90% in the CYP2C9*2 and CYP2C9*3 variants, which leads to a decrease in the xenobiotic metabolism rate, and that in turn can lead to the accumulation of various xenobiotics. This mechanism underlies the changes of the effectiveness of a number of drugs and results in the need to reduce the dosage of the corresponding drugs. It is possible that the polymorphism in the CYP2C9 gene modifies the effectiveness of pharmacological support for the oocytes maturation.
According to the literature, the role of gene polymorphism of the detoxification system was demonstrated in the development of gestational complications [14]. There is also evidence of the significance of this system for conducting ART programs. Malyshkina A.I. et al., in their work on the detoxification genes polymorphism in couples participating in the IVF program, demonstrated the role of the detoxification system in women with tuboperitoneal infertility and low-quality embryos obtained during the IVF procedure [15].
The study also revealed the dependence of the probability of pregnancy on the EPHX1 genotype. The microsomal epoxide hydrolase gene is an enzyme in the xenobiotic biotransformation system. The T-337C polymorphism is responsible for reducing the enzyme activity by 50% (the “slow” allele), resulting in a decrease in the inactivation efficiency of toxic metabolites. It leads to the development of “oxidative stress”, the essence of which is the release of a large number of free radicals that significantly exceed physiological needs. It was demonstrated that there is an association of the EPHX gene polymorphism with the development of the chronic obstructive pulmonary disease, bronchial asthma, pathology of the female reproductive system: endometriosis, complicated pregnancy (gestosis, miscarriage) implemented via the powerful damaging effect of free radicals.
The detoxification system genes are involved in the mechanisms of cell protection against the damaging effects of xenobiotics and endogenous substances, including products of peroxidation. Probably, the lack of genetic “protection” of the body from environmental pressure on the one hand, and the inability of the enzyme systems of the cell to adequately neutralize endogenous toxic substances during their metabolic transformations on the other hand, negatively affect the quality of oocytes, and as a result, the quality of embryos in ART program.
Conclusion
Thus, considering the results of this study, it can be assumed that the serum vitamin K levels may be a prognostic value for determining the quality of the embryo in the IVF/ICSI program. The question of the effect of the detoxification system genes on the quality of oocytes, and, as a consequence, the quality of embryos and the chance of getting pregnant in ART programs is also noteworthy.
References
1. Pandey S., Shetty A., Hamilton M., Bhattacharya S., Maheshwari A. Obstetric and perinatal outcomes in singleton pregnancies resulting from IVF/ICSI: a systematic review and meta-analysis. Hum. Reprod. Update. 2012; 18(5): 485-503. doi: 10.1093/humupd/dms018.
2. Calhaz-Jorge C., De Geyter C., Kupka M.S., de Mouzon J., Erb K., Mocanu E. et al.; European IVF-monitoring Consortium (EIM); European Society of Human Reproduction and Embryology (ESHRE). Assisted reproductive technology in Europe, 2013: results generated from European registers by ESHRE. Hum. Reprod. 2017; 32(10): 1957-73. doi: 10.1093/humrep/dex264.
3. Калинина Е.А., Эбзеева М.В., Кузьмичев Л.Н. Опыт применения «мягких» схем стимуляции суперовуляции у пациенток группы риска развития синдрома гиперстимуляции яичников. Акушерство и гинекология. 2010; 6: 60-4.
4. Кузьмичев Л.Н., Смольникова В.Ю., Калинина Е.А., Дюжева Е.В. Принципы комплексной оценки и подготовки эндометрия у пациенток программ вспомогательных репродуктивных технологий. Акушерство и гинекология. 2010; 5: 32-6.
5. Краснощока О.Е., Смольникова В.Ю., Калинина Е.А., Елагин В.В. Роль морфологической оценки ооцита и эмбриона при использовании вспомогательных репродуктивных технологий (обзор литературы). Проблемы репродукции. 2015; 21(1): 54-8.
6. Davidson L.M., Coward K. Molecular mechanisms of membrane interaction at implantation. Birth Defects Res. C Embryo Today. 2016; 108(1): 19-32. doi: 10.1002/bdrc.21122.
7. Murota H., Lingli Y., Katayama I. Periostin in the pathogenesis of skin diseases. Cell. Mol. Life Sci. 2017; 74(23): 4321-8. doi: 10.1007/s00018-017-2647-1.
8. Di Cello A., Rania E., Di Sanzo M., Alviggi E., Rienzi L., Morelli M. et al. Periostin a new non-invasive parameter in addition to the morphologic criteria for evaluating oocyte/blastocyst quality and its impact on endometrial receptivity. In: Abstract book of the 31st ESHRE Annual Meeting, Lisbon, Portugal, 14-17 June 2015. Hum. Reprod. 2015; 30(Suppl. 1): i1-i501. doi: 10.1093/humrep/30.Supplement_1.1.
9. Coutu D.L., Wu J.H., Monette A., Rivard G.E., Blostein M.D., Galipeau J. Periostin, a member of a novel family of vitamin K-dependent proteins, is expressed by mesenchymal stromal cells. J. Biol. Chem. 2008; 283(26): 17991-8001. doi: 10.1074/jbc.M708029200.
10. Загорская В.Л. Изучение полиморфизма гена, кодирующего витамин К эпоксид-редуктазу (VKORC1) и его значение в клинической практике. Рефлексология. 2007; 1-2: 20-2.
11. Лавринов П.А., Белова Н.И., Воробьева Н.А. Полиморфизмы гена VKORC1 -1639 G/A и 1173 C/Т в популяции коренных жителей Ненецкого автономного округа. Ученые записки Санкт-Петербургского государственного медицинского университета имени академика И.П. Павлова. 2014; 21(2): 33-6. doi: 10.24884/1607-4181-2014-21-2-33-36.
12. Azuma K., Urano T., Watabe T., Ouchi Y., Inoue S. PROX1 suppresses vitamin K-induced transcriptional activity of Steroid and Xenobiotic Receptor. Genes Cells. 2011; 16(11): 1063-70. doi: 10.1111/j.1365-2443.2011.01551.x.
13. Huang P., Chandra V., Rastinejad F. Structural overview of the nuclear receptor superfamily: insights into physiology and therapeutics. Ann. Rev. Physiol. 2010; 72: 247-72. doi: 10.1146/annurev-physiol-021909-135917.
14. Казанцева Е.В., Долгушина Н.В., Донников А.Е., Беднягин Л.А., Баранова Е.Е., Терешков П.П. Влияние пренатальной экспозиции бенз(а)пирена, стриола и формальдегида на массу тела при рождении в зависимости от полиморфизмов генов системы детоксикации. Акушерство и гинекология. 2016; 7: 68-78.
15. Малышкина А.И., Фетисова И.Н., Липин М.А. Полиморфизм генов детоксикации в супружеских парах, участвующих в программе ЭКО. Детская медицина Северо-Запада. 2012; 3(2): 24-6.
Received 21.03.2018
Accepted 20.04.2018
About the Authors
Khechumyan, Lusine R., graduate student of department of assisted reproductive technology in treating sterility Academician V.I. Kulakov Medical Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina 4. Tel. +7 (495) 438-25-01 E-mail: khechumyan_l@mail.ruKalinina, Elena A., MD, Associate Professor, The chief of department of assisted reproductive technology in treating sterility Academician V.I. Kulakov Medical Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia.
117997, Russia, Moscow, Ac. Oparina 4. Tel. +7(495) 438-13-41 E-mail: e_kalinina@oparina4.ru
Donnikov, Andrew E., PhD, Head of laboratory of molecular-genetical methods Academician V.I. Kulakov Medical Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina 4. Tel. +7(495) 438-13-41 E-mail: a_donnikov@oparina4.ru
Ivanets, Tatiana Yu., PhD, The head of the clinical laboratory of the Academician V.I. Kulakov Medical Research Center of Obstetrics, Gynecology, and Perinatology,
Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina 4. Tel.: +7(495) 438-25-66, E-mail: t_ivanets@oparina4.ru.
Kulakova, Elena V., PhD, Senior Researcher of department of assisted reproductive technology in treating sterility Academician V.I. Kulakov Medical Research
Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia.
117997, Russia, Moscow, Ac. Oparina 4. Tel. +7(495) 438-13-41 E-mail: evkulakova@mail.ru
For citations: Khechumyan L.R., Kalinina E.A., Donnikov A.E., Ivanets T.Yu., Kulakova E.V. Association of blood vitamin K level and polymorphism of detoxification genes with the outcomes of an assisted reproductive technology program. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2018; (11): 80-5. (in Russian)
https://dx.doi.org/10.18565/aig.2018.11.80-85



