Association of polymorphism of genes in the xenobiotic biotransformation system with early first pregnancy loss in a large industrial region
Shramko S.V., Matoshin S.V., Gulyaeva O.N., Samus I.V.
Relevance: The 15–20-fold increase in the number of missed miscarriages in Novokuznetsk over the past 25 years amid deteriorating environmental conditions is of great public health concern.
Objective: To conduct a molecular genetic analysis of gene polymorphisms in the xenobiotic metabolism enzyme system, specifically focusing on CYP1A1 (3798 A>G rs4646903), CYP1A2 (-163 A>C, rs762551), GSTM1 (deletion), and GSTT1 (deletion), as potential genetic risk factors for early first pregnancy loss among women living in a large industrial region.
Materials and methods: This case-control study included 358 primigravida women, matched for age, residing in a large industrial region from 2021 to 2023. Among them, 186 had normal births, while 172 had missed miscarriages before the 12th week of gestation. Gene polymorphisms in the xenobiotic biotransformation system (glutathione-S-transferases and cytochrome P450) were examined using venous blood samples from 102 women (53 with normal births and 49 with missed miscarriages).
Results: Women with normal childbirth were significantly more likely to have the AA genotype of CYP1A1 and the normal genotypes of GSTM1 and GSTT1 (p=0.016 and p=0.002, respectively). In contrast, patients with missed miscarriages had the AG genotype of CYP1A1 (p=0.046), the AA genotype of CYP1A2 (p=0.03), and deletion polymorphisms in GSTM1 and GSTT1 (p<0.001 and p=0.004, respectively).
Conclusion: The combination of the AG genotype of CYP1A1 and the AA genotype of CYP1A2, along with deletion polymorphisms in GSTM1 and GSTT1, is associated with an increased risk of miscarriage. The findings of this study can be utilized for biological optimization, career guidance, and the prevention of missed miscarriages among women living in large industrial regions.
Authors' contributions: Shramko S.V., Matoshin S.V. – conception and design of the study; Matoshin S.V. – data collecting and processing; Gulyaeva O.N. – blood plasma samples preparation and analysis; Samus I.V. – statistical analysis; Shramko S.V., Matoshin S.V., Gulyaeva O.N. – drafting of the manuscript; Shramko S.V., Gulyaeva O.N. – editing of the manuscript, final approval for submission.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the Novokuznetsk State Institute of Advanced Medical Training (Ref. No: 3 of 10.04.2024).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Shramko S.V., Matoshin S.V., Gulyaeva O.N., Samus I.V.
Association of polymorphism of genes in the xenobiotic biotransformation system
with early first pregnancy loss in a large industrial region.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (8): 58-68 (in Russian)
https://dx.doi.org/10.18565/aig.2024.91
Keywords
Currently, Russia is experiencing an increase in depopulation [1]. The country is facing a demographic crisis owing to low birth rates and high levels of reproductive losses [2]. In 13 Russian cities, including Novokuznetsk, this crisis is exacerbated by low birth rates, high mortality rates, large migration outflows, and environmental deterioration [3]. Pregnancy loss has not only medical but also significant social implications, especially in terms of demographic outcomes [4, 5].
It is important to note that around 30% of pregnancies in healthy women are terminated at the preclinical stage due to "genetic reset." In addition, every fifth diagnosed and desired pregnancy ends unfavorably, with death of the embryo in the early stages. This includes missed miscarriages (MM), which account for up to 10% of these cases. Over the past decade, both absolute and relative MM rates have increased [6, 7]. In Novokuznetsk, according to the reporting documentation of the pregnancy pathology department of the G.P. Kurbatov First City Clinical Hospital, the number of MM has increased 15–20 times since 1997.
Although the main groups of causal factors of reproductive losses have been well studied, genetic factors are considered the leading cause. However, in one-third of cases, the causes of MM remain unknown [8–10].
To date, numerous ecological and epidemiological studies have confirmed the detrimental effects of environmental chemical pollutants on gestational processes, pregnancy outcomes, and the development of congenital defects [11, 12]. Xenobiotics that enter the body are metabolized and eliminated by xenobiotic biotransformation systems. In phase I of metabolism, xenobiotic molecules undergo oxidation, reduction, or hydrolysis involving cytochrome P450 (CYP) enzymes, resulting in the formation of chemically inert metabolites as well as reactive electrophilic, toxic, mutagenic, and carcinogenic compounds. Phase II involves conjugation of these compounds and an increase in their hydrophilicity through transferase enzymes (GSTM1, GSTT1, and GSTP1), followed by detoxification and elimination from the body [13]. Imbalances in the activity of phase I and II enzymes can lead to the accumulation of toxic metabolites capable of damaging DNA and proteins, thus triggering teratogenic and carcinogenic processes [14].
Under normal conditions, the embryo is protected by the mother's detoxifying enzyme system owing to the effects of toxins and teratogens. However, in the context of modern technogenic civilization, it is believed that genes related to xenobiotic biotransformation systems may contribute to disruptions in normal pregnancy, particularly when "functionally weakened" gene variants (alleles) are present [15, 16]. The significance of gene polymorphisms in the xenobiotic biotransformation system in early reproductive losses and their potential damaging mechanisms during gametogenesis, fertilization, implantation, placentation, and embryogenesis are being actively studied; however, the findings thus far are ambiguous [10, 17, 18].
There is scientific and practical interest in exploring various genetic aspects of early reproductive losses with the aim of developing personalized approaches to predict such losses. However, a comprehensive assessment of the involvement of polymorphic variants of genes in xenobiotic biotransformation systems in the development of early miscarriages has not yet been conducted in large industrial regions.
In this study, we conducted a molecular genetic analysis of gene polymorphisms in the xenobiotic metabolism enzyme system, specifically focusing on CYP1A1 (3798 A>G rs4646903), CYP1A2 (-163 A>C, rs762551), GSTM1 (del), and GSTT1 (del) as potential genetic risk factors for early first pregnancy loss among women living in a large industrial region.
Materials and methods
This study was conducted in two stages. The first stage analyzed clinical and medical history data, and the second included a molecular genetic analysis of the polymorphism of the genes of the xenobiotic metabolism system; both stages corresponded to the case-control study type. The first-stage sample included 358 primigravida women living in a large industrial region. The sample was formed during the period 2021–2023. During this period, 228 cases of MM were registered among primigravida women aged <23 years in the Novokuznetsk and Novokuznetsk districts. Of these, 214 provided informed consent to participate in the study and 42 patients were excluded in accordance with the criteria. The group of patients included 172 patients with MM before 12 weeks of gestation: 15/8 (7%) before 7+6 weeks, 116/67 (4%) before 8–10+6 weeks, and 41/23 (8%) before 11–12+6 weeks. The control group included 186 women with physiologic birth (PB), who were comparable to the case group by age, place of residence, and gynecological history.
The inclusion criteria for the groups were the first pregnancy, age up to 23 years, desire to participate in the study; for the MM group signs of non-viable pregnancy according to ultrasound examination, for the PB group normal pregnancy without threat of termination, ending in PB with an Apgar score of 8–10.
The exclusion criteria were acute and chronic infectious diseases in the acute stage, decompensated extragenital diseases, and refusal to participate in the study. The study was reviewed and approved by the Research Ethics Committee of Novokuznetsk State Institute of Advanced Medical Training.
A sample size calculation was not performed, and all information available in the database was used to maximize the power and generalizability of the results. There were no missing values (for the analyzed variables) in the database.
In the second phase of the study, subgroups were created from the original study groups for genetic analysis. The patients were selected by simple randomization, and the MM and PB groups included 49 and 53 patients, respectively. For analysis of allelic variants of genes encoding CYP1A1, CYP1A2, GSTM1, and GSTT1 enzymes involved in xenobiotic biotransformation, venous blood was collected in a sterile disposable 5 ml tube containing a preservative (K3EDTA solution) in a 1:10 ratio. Samples were stored at -20°C until the reaction. The gene characteristics are shown in Table 1 (https://www.ncbi.nlm.nih.gov).
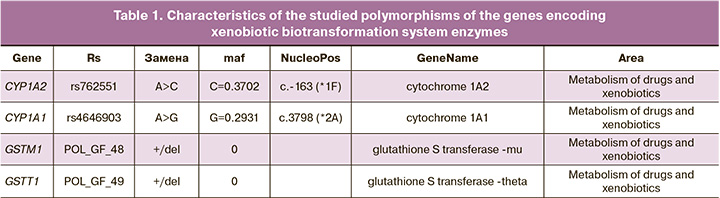
Genomic DNA from peripheral blood leukocytes was isolated using the phenol-chloroform extraction method, followed by ethanol precipitation [19]. DNA samples were stored at -20°C. Genotyping of single nucleotide substitutions in GSTM1, GSTT1, CYP1A2*1F, and CYP1A1*2C was performed using the RealTime method on a DTprime 4 device owned by NPO DNA-Technology LLC using competing TaqMan probes complementary to polymorphic DNA regions. Test systems for the molecular genetic analysis of polymorphisms were developed by the Institute of Chemical Biology and Fundamental Medicine SB RAS and synthesized by SibDNA LLC. Each sample was amplified using a pair of primers and two probes carrying a "quencher" at the 3' end and different fluorescent dyes (FAM or R6G) at the 5' end. The total volume of the reaction mixture was 25 μl, the mixture contained 40–100 ng of DNA; 300 nM of each primer; 100–200 nM Taqman probes conjugated with FAM or R6G; 200 μM dNTP, amplification buffer, thermostable Taq polymerase – 0.5 units of activity per reaction. The main parameter that we considered for each reaction was the ratio of fluorescence values (relative fluorescence unit, RFU) in the emission ranges of the FAM and R6G dyes. For the C/C genotype (CYP1A1*2C), the fluorescence intensity increased mainly in the FAM range; for the T/T genotype (CYP1A1*2C), mainly in the R6G range; and for the heterozygous genotype, the fluorescence intensity increased in both ranges. When typing the GSTM (del) and GSTT (del) genes, the absence of a fluorescent signal indicated homozygosity of the individual for the deletion (mutation) of the GSTM1 and GSTT1 genes – 0/0. Heterozygotes for the mutation (genotype +/0) were considered in one group with homozygous carriers of "normal" genes (+/+). Polymorphisms in the genes of the xenobiotic biotransformation system were studied at the Research Institute for Complex Problems of Hygiene and Occupational Diseases in Novokuznetsk (Director – Prof. N.N. Mikhailova).
Statistical analysis
All analyzed variables were categorical. Descriptive statistics are presented as frequencies (n) and percentages (%). The chi-square test for 2×2 contingency tables was used to compare the categorical variables. Fisher’s exact test was used when the expected frequency of one or more cells was less than five.
The second part of the study (genetic analysis) was conducted in accordance with modern trends in the statistical analysis of genetic and epidemiological studies [20]. Groups were compared according to five possible inheritance models (codominant, dominant, recessive, overdominant, and log-additive). In the absence of homozygous recessive genotypes, only the codominant model was analyzed. The Akaike information criterion was calculated for each model. Statistical significance was assessed using a likelihood ratio test. The most probable inheritance model for each specific gene polymorphism had the lowest Akaike information criterion value. For each inheritance model, odds ratios and their 95% confidence intervals were presented, and the homozygous dominant genotype was selected as the reference.
Differences were considered to be statistically significant at p≤0.05. The calculations were performed using the R statistical environment (v.3.6, GNU GPL2 license).
Results
The study groups were comparable in terms of age, place of residence, and gynecological history (Table 2).
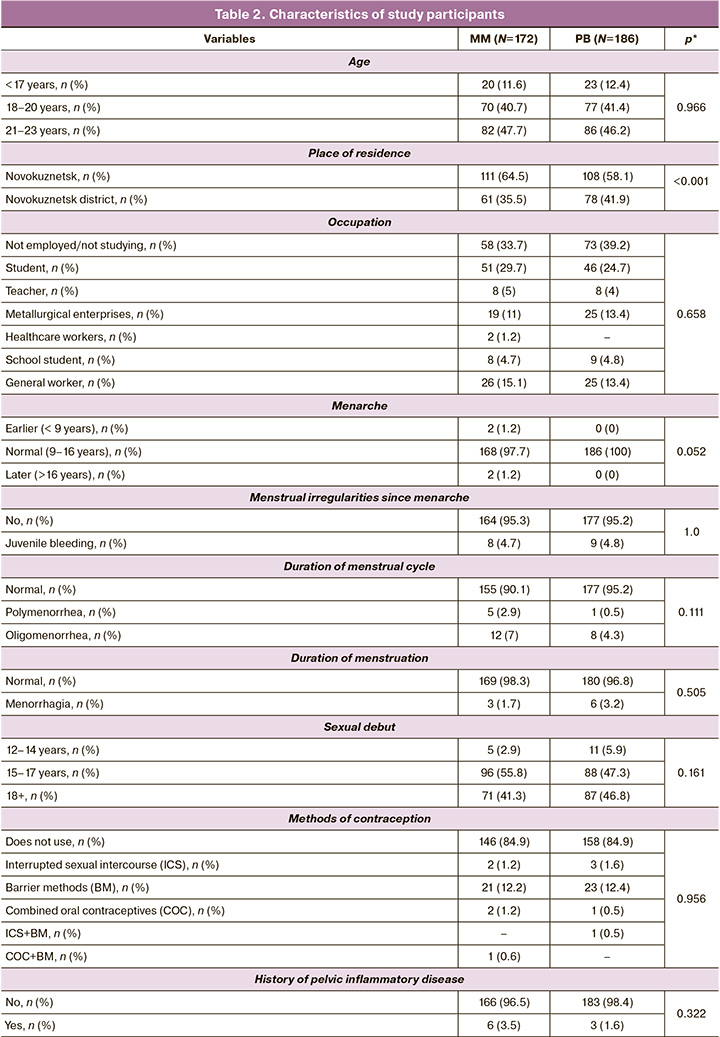
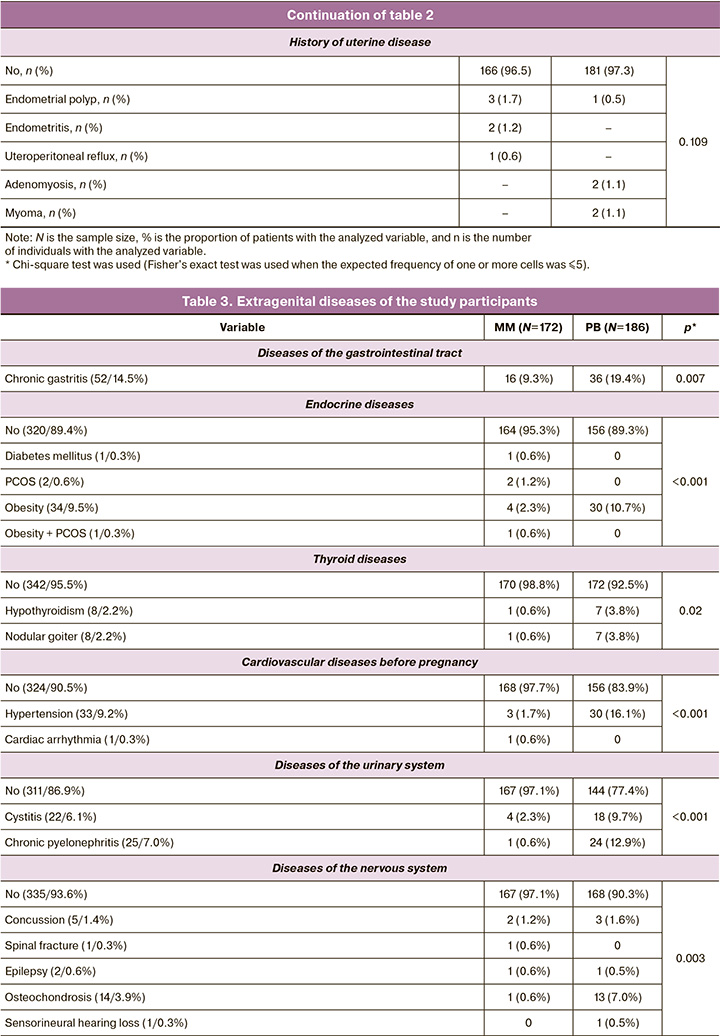
Patients with MM were found to have good somatic health, with significantly lower rates of obesity (p<0.001), endocrine (p<0.001), respiratory (p<0.001), cardiovascular (p<0.001), gastrointestinal (p=0.007), nervous (p<0.001), visual (p<0.001), and urinary (p<0.001) disorders (Table 3).
Analysis of the vaginal microbiota showed a predominance of opportunistic flora (p<0.001), and candidal vulvovaginitis was significantly more frequent (p<0.001) (Table 4). On the other hand, morphological examination of the abortive material of patients with MM revealed an inflammatory process in 14.0% (24/172) of cases, while in PB, inflammatory changes in the placenta were observed twice less often, 6.5% (12/186) (p<0.001), which confirms the multifactorial genesis of MM and allows consideration of inflammation as a risk factor for early reproductive losses.
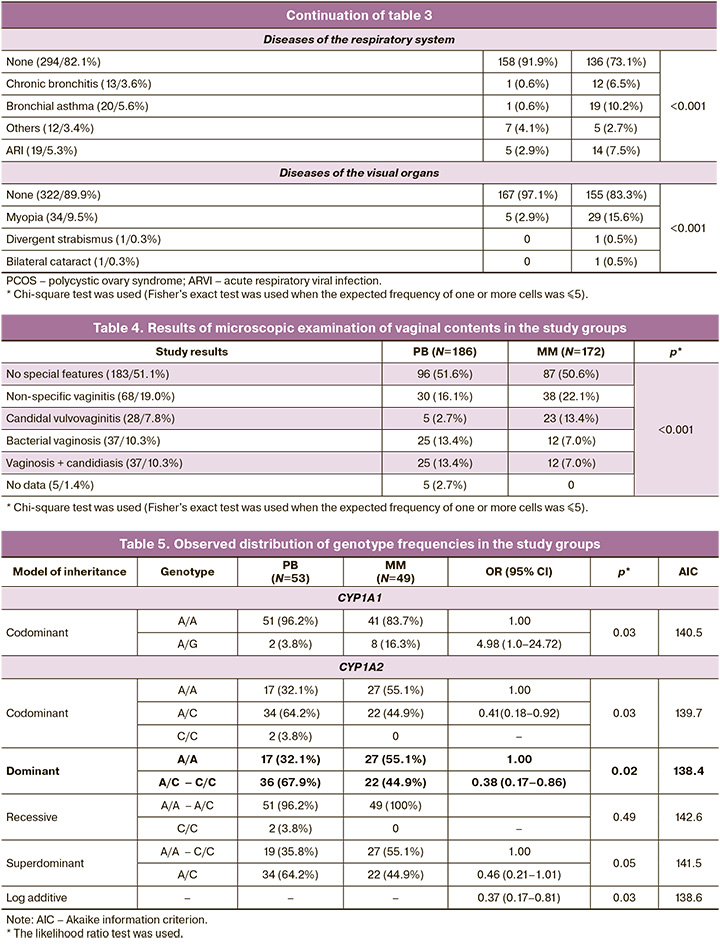
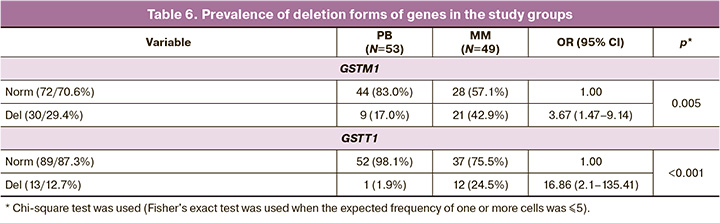
In addition, our study established different frequencies of polymorphic variants of genes encoding enzymes of phases I and II of the xenobiotic biotransformation system (Tables 5 and 6).
Analysis of the CYP1A2 gene showed that the most probable inheritance model was the dominant model (Akaike criterion is 138.4, the lowest among other models), indicating that an association of the homozygous genotype A/A of the CYP1A2 gene with ST: A/C – C/C relative to A/A is protective (OR=0.38, 95% CI 0.17–0.86, p=0.018). The AA genotype of CYP1A2 is associated with a high inducibility and activity of the encoded enzyme. This leads to an increased risk of developing various forms of complicated obstetric history, including MM, due to an increased rate of mutations resulting from damage to DNA molecules by PAH-DNA adducts. The activity of this enzyme is normally regulated by the body of the woman, leading to a decrease in its production throughout pregnancy. In this context, the maternal homozygous AA genotype increases the mutation load of the fetus, especially under adverse environmental conditions, and is a risk factor for pregnancy-related complications [21].
Of particular interest is the heterozygous AG genotype of the CYP1A1 gene, associated with the death of the ovum in primigravida women, since the G allele is extremely rare in the population and was found only in the heterozygous AG form of the CYP1A1 gene in our sample [22]. The A/A genotype of the CYP1A1 gene, which is common in the population, was observed in our study significantly more often in women with PB (OR=4.98, 95% CI 1.0–24.72, p=0.005), which is associated with resistance to early reproductive losses.
Analysis of the incidence of the GSTM1 "0" (OR=3.67, 95% CI 1.47–9.14, p=0.005) and GSTT1 "0" (OR=16.86, 95% CI 2.1–135.41, p<0.001) gene polymorphisms showed a statistically significant association between the deletion forms of these genes and the death of the ovum. Carriers of these alleles have an extended deletion in the structural part of both genes, which leads to the absence of enzymatic activity, a decrease in the conjugation of toxic phase I products with mutagenic and carcinogenic properties, and their accumulation in the body of pregnant women. Thus, in women with MM, we observed an increase in the incidence of gene forms that ensure the activity of phase I enzymes of the xenobiotic biotransformation system, which is beneficial in conditions of increased anthropogenic load. At the same time, the increased frequency of deletion polymorphisms of the genes of phase II of the xenobiotic biotransformation system indicates a decrease in the activity of glutathione S-transferases and an impairment in the elimination of toxic products.
Discussion
CYP1A1 and CYP1A2 are members of the human cytochrome P450 subfamily. They are located on chromosome 15q24.1 and 15q22. These genes encode monooxygenases that play a critical role in the biotransformation of various substances in the body. These include endogenous substrates such as estradiol, arachidonic acid, and cholesterol as well as exogenous compounds such as smoke components, polycyclic aromatic hydrocarbons, and therapeutic drugs [14, 23].
Our study found a significant increase in the frequency of certain forms of the CYP1A1 and CYP1A2 genes in patients with MM. This supports the idea that polymorphisms in these genes are involved in early reproductive losses, a finding that is consistent with that of other studies [24]. These enzymes are crucial for the body to detoxify harmful environmental factors. However, if the activity of phase I enzymes is too high and that of phase II detoxification enzymes is low, toxic metabolites can accumulate in the body and cause damage [25].
Our study also revealed a link between specific forms of genes related to phases I and II of the xenobiotic biotransformation system and a high risk of ovum death in young primigravida women. Deletions in GSTM1 and GSTT1 were found to be significantly more common in patients with MM than in women with PB. These genes belong to the glutathione-S-transferase (GST) family, which includes important antioxidant enzymes involved in stage II detoxification. These proteins are also expressed during embryonic development. Loss of functional activity due to deletions in the GSTM1 (chromosome 1p13.3) and GSTT1 (chromosome 22q11.2) genes can lead to an imbalance of enzymes and accumulation of toxins in the mother and fetus, increasing the risk of miscarriage and pregnancy complications [26, 27].
The association between weakened alleles of the GSTM1 gene and miscarriage was first discovered by Hirvonen A. et al. in 1996 [29] and has since been confirmed by other researchers, including those at the Research Institute of Obstetrics and Gynecology named after D.O. Otta in St. Petersburg [10, 30]. Several studies have investigated the role of xenobiotic biotransformation system genes in reproductive losses; however, the results have been contradictory. This is likely due to differences in population background, level of environmental pollution, and criteria used for selecting study participants. Notably, Novokuznetsk, where our study was conducted, is located in an area with high levels of anthropogenic pollution [31]. Furthermore, the southern Kemerovo region is the original home of the Shor ethnic group, which may affect the frequency of deletion polymorphisms in phase II xenobiotic biotransformation system genes within the population [32].
Patients in our study with the first MM had no underlying health issues and no history of obstetric and gynecological problems. However, they possess genes from phases I and II of the xenobiotic biotransformation system that can potentially disrupt the balance of detoxification enzymes, making the female body vulnerable and unstable during pregnancy, particularly in environments with high levels of pollution. This suggests that external factors (the environment) may pose a greater risk than internal factors (homeostasis) for reproductive losses.
Conclusion
The presence of the heterozygous A/G variant of CYP1A1 and the homozygous A/A variant of CYP1A2, combined with deletions in GSTM1 and GSTT1, may create a genetic foundation for an imbalance between toxicity and detoxification processes. This imbalance can lead to the increased formation of highly toxic xenobiotic metabolites and death of the embryo at early stages due to mutations that are incompatible with life. In contrast, individuals with the homozygous A/A genotype of the CYP1A1 gene, along with the normal genotypes of GSTM1 and GSTT1, have a successful pregnancy outcome and are not at risk for MM. This study highlights the need to develop genetic monitoring methods that consider the molecular genetic characteristics of women who are planning to become pregnant. The identified risk markers for reproductive loss can be used to assess individual predispositions to MM and guide career choices for young women. The authors believe that further research on the mechanisms of the genetic control of MM is necessary.
References
- Ткаченко А.А. Социально-экономическая оценка развития демографической ситуации в России. Социально-трудовые исследования. 2021; 4(45): 89-97. [Tkachenko A.A. Socio-economic assessment of the development of the demographic situation in Russia. Social and labor research. 2021; 4(45): 89-97. (in Russian)]. https://dx.doi.org/10.34022/2658-3712-2021-45-4-89-97.
- Федеральная служба государственной статистики. Естественное движение населения РФ за 2021 год (статистический бюллетень). 2022. Доступно по: https://rosstat.gov.ru/compendium/document/13269 [Federal State Statistics Service. Natural movement of the population of the Russian Federation for 2021 (Statistical Bulletin). 2022. Available at: https://rosstat.gov.ru/compendium/document/13269 (in Russian)].
- Федеральная служба государственной статистики. Естественное движение населения Кузбасса. 2022. Доступно по: https://42.rosstat.gov.ru/storage/mediabank/ЕДН%20и%20миграция%20в%20Кузбассе%20_%20декабрь_2022%20(3).pdf [Federal State Statistics Service. The natural movement of the Kuzbass population. 2022. Available at: https://42.rosstat.gov.ru/storage/mediabank/ЕДН%20и%20миграция%20в%20Кузбассе%20_%20декабрь_2022%20(3).pdf (in Russian)].
- Chichester M., Harding K.M. Early pregnancy loss: invisible but real. Nursing. 2021; 51(12) 28-32. https://dx.doi.org/10.1097/01.NURSE.0000800080.92781.c5.
- The long, good life. Demographics and economic well-being. Finance & Development. A quarterly publication of the International Monetary Fund. 2020; 57(1). https://dx.doi.org/10.5089/9781513528861.022.
- Олина А.А. Неразвивающаяся беременность и гиперпролактринемия. Есть ли место растительным препаратам в терапии? РМЖ. Мать и дитя. 2020; 3(2): 64-9. [Olina A.A. Non-developing pregnancy and hyperprolactrinemia. Is there a place for herbal medicines in therapy? RMJ. Mother and child. 2020; 3(2): 64-69. (in Russian)]. https://dx.doi.org/10.32364/2618-8430-2020-3-2-64-69.
- Олина А.А., Садыкова Г.К., Галинова И.В. Структура репродуктивных потерь. Пермский медицинский журнал. 2017; 34(6): 59-66. [Olina A.A., Sadykova G.K., Galinova I.V. Structure of reproductive losses. Perm Medical Journal. 2017; 34(6): 59-66. (in Russian)]. https://dx.doi.org/10.17816/pmj34659%66.
- Междисциплинарная ассоциация специалистов репродуктивной медицины (МАРС). Неразвивающаяся беременность в анамнезе: реабилитация и подготовка к следующей гестации. Методические рекомендации МАРС. Версия 2.0. М.: Редакция журнала StatusPraesens; 2021. 68 с. [Interdisciplinary Association of Reproductive Medicine Specialists (MARS). History of non-developing pregnancy: rehabilitation and preparation for the next gestation. MARS Guidelines. Version 2.0. Moscow: StatusPraesens; 2021. 68 p. (in Russian)].
- Министерство здравоохранения Российской Федерации. Выкидыш (самопроизвольный аборт). Клинические рекомендации. 2021. 25 с. [Ministry of Health of the Russian Federation. Miscarriage (spontaneous abortion). Clinical guidelines. 2021. 25 p. (in Russian)].
- Беспалова О.Н. Генетика невынашивания беременности. Журнал акушерства и женских болезней. 2007; 1: 81-95. [Bespalova O.N. Genetics of pregnancy miscarriage. Journal of Obstetrics and Women's Diseases. 2007; 1: 81-95. (in Russian)].
- Liu B., Lu X., Jiang A., Lv Y., Zhang H., Xu B. Influence of maternal endocrine disrupting chemicals exposure on adverse pregnancy outcomes: a systematic review and meta-analysis. Ecotoxicol. Environ. Saf. 2024; 270: 115851. https://dx.doi.org/10.1016/j.ecoenv.2023.115851.
- Шабалдин А.В., Глебова Л.А., Бачина А.В., Цепокина А.В., Счастливцев Е.Л., Потапов В.П. Сравнительная характеристика встречаемости различных врожденных пороков развития плода с позиции оценки экологической опасности в крупном промышленном центре. Мать и дитя в Кузбассе. 2014; 4(59): 19-24. [Shabaldin A.V., Glebova L.A., Bachina A.V., Cepokina A.V., Schastlivcev E.L., Potapov V.P. Comparative characteristics of the incidence of various congenital malformations of the fetus from the perspective of assessing environmental hazards in the large industrial center. Mother and Child in Kuzbass. 2014; 4(59): 19-24. (in Russian)].
- Чурносов М.И., Полякова И.С., Пахомов С.П., Орлова В.С. Молекулярные и генетические механизмы биотрансформации ксенобиотиков. Актуальные проблемы медицины. 2011; 15(16(111)): 223-8. [Churnosov M.I., Poliakova I.S., Pachomov S.P., Orlova V.S. Molecular and genetic mechanisms of xenobiotic biotransformation. Challenges in modern medicine. 2011; 15(16(111)): 223-8. (in Russian)].
- Гуляева Л.Ф., Вавилин В.А., Ляхович В.В. Ферменты биотрансформации ксенобиотиков в химическом канцерогенезе. Новосибирск: серия «Экология»; 2000. 85c. [Gulyaeva L.F., Vavilin V.A., Lyakhovich V.V. Xenobiotic biotransformation enzymes in chemical cancerogenesis. Novosibirsk: seriya «Ekologiya»; 2000; 85 p. (in Russian)].
- Супрун С.В., Кудряшова О.С., Наговицына Е.Б., Власова М.А., Морозова О.Н. Роль генов детоксикации в формировании осложнений гестационного процесса у женщин. Таврический медико-биологический вестник. 2017; 20(2-2): 154-9. [Suprun S.V., Kudryashova O.S., Nagovicyna E.B., Vlasova M.A., Morozova O.N. The role of detoxification genes in the formation of complications of the gestational process in women. Tauride Medical and Biological Bulletin. 2017; 20(2-2): 154-9. (in Russian)].
- Гордеева Л.А., Воронина Е.Н., Глушков А.Н. Генетические особенности метаболизма ксенобиотиков и предрасположенность к патологии беременности. Часть 1. Медицина в Кузбассе. 2016; 15(2): 8-16. [Gordeeva L.A., Voronina E.N., Glushkov A.N. Genetic features of xenobiotic metabolism and susceptibility to the pathology of pregnancy. Part 1. Medicine in Kuzbass. 2016; 15(2): 8-16. (in Russian)].
- Kobayashi S., Sata F., Sasaki S., Ban S., Miyashita C., Okada E. et al. Maternal genetic polymorphisms and unexplained recurrent miscarriage: a systematic review and meta-analysis. Clin. Genet 2017; 91(2): 265-284. https://dx.doi.org/10.1111/cge.12910.
- Гордеева Л.А., Попова О.С., Воронина Е.Н., Шаталина И.В., Оленникова Р.В., Нерсесян С.Л., Филипенко М.Л., Глушков А.Н. Ассоциации полиморфизма генов ферментов биотрансформации ксенобиотиков с невынашиванием беременности в ранние сроки. Молекулярная медицина. 2017; 15(3): 37-44. [Gordeeva L.A., Popova O.S., Voronina E.N., Shatalina I.V., Olennikova R.V., Nersesyan S.L., Filipenko M.L., Glushkov A.N. Association of gene polymorphisms of xenobiotics-metabolizing enzymes with the recurrent miscarriage. Molecular Medicine. 2017; 15(3): 37-44. (in Russian)].
- Маниатис Т., Фрич Э., Сэмбрук Дж. Молекулярное клонирование. М.: Мир; 1984. 480 с. [Maniatis T., Frich E., Sembruk Dzh. Molecular cloning. Moscow: Mir; 1984. 480 p. (in Russian)].
- Кутихин А.Г., Южалин А.Е., Понасенко А.В. Современные тенденции статистической обработки данных и представления результатов в кандидатных генетико-эпидемиологических исследованиях. Фундаментальная и клиническая медицина. 2017; 2(2): 77-82. [Kutihin A.G., Yuzhalin A.E., Ponasenko A.V. Current trends in statistical data processing and presentation of results in candidate genetic and epidemiological studies. Fundamental and Clinical Medicine. 2017; 2(2): 77-82. (in Russian)]. https://dx.doi. org/10.23946/2500-0764-2017-2-2-77-82.
- Tracy T.S., Venkataramanan R., Glover D.D., Caritis S.N. Temporal changes in drug metabolism (CYP1A2, CYP2D6 and CYP3A activity) during pregnansy. Am. J. Obstet Gynecol. 2005; 192(2): 633-9. https://dx.doi.org/10.1016/j.ajog.2004.08.030.
- Uittenboogaard A., Neutel C.L.G., Ket J.C.F., Njuguna F., Huitema A.D.R., Kaspers G.J.L. et al. Pharmacogenomics of vincristine-induced peripheral neuropathy in children with cancer: a systematic review and meta-analysis. Cancers (Basel). 2022; 14(3): 612. https://dx.doi.org/10.3390/cancers14030612.
- Kukal S., Thakran S., Kanojia N., Yadav S., Mishra M.K., Guin D. et al. Genic-intergenic polymorphisms of CYP1A genes and their clinical impact. Gene. 2023; 857: 147171. https://dx.doi.org/10.1016/j.gene.2023.147171.
- Saijo Y., Sata F., Yamada H., Suzuki K., Sasaki S., Kondo T. et al. Ah receptor, CYP1A1, CYP1A2 and CYP1B1 gene polymorphisms are not involved in the risk of recurrent pregnancy loss. Mol. Hum. Reprod. 2004; 10(10):729-33. https://dx.doi.org/10.1093/molehr/gah09.
- Сальникова Л.Е., Замулаева И.А., Белопольская О.Б., Иванова Т.И., Кузнецова Г.И., Саенко А.С., Абилев С.К., Рубанович А.В. Встречаемость TCR-мутантных лимфоцитов у человека в зависимости от генотипов по локусам детоксикации ксенобиотиков. Экологическая генетика человека. 2010; 8(2): 18-23. [Sal'nikova L.E., Zamulaeva I.A., Belopol'skaya O.B., Ivanova T.I., Kuznetsova G.I., Saenko A.S., Abilev S.K., Rubanovich A.V. Frequency of TCR mutant human lymphocytes depending on genotypes by loci of xenobiotics detoxication. Ecological Genetics. 2010; 8(2): 18-23. (in Russian)].
- Hayes J.D., Flanagan J.U., Jowsey I.R. Glutathione transferases. Ann. Rev. Pharmacol. Toxicol. 2005; 45: 51-88. https://dx.doi.org/10.1146/annurev.pharmtox.45.120403.095857.
- Van Lieshout E., Knapen M., Lange W. Localization of glutathione S-transferase α and π in human embryonic tissues at 8 weeks gestational age. Hum. Reprod. 1998; 13(5): 1380-6. https://dx.doi.org/10.1093/humrep/13.5.1380.
- Hayes J.D., Strange R.C. Glutathione S-transferase polymorphisms and their biological consequences. Pharmacology. 2000; 61(3): 154-66. https://dx.doi.org/10.1159/000028396.
- Hirvonen A., Taylor J.A., Wilcox A., Berkowitz G., Schachter B., Chaparro C. et al. Xenobiotic metabolism genes and the risk of recurrent spontaneous abortion. Epidemiology. 1996; 7(2): 206-8. https://dx.doi.org/10.1097/00001648-199603000-00018.
- Nair R.R, Khanna A., Singh K. Association of GSTT1 and GSTM1 polymorphisms with early pregnancy loss in an Indian population and a meta-analysis. Reprod. Biomed. Online. 2013; 26(4): 313-22. https://dx.doi.org/10.1016/j.rbmo.
- Гуляева О.Н., Жукова А.Г., Казицкая А.С., Лузина Ф.А., Алексеева М.В., Ренге Л.В., Рябов В.А. Степень антропогенной нагрузки, полиморфизм генов системы биотрансформации ксенобиотиков и врожденные пороки развития плода как звенья одной цепи. Гигиена и санитария. 2021; 100(7): 658-62. [Gulyaeva O.N., Zhukova A.G., Kazickaya A.S., Luzina F.A., Alekseeva M.V., Renge L.V., Ryabov V.A. The degree of anthropogenic load, polymorphism of genes of the xenobiotic biotransformation system and congenital malformations of the fetus as links in one chain. Hygiene and Sanitation. 2021; 100(7): 658-62. (in Russian)]. https://dx.doi.org/10.47470/0016-9900-2021-100-7-658-662.
- Викторова Т.В., Исхакова Г.М. Ассоциация полиморфных вариантов генов глутатион-зависимых ферментов с репродуктивной патологией у женщин. Современные проблемы науки и образования. 2015; 3: 175. [Viktorova T.V., Iskhakova G.M. Association polymorphism of genes of glutathione-dependent enzymes with reproductive pathology in women. Modern Problems of Science and Education. 2015; (3): 175. (in Russian)].
Received 15.04.2024
Accepted 26.07.2024
About the Authors
Svetlana V. Shramko, Dr. Med. Sci., Associate Professor, Professor at the Department of Obstetrics and Gynecology, Novokuznetsk State Institute of Advanced Medical Training, branch of RMACPE, Ministry of Health of Russia; 654005, Russia, Kemerovo region, Novokuznetsk, Stroiteley Ave., 5, +7(961)714-00-13, shramko_08@mail.ru, https://orcid.org/0000-0003-1299-165XSergey V. Matoshin, Obstetrician-Gynecologist, Pр Student at the Department of Obstetrics and Gynecology, Novokuznetsk State Institute of Advanced Medical Training, branch of RMACPE, Ministry of Health of Russia; 654005, Russia, Kemerovo region, Novokuznetsk, Stroiteley Ave., 5, +7(913)987-73-00, matoshin94@bk.ru,
https://orcid.org/0000-0002-2805-6829
Olga N. Gulyaeva, Senior Researcher at the Laboratory for Molecular-Genetic and Experimental Researches, Research Institute for Complex Problems of Hygiene and Occupational Diseases, 654041, Russia, Kemerovo region, Novokuznetsk, Kutuzova str., 23, +7(903)984-87-04, Gulyaich1973@mail.ru, https://orcid.org/0000-0003-2225-6923
Irina V. Samus, PhD, Senior Researcher, Research Institute for Complex Issues of Cardiovascular Diseases, 650002, Russia, Kemerovo Region, Kemerovo,
Sosnovy Boulevard, 6, +7(923)613-63-16, stat-for-you@yandex.ru, https://orcid.org/0000-0002-3293-5746
Corresponding author: Svetlana V. Shramko, shramko_08@mail.ru



