Antibacterial activity of probiotic strains of lactobacilli included in the MRM Nutrition Women’s Probiotic complex (Lactobacillus plantarum LP01 and Lactobacillus paracasei LPC09)
Budilovskaya O.V., Spasibova E.V., Khusnutdinova T.A., Krysanova A.A., Shalepo K.V., Savicheva A.M.
Lactobacilli, recognized as probiotics, are regarded as promising instruments, both for the prevention and treatment of vaginal dysbiosis and urinary tract infection. Given the ineffectiveness of therapy and the frequent recurrence of these infections, the use of probiotics has become a progressive solution to this problem.
Objective: To evaluate the antibacterial activity of probiotic strains of lactobacilli included in the MRM Nutrition Women's Probiotic complex (Lactobacillus plantarum LP01 and Lactobacillus paracasei LPC09) against opportunistic bacteria isolated from urogenital clinical materials, and to determine the sensitivity of these lactobacilli to antibacterial drugs.
Materials and methods: Probiotic strains of Lactobacillus plantarum LP01 and Lactobacillus paracasei LPC09 were cultivated and the grown colonies were identified using the MALDI-TOF mass spectrometry method (Bruker, Germany). To study the antagonism between probiotic strains of lactobacilli and opportunistic microorganisms, a collection of 16 clinical isolates of opportunistic microorganisms was made: Escherihia coli (3), Klebsiella pneumoniae (2), Morganella morganii (1), Enterococcus faecalis (3), Enterococcus raffinosus (1), Streptococcus agalactiae (3), Staphylococcus epidermidis (1) and Candida albicans (2). To study the sensitivity of probiotic strains of lactobacilli to antimicrobial drugs, a panel for determining the sensitivity of gram-positive bacteria to antimicrobial drugs "Gram-positive AST" (AutoBio Diagnostics, China) was used.
Results: When co-cultivated with probiotic lactobacilli (Lactobacillus plantarum LP01 and Lactobacillus paracasei LPC09), a decrease in the pH of the medium was noted, and a direct antibacterial effect of these lactobacilli against all tested opportunistic microorganisms was detected. When studying antibiotic resistance, it was marked that both strains of lactobacilli were resistant to antibacterial drugs of the penicillin group, cephalosporins, and fluoroquinolones.
Conclusion: Oral administration of the MRM Nutrition Women's Probiotic complex, containing a combination of Lactobacillus plantarum LP01 and Lactobacillus paracasei LPC09, cranberry extract and D-mannose, has significant antibacterial activity against opportunistic microorganisms isolated from women’s urogenital tract. These lactobacilli strains are resistant to some antibacterial drugs widely used in clinical practice, which may be the ground for taking this probiotic drug together with prescribed antibiotics.
Authors' contributions: Budilovskaya O.V. – literature review, data analysis, text composition; Shalepo K.V., Spasibova E.V. – study conduction, data analysis; Khusnutdinova T.A., Krysanova A.A. – data analysis, text editing, final approval of the article; Savicheva A.M. – study concept and design, data analysis, final editing. All authors have made significant contribution to the study concept and conduction as well as article conduction, have read and approved the final version of the article before publication.
Conflicts of interest: The authors declare no conflicts of interest in connection with the publication of this article.
Funding: The study was performed without external funding.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Budilovskaya O.V., Spasibova E.V., Khusnutdinova T.A., Krysanova A.A., Shalepo K.V., Savicheva A.M. Antibacterial activity of probiotic strains of lactobacilli included in the MRM Nutrition Women’s Probiotic complex (Lactobacillus plantarum LP01 and Lactobacillus paracasei LPC09).
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (8): 187-194 (in Russian)
https://dx.doi.org/10.18565/aig.2025.204
Keywords
The growth of antibacterial resistance caused by overuse of antibacterial drugs is considered to be a serious threat to global public health. Reproductive tract and urinary tract infections (UTIs) affect millions of people worldwide [1]. The most vulnerable groups of the population are women of reproductive age, postmenopausal women, pregnant women, patients with diabetes mellitus and others. They are at increased risk of infection due to various physiological, hormonal and anatomical factors. Lactobacillus-based drugs are believed to be high-potential for preventing recurrent UTIs and vaginal infections, as well as reducing the risk of developing antibiotic resistance. Encouraging results have also been obtained with cranberry extracts. Today, many scientists are exploring the therapeutic and preventive potential of probiotics and plant extracts, which are increasingly used in clinical practice to maintain urinary tract health and prevent bacterial colonization [2].
Probiotics are preparations containing live bacteria that are not pathogenic for humans and have antagonistic activity against pathogenic and opportunistic microorganisms, ensuring the restoration of normal microflora. Probiotics can be prescribed both in pathological conditions and after taking certain medications.
The vaginal microbiome is a balanced ecosystem with the domination of Lactobacillus spp., the composition of which changes over time and varies among women [3]. Under physiological conditions, the predominance of lactobacilli significantly reduces the pH of the vaginal environment, suppressing the proliferation of other microorganisms and thereby promoting the maintenance of a eubiotic vaginal microbiome [4]. The ability of lactobacilli to produce lactic acid, biosurfactants, bacteriocin-like substances and hydrogen peroxide depends on the strain used. [5].
Given that lactobacilli in the vagina produce a defense effect against infections, enrichment of the vaginal microbiome with lactobacilli is a promising strategy for the prevention of urogenital infections [6].
Today, the Lactobacillus is one of the most numerous bacterial genera, including over 260 species, of which about 20 can inhabit the vaginal biotope [7]. But some of them such as L. acidophilus, L. brevis, L. crispatus, L. delbrueckii, L. fermentum, L. gasseri, L. helveticus, L. jensenii, L. johnsonii, L. plantarum, L. paracasei, L. reuteri, L. Rhamnosus и L. salivarius, are used as probiotic stains to treat vaginal infections [8, 9]. Despite L. iners is the most common bacterium found in the vagina, it is not used as a vaginal probiotic, which is due to the cultural properties and uncertainty about the importance of L. iners [10].
Probiotics with lactobacilli for the restoration of vaginal microbiota are used either intravaginally or orally. Most studies show that oral administration is more effective than vaginal forms [11, 12].
Probiotic strains of lactobacilli must strictly meet the following criteria: have a marked adhesive capacity and provide colonization resistance; survive and reproduce; synthesize organic acids, hydrogen peroxide and bacteriocins; be resistant to vaginal bactericidal agents; coaggregate with endogenous bacteria, forming biofilms; be non-invasive, non-pathogenic, non-carcinogenic [13]. The most significant quality of oral probiotics strains is their resistance to gastric hydrochloric acid, bile acids and intestinal microbiota in order to survive during transit through the gastrointestinal tract (GI) [14].
Among all lactobacilli, two strains stand out for their ability to produce lactic acid in the vaginal environment: Lactobacillus plantarum LP01 and Lactobacillus paracasei LPC09. These strains are included in MRM Nutrition Women’s Probiotic.
MRM Nutrition Women's Probiotic is produced using the patented Microbac technology, which includes microencapsulation of each bacterium with a plant-based lipid. Due to this, the bacteria are not damaged in the stomach and small intestine, and reach the large intestine intactly. Research has shown that the use of Microbac technology increases the effectiveness and improves preservation of probiotics by 5 times. Cranberry extract, which is part of MRM Nutrition Women's Probiotic, is a unique source of polyphenols, including flavonoids and phenolic acids, which, as many studies have shown, have a beneficial effect on UTIs [15]. Another important component of this probiotic is D-mannose, which is a metabiotic or prebiotic that supports the growth of normal intestinal microflora [16]. D-mannose and cranberry extract reduce the binding of pathogenic microorganisms and yeast-like fungi to the epithelium of the urogenital tract [17].
Study objective: To evaluate the antibacterial activity of probiotic strains of lactobacilli included in the MRM Nutrition Women's Probiotic complex (Lactobacillus plantarum LP01 and Lactobacillus paracasei LPC09) against opportunistic bacteria isolated from urogenital clinical materials, and to determine the sensitivity of these lactobacilli to antibacterial drugs.
Materials and methods
In the first stage, we isolated and identified lactobacilli from the capsules of the Women’s Probiotic preparation and specified their concentration. For this purpose, the contents of the MRM Nutrition Women’s Probiotic capsule were added to the MRS lactobacillus culture broth and incubated for 24 hours at 37°C. The next day, the broth was seeded onto a dense culture MRS media and incubated for another 24 hours at 37°C. On the 3rd day, the grown colonies of lactobacilli were identified. Both separately grown strains of lactobacilli Lactobacillus plantarum LP01 and Lactobacillus paracasei LPC09 and a complex of these lactobacilli were studied. Identification of the isolated lactobacilli was performed using MALDI-TOF mass spectrometry (Bruker, Germany).
Next, the pH was measured during the growth and reproduction of lactobacilli using test strips by applying a drop of the culture medium to the area with the indicator and comparing it with the color scale. To study the antibacterial activity of lactobacilli included in MRM Nutrition Women's Probiotic, a collection of opportunistic bacteria was made, consisting of 16 clinical isolates of microorganisms isolated from the women’s urogenital tract, including: Escherihia coli (3), Klebsiella pneumoniae (2), Morganella morganii (1), Enterococcus faecalis (3), Enterococcus raffinosus (1), Streptococcus agalactiae (3), Staphylococcus epidermidis (1) и Candida albicans (2).
For cultivation, storage and further study of microorganisms, solid and liquid culture media were used: Levine agar, chromogenic agar for uropathogens, blood agar with 5% defibrinated blood (BA), yolk-salt agar, Sabouraud medium (agar and broth), MRS medium for culturing lactobacilli (agar and broth), trypticase soy agar (TSA), trypticase soy broth (TSB), trypticase soy broth with 10% glycerol (TSB-G), heart-brain broth (BHB).
A suspension of the grown cultures was prepared in a sterile isotonic solution, bringing the inoculum density to 0.5 MF (according to the McFarland standard). 1.0 ml of each lactobacillus strain separately and their mixture (Lactobacillus plantarum LP01 and Lactobacillus paracasei LPC09) and 1.0 ml of the suspension of the studied opportunistic microorganisms were added to a test tube with 8.0 ml of liquid MRS medium. The results of co-cultivation were assessed after 48 hours of incubation at 37°C in 5% CO2. For this purpose, inoculations made on chromogenic agar (Oxoid, UK) were incubated for 24 hours, and the growth of the microorganisms was assessed. This chromogenic agar is intended for the cultivation of uropathogens, yeast-like fungi and lactobacilli. With the use of this medium Lactobacilli grow in the form of small colonies of blue, violet or green color. Identification of both all microorganisms and lactobacilli grown on chromogenic agar was identified by the MALDI-TOF mass spectrometry method (Bruker, Germany). Simultaneously, the grown colonies of lactobacilli were seeded on dense culture MRS media for lactobacilli cultivation.
pH measurements were performed before and after co-cultivation using test strips by applying a drop of culture medium to a pad with an indicator and comparing with a color scale.
To study sensitivity of probiotic lactobacilli strains, comprising MRM Nutrition Women’s Probiotic (Lactobacillus plantarum LP01 и Lactobacillus paracasei LPC09), to antibacterial drugs we used a panel to detect sensitivity of gram-positive bacteria to antibacterial drugs «Gram-positive AST» (AutoBio Diagnostics, Китай) and assessed minimal inhibiting concentrations of antibiotics in accordance with the test-system instruction. Interpretation of the results according to the activity level of the antimicrobial drugs that are included into this test-system, on probiotic lactobacilli (S – sensitive, I – intermediate, R – resistant) was carried out as per the table in the instruction.
Results
When cultivating on the MRS culture medium the growth of the certain lactobacilli in concentration of more than 109 KOE/ml was noted. As a result of identification of the grown lactobacilli using the MALDI-TOF mass-spectrometry method it was specified that, indeed, lactobacilli Lactobacillus plantarum and Lactobacillus paracasei are present in certain capsules.
On pH measurement of culture media prior to and after cultivation of these lactobacilli, the decrease of this parameter from neutral to acid was identified, which was demonstrated the change of color of the test strip from green (7,0) to orange (5,0).
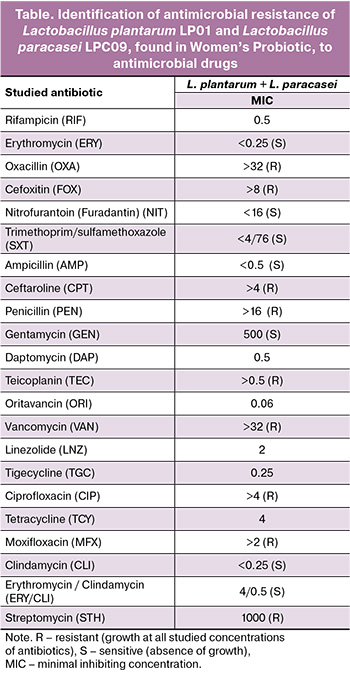
The results of co-cultivation of lactobacilli and opportunistic bacteria are presented in Figure 1.
As per the data in Figure 1, bacteria of various species on chromogenic agar look differently. In the upper line of the picture one may see the growth of 16 microorganism strains prior to their co-cultivation with lactobacilli. In the lower left picture there are the following microorganisms: 1. Escherichia coli, 2. Escherichia coli, 3. Morganella morganii, 4. Enterobacter cloacae, 5. Enterococcus raffinosus, 6. Staphylococcus epidermidis, 7. Candida albicans, 8. Klebsiella pneumonia. Справа вверху: 9. Enterococcus faecalis, 10. Candida albicans, 11. Enterococcus faecalis, 12. Escherichia coli, 13. Streptococcus agalactiae, 14. Streptococcus agalactiae, 15. Streptococcus agalactiae, 16. Enterococcus faecalis.
After the co-cultivation of bacteria and yeast-like fungi with lactobacilli the growth of only probiotic lactobacilli strains was noted. These were identified using mass-spectrometry and seeding onto the dense culture media for lactobacilli growth. All opportunistic microorganisms died after co-cultivation with lactobacilli included in the drug composition. In the lower line of Pic.1 one can see the results obtained after co-cultivation all the studied bacteria and clinical isolates Candida albicans with lactobacilli, comprising MRM Nutrition Women’s Probiotic. На In the left and right part of the picture there is only the growth of Lactobacillus plantarum and Lactobacillus paracasei and the absence of growth of other bacteria and yeast-like fungi.
Thus, due to co-cultivation of probiotic lactobacilli (Lactobacillus plantarum LP01 and Lactobacillus paracasei LPC09) with the studied urogenital bacteria and yeast-like fungi the suppression of all 16 clinical isolates was detected.
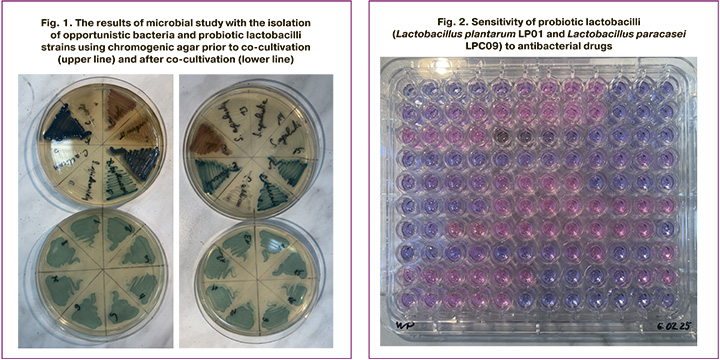
While studying the sensitivity of probiotic lactobacilli strains presented in MRM Nutrition Women’s Probiotic (Lactobacillus plantarum LP01 and Lactobacillus paracasei LPC09), to antimicrobial drugs, we obtained the following results (Fig.2, Table). Pic.2 demonstrates the results of sensitivity detection to antimicrobial drugs in probiotic bacteria. The pink cups show the resistant of Lactobacillus plantarum LP01 and Lactobacillus paracasei LPC09 to antibiotics. The purpur cups demonstrate sensitivity of Lactobacillus plantarum LP01 and Lactobacillus paracasei LPC09 to antibiotics. These data are summed up in the Table.
When performing an antibiotic susceptibility test, Lactobacillus plantarum LP01 and Lactobacillus paracasei LPC09 were found to be resistant to penicillin, cephalosporin, and fluoroquinolone antimicrobial drugs.
Thus, the MRM Nutrition Women's Probiotic, containing a combination of two lactobacilli Lactobacillus plantarum LP01 and Lactobacillus paracasei LPC09, as well as cranberry fruit extract, has marked antimicrobial activity against opportunistic microorganisms and yeast-like fungi. These lactobacilli strains are resistant to most antimicrobial drugs used in widespread clinical practice, which may be the basis for recommending the use of this probiotic drug together with prescribed antibiotics.
Discussion
Low efficiency of antibiotics against opportunistic microorganisms and elimination of lactobacilli are the main reasons for the failure of traditional antimicrobial treatment of urogenital infections. Antibiotic therapy does not promote the restoration of normal microbiota with the dominance of lactobacilli. Numerous studies on the search for the restoration mechanisms of vaginal eubiosis have resulted in the use of probiotic drugs that have proven their effectiveness.
We evaluated the antagonistic and antibacterial activity of probiotic lactobacilli strains included in the MRM Nutrition Women’s Probiotic complex (Lactobacillus plantarum LP01 and Lactobacillus paracasei LPC09) against opportunistic microorganisms isolated from women’s urogenital tract: Escherichia coli, Klebsiella pneumoniae, Morganella morganii, Enterococcus faecalis, Enterococcus raffinosus, Streptococcus agalactiae, Staphylococcus epidermidis and Candida albicans. We also identified the sensitivity of these probiotic lactobacilli strains to antimicrobial drugs.
For the clarity of the experiment, we chose chromogenic agar, on which the isolated microorganisms were stained in different colors. When cultivated, lactobacilli are known to be fastidious microorganisms that gave rise to colonies of blue, purple or green color. In order to understand that these particular lactobacilli strains grew on chromogenic agar, we seeded them on dense culture media for lactobacilli – MRS medium, and also identified the grown colonies using MALDI-TOF mass spectrometry.
The studied probiotic strains of Lactobacillus plantarum LP01 and Lactobacillus paracasei LPC09 showed high inhibitory activity against the studied opportunistic microorganisms. After co-cultivation with probiotic lactobacilli, only the growth of probiotic lactobacilli was detected; all opportunistic bacteria and yeast-like fungi were not isolated.
The antimicrobial and antagonistic activity of the Women’s Probiotic depends on several mechanisms. This is the modulation of the immune system and the action of biologically active compounds: organic acids, hydrogen peroxide and bacteriocins, which are enhanced by the symbiosis of Lactobacillus plantarum and Lactobacillus paracasei [19]. Cranberry polyphenols act during the bacterial attachment to the epithelial cells of the urogenital tract, blocking or inhibiting the attachment of uropathogens, thereby preventing bacterial colonization and the development of urinary tract infections [20].
Our data confirm the effect of probiotic lactobacilli against pathogens causing aerobic vaginitis and UTI, such as Escherichia coli, Enterococcus spp. and Streptococcus agalactiae, which have also been the subject of similar studies [21, 22]. The activity of the studied probiotic strains against opportunistic microorganisms colonizing the gastrointestinal tract emphasizes the advantage of oral administration. Many studies show that the main action of probiotics is primarily aimed at maintaining intestinal homeostasis, since the inflammation in intestinal dysbiosis is associated with increased permeability of the intestinal barrier, tissue damage and the spread of bacterial infection [23].
The results of the study show the effectiveness of lactobacilli against Candida albicans, which may be useful for the treatment and prevention of vulvovaginal candidiasis (VVC) that is considered to be a common and severe vaginal infection. Despite the use of antifungal drugs, it may occur in a recurrent form in a significant number of patients and requires additional treatment methods. Previously, we conducted a study on the effect of the drug on bacterial films formed by yeast-like Candida fungi [24]. Probiotics represent a promising complementary or alternative therapeutic strategy to antifungal drugs in the treatment of VVC.
Probiotics represent a promising complementary or alternative therapy to antifungal drugs for the treatment of VVC [25]. This is supported by the results obtained in a study of L. plantarum strains that effectively inhibited biofilm formation and eliminated mature biofilms formed by C. albicans, which is often observed in recurrent VVC [26].
The meaningful outcome of our study turned out to be the identification of resistance of Lactobacillus plantarum LP01 and Lactobacillus paracasei LPC09 to penicillin, cephalosporin and fluoroquinolone antibacterial drug groups. This opens up the prospect of using probiotics and antibiotics together for the treatment of vaginal infections and UTIs. However, despite the fact that probiotics are becoming increasingly popular and have the potential for the use in medicine and the food industry, there is a risk of transferring antibiotic resistance genes to opportunistic bacteria [27]. These data provide a basis for future studies to be conducted in animal models and human volunteers using advanced metagenomics approaches to clarify the long-term risks associated with probiotic consumption.
Thus, the potential health benefits of probiotics in women highlight the need for further research to complement existing knowledge and promote clinical use of probiotics as preventive and therapeutic agents.
Conclusion
Oral MRM Nutrition Women's Probiotic complex, containing a combination of two lactobacilli Lactobacillus plantarum LP01 and Lactobacillus paracasei LPC09, cranberry extract and D-mannose, has pronounced antibacterial activity against opportunistic microorganisms isolated from the women’s urogenital tract. These lactobacilli strains are resistant to some antibacterial drugs widely used in clinical practice, which may be the basis for recommending to take this probiotic drug together with prescribed antibiotics.
References
- Öztürk R., Murt A. Epidemiology of urological infections: a global burden. World J. Urol. 2020; 38(11): 2669-79. https://dx.doi.org/10.1007/s00345-019-03071-4
- Çelik H., Kozan E., Caf B.B., Çebi G., Koҫ M. The association between urinary tract infections and diet: a literature review. Discov. Med. 2025; 2: 91. https://dx.doi.org/10.1007/s44337-025-00272-2
- Lehtoranta L., Ala-Jaakkola R., Laitila A., Maukonen J. Healthy vaginal microbiota and influence of probiotics across the female life span. Front. Microbiol. 2022; 13: 819958. https://dx.doi.org/10.3389/fmicb.2022.819958
- Cappello C., Acin-Albiac M., Pinto D., Polo A., Filannino P., Rinaldi F. et al. Do nomadic lactobacilli fit as potential vaginal probiotics? the answer lies in a successful selective multi-step and scoring approach. Microb. Cell Fact. 2023; 22(1): 27. https://dx.doi.org/10.1186/s12934-023-02030-4
- Das S., Bhattacharjee M.J., Mukherjee A.K., Khan M.R. Recent advances in understanding of multifaceted changes in the vaginal microenvironment: implications in vaginal health and therapeutics. Crit. Rev. Microbiol. 2023; 49(2): 256-82. https://dx.doi.org/10.1080/1040841X.2022.2049696
- Mei Z., Li D. The role of probiotics in vaginal health. Front. Cell. Infect. Microbiol. 2022; 12: 963868. https://dx.doi.org/10.3389/fcimb.2022.963868
- Zheng J., Wittouck S., Salvetti E., Franz C.M.A.P., Harris H.M.B., Mattarelli P. et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020; 70(4): 2782-858. https://dx.doi.org/10.1099/ijsem.0.004107
- Piccioni A., Franza L., Vaccaro V., Saviano A., Zanza C., Candelli M. et al. Microbiota and probiotics: the role of Limosilactobacillus reuteri in diverticulitis. Medicina (Kaunas). 2021; 57(8): 802. https://dx.doi.org/10.3390/medicina57080802
- Будиловская О.В., Спасибова Е.В., Шалепо К.В., Хуснутдинова Т.А., Крысанова А.А., Синякова А.А., Беспалова О.Н., Савичева А.М. Антагонистическая и антибактериальная активность Lactobacillus rhamnosus HN001 и Lactobacillus acidophilus La-14, входящих в состав перорального пробиотика. Журнал акушерства и женских болезней. 2024; 73(3): 27-39. [Budilovskaya O.V., Spasibova E.V., Shalepo K.V., Khusnutdinova T.A., Krysanova A.A., Siniakova A.A., Bespalova O.N., Savicheva A.M. Antagonistic and antibacterial activity of Lactobacillus rhamnosus HN001 and Lactobacillus acidophilus La-14 included in the oral probiotic. Journal of obstetrics and women's diseases. 2024; 73(3): 27-39 (in Russian)]. https://dx.doi.org/10.17816/JOWD630698
- Novak J., Ravel J., Ma B., Ferreira C.S.T., Tristão A.D.R., Silva M.G. et al. Characteristics associated with Lactobacillus iners-dominated vaginal microbiota. Sex. Transm. Infect. 2022; 98(5): 353-9. https://dx.doi.org/10.1136/sextrans-2020-054824
- Marrazzo J.M., Fiedler T.L., Srinivasan S., Thomas K.K., Liu C., Ko D. et al. Extravaginal reservoirs of vaginal bacteria as risk factors for incident bacterial vaginosis. J. Infect. Dis. 2012; 10(205): 1580-8. https://dx.doi.org/10.1093/infdis/jis242
- Леонова М.В. Пробиотики в лечении вагинальных инфекций: эффективность с позиции доказательной медицины. Медицинский совет. 2020; 13: 148-54. [Leonova M.V. Probiotics in the treatment of vaginal infections: efficacy from the perspective of evidence-based medicine. Medical Council. 2020; (13): 148-54 (in Russian)]. https://dx.doi.org/10.21518/2079-701X-2020-13-148-154
- Reid G. The scientific basis for probiotic strains of lactobacillus. Appl. Environ. Microbiol. 1999; 65(9): 3763-6. https://dx.doi.org/10.1128/AEM.65.9.3763-3766.1999
- Conway P.L., Gorbach S.L., Goldin B.R. Survival of lactic acid bacteria in the human stomach and adhesion to intestinal cells. J. Dairy Sci. 1987; 70(1): 1-12. https://dx.doi.org/10.3168/jds.S0022-0302(87)79974-3
- González de Llano D.G., Moreno-Arribas M.V., Bartolomé B. Cranberry polyphenols and prevention against urinary tract infections: relevant considerations. Molecules. 2020; 25(15): 3523. https://dx.doi.org/10.3390/molecules25153523
- Тетерина Т.А., Тарнаева Л.А., Аполихина И.А. Применение D- маннозы в профилактике рецидивирующих инфекций мочевыводящих путей. Фарматека. 2024; 31(3): 26-35. [Teterina T.A., Tarnaeva L.A., Apolikhina I.A. The use of D-mannose in the prevention of recurrent urinary tract infections. Pharmateca. 2024; 31(3): 26-35 (in Russian)]. https://dx.doi.org/10.18565/pharmateca.2024.3.26-35
- González de Llano D., Liu H., Khoo C., Moreno-Arribas M.V., Bartolomé B. Some new findings regarding the antiadhesive activity of cranberry phenolic compounds and their microbial-derived metabolites against uropathogenic bacteria. J. Agric. Food Chem. 2019; 67(8): 2166-74. https://dx.doi.org/10.1021/acs.jafc.8b05625
- Китайский комитет содействия развитию международной торговли. Китайская палата международной торговли. Инструкция по применению. Набор реагентов Gram Positive bacteria AST для определения чувствительности к антимикробным препаратам на анализаторе AutoMic-i600 для in vitro диагностики. Китай; 2023. 23 с. [China Council for the Promotion of International Trade. China Chamber of International Commerce. Instruction for use. Gram Positive bacteria AST reagent for sensitivity testing to antimicrobial drug on the analyzer AutoMic-i600 for in vitro diagnostics. China; 2023. 23 p. (in Russian)]. https://docs.nevacert.ru/files/med_reestr_v2/73173_instruction.pdf
- Nader-Macías M.E.F., De Gregorio P.R., Silva J.A. Probiotic lactobacilli in formulas and hygiene products for the health of the urogenital tract. Pharmacol. Res. Perspect. 2021; 9(5): e00787. https://dx.doi.org/10.1002/prp2.787
- Lewis A.J., Richards A.C., Mendez A.A., Dhakal B.K., Jones T.A., Sundsbak J.L. et al. Plant phenolics inhibit focal adhesion kinase and suppress host cell invasion by uropathogenic Escherichia coli. Infect. Immun. 2024; 92(5): 0008024. https://dx.doi.org/10.1128/iai.00080-24
- Pino A., Vaccalluzzo A., Caggia C., Balzaretti S., Vanella L., Sorrenti V. et al. Lacticaseibacillus rhamnosus CA15 (DSM 33960) as a candidate probiotic strain for human health. Nutrients. 2022; 14(22): 4902. https://dx.doi.org/10.3390/nu14224902
- Leccese Terraf M.C., Juarez Tomás M.S., Rault L., Le Loir Y., Even S., Nader-Macías M.E.F. In vitro effect of vaginal lactobacilli on the growth and adhesion abilities of uropathogenic Escherichia coli. Arch. Microbiol. 2017; 199(5):767-74. https://dx.doi.org/10.1007/s00203-016-1336-z
- Zhang Z., Wang X., Li F. An exploration of alginate oligosaccharides modulating intestinal inflammatory networks via gut microbiota. Front. Microbiol. 2023; 14: 1072151. https://dx.doi.org/10.3389/fmicb.2023.1072151
- Шалепо К.В., Спасибова Е.В., Будиловская О.В., Крысанова А.А., Хуснутдинова Т.А., Чеберя А.С., Чеберя А.Р., Савичева А.М. Оценка эффективности in vitro компонентов препарата «Депантол» против биопленок, сформированных вагинальными микроорганизмами. Акушерство и гинекология. 2024; 10: 158-66. [Shalepo K.V., Spasibova E.V., Budilovskaya O.V., Krysanova A.A., Khusnutdinova T.A., Cheberya A.S., Cheberya A.R., Savicheva A.M. Evaluation of the in vitro effectiveness of the Depantol components on biofilms produced by vaginal microorganisms. Obstetrics and Gynecology. 2024; (10): 158-66 (in Russian)]. https://dx.doi.org/10.18565/aig.2024.256
- Akinosoglou K., Schinas G., Polyzou E., Tsiakalos A., Donders G.G.G. Probiotics in the management of Vulvovaginal Candidosis. J. Clin. Med. 2024; 13(17): 5163. https://dx.doi.org/10.3390/jcm13175163
- Bae W.Y., Lee Y.J., Jo S., Shin S.L., Kim T.R., Sohn M. et al. Effects of Lactiplantibacillus plantarum LM1215 on Candida albicans and Gardnerella vaginalis. Yonsei Med. J. 2024; 65(12): 727-40. https://dx.doi.org/10.3349/ymj.2023.0490
- Tian Q., Ye H., Zhou X., Wang J., Zhang L., Sun W. et al. Evaluating the health risk of probiotic supplements from the perspective of antimicrobial resistance. Microbiol. Spectr. 2025; 13(1): e0001924. https://dx.doi.org/10.1128/spectrum.00019-24
Received 28.07.2025
Accepted 11.08.2025
About the Authors
Olga V. Budilovskaya, PhD, Senior Researcher at the Experimental Microbiology Group, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology,199034, Russia, St. Petersburg, Mendeleevskaya Line, 3; Teaching Assistant at the Department of Medical Microbiology and Clinical Laboratory Diagnostics of AF and DPO, St. Petersburg State Pediatric Medical University, Ministry of Health of Russia, 194100, Russia, St. Petersburg, Litovskaya str., 2, +7(911)912-77-52, o.budilovskaya@gmail.com, https://orcid.org/0000-0001-7673-6274
Elena V. Spasibova, Bacteriologist at the Laboratory of Clinical Microbiology, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, 199034, Russia,
St. Petersburg, Mendeleevskaya Line, 3; Teaching Assistant at the Department of Medical Microbiology and Clinical Laboratory Diagnostics of FP and DPO, St. Petersburg State Pediatric Medical University, Ministry of Health of Russia, 194100, Russia, St. Petersburg, Litovskaya str., 2, elena.graciosae@gmail.com,
https://orcid.org/0009-0002-6070-4651
Tatiana A. Khusnutdinova, PhD, Senior Researcher at the Experimental Microbiology Group, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, 199034, Russia, St. Petersburg, Mendeleevskaya Line, 3; Teaching Assistant at the Department of Medical Microbiology and Clinical Laboratory Diagnostics of AF and DPO, St. Petersburg State Pediatric Medical University, Ministry of Health of Russia, 194100, Russia, St. Petersburg, Litovskaya str., 2, +7(981)725-96-90, husnutdinovat@yandex.ru, https://orcid.org/0000-0002-2742-2655
Kira V. Shalepo, PhD, Senior Researcher at the Experimental Microbiology Group, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, 199034, Russia, St. Petersburg, Mendeleevskaya Line, 3; Teaching Аssistant at Department of Medical Microbiology and Clinical Laboratory Diagnostics of AF and DPO, St. Petersburg State Pediatric Medical University, Ministry of Health of Russia, 194100, Russia, St. Petersburg, Litovskaya str., 2, +7(911)247-41-51, 2474151@mail.ru,
https://orcid.org/0000-0002-3002-3874
Anna A. Krysanova, PhD, Senior Researcher at the Experimental Microbiology Group, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology,
199034, Russia, St. Petersburg, Mendeleevskaya Line, 3; Teaching Assistant at the Department of Medical Microbiology and Clinical Laboratory Diagnostics of AF and DPO, St. Petersburg State Pediatric Medical University, Ministry of Health of Russia, 194100, Russia, St. Petersburg, Litovskaya str., 2, +7(911)737-96-93, krusanova.anna@mail.ru, https://orcid.org/0000-0003-4798-1881
Alevtina M. Savicheva, Dr. Med. Sci., Professor, Head of the Laboratory of Microbiology, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology,
199034, Russia, St. Petersburg, Mendeleevskaya Line, 3; Head of the Department of Medical Microbiology and Clinical Laboratory Diagnostics, St. Petersburg State Pediatric Medical University, Ministry of Health of Russia, 194100, Russia, St. Petersburg, Litovskaya str., 2, +7(921)944-15-47, savitcheva@mail.ru,
https://orcid.org/0000-0003-3870-5930
Corresponding author: Olga V. Budilovskaya, o.budilovskaya@gmail.com



