Androgen profile in infertile women undergoing treatment with assisted reproductive technologies depending on the ovarian response
Objective: To evaluate the androgen profile in infertile women undergoing treatment with assisted reproductive technologies (ART) depending on the ovarian response. Materials and methods: The study included 150 women who underwent IVF/ICSI treatment at the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, and met the inclusion criteria. The groups were stratified depending on the ovarian reserve on the basis of the POSEIDON classification and ovarian response to the stimulation in accordance with the Bologna criteria: group 1 included 50 patients with diminished ovarian reserve (AMH<1.2 ng/ml, CAF<5) and poor ovarian response to stimulation (≤3 oocytes during transvaginal puncture); group 2 consisted of 100 patients with normal ovarian reserve (AMH≥1.2 ng/ml, CAF≥5), among them there were 50 patients with normal ovarian response (>9 oocytes) and 50 patients with reduced ovarian response (<9 oocytes). The androgen profile in the follicular fluid and blood serum was studied using mass spectrometry data. Results: Depending on the decrease in the ovarian reserve and ovarian response, there was a statistically significant decrease in the concentration of total testosterone and androstenedione in the blood. The women with reduced ovarian response had a statistically significant decrease in the blood level of total testosterone and androstenedione compared to women with normal ovarian reserve and normal response. There was also a decrease in the concentration of DHEA-C in the follicular fluid associated with a decrease in ovarian reserve and ovarian response. However, there was a reverse trend for androstenedione in the follicular fluid: women with normal ovarian response and normal ovarian reserve had lower levels of androstenedione. Conclusion: This study was the first comparative assessment of the androgen profile in infertile patients using mass spectrometry which is considered to be the gold standard. This analysis indicates changes in the levels of androgens and confirms their role in the emerging androgen deficiency. Our study also confirms the hypothesis about the influence of androgens on the processes of folliculogenesis and their significant contribution to the ovarian response. It is possible to explain pathogenetically the value of studies on the effect of hormonal priming in women with infertility and diminished ovarian reserve in order to block apoptosis of follicles and increase sensitivity to FSH.Gavisova A.A., Kindysheva S.V., Starodubtseva N.L., Frankevich V.E., Dolgushina N.V.
Keywords
Androgens are sex hormones that are important in the functioning of both male and female reproductive systems. In women, androgens play a dominant role in the processes of folliculogenesis, which can be clearly demonstrated by polycystic ovary syndrome (PCOS) [1]. The main circulating androgens in the serum of women are dehydroepiandrosterone sulfate (DHEAS), dehydroepiandrosterone (DHEA), androstenedione (A), testosterone (T) and dihydrotestosterone (DHT) [2]. Androgens are effective by means of binding to androgen receptors (AR), which belong to the nuclear receptor superfamily [3]. Only bioactive androgens, testosterone and DHT bind directly to AR, while proandrogens, DHEA and A, require some transformation to be effective as androgens [4].
When women get older, there is a progressive decrease in the follicular reserve in the ovaries, which correlates with a decrease in fertility, maximum in the period from 30 to 40 years. The quality of oocytes also decreases along with the decrease in the antral follicle count (AFC) [5]. Age is known to be the main risk factor for poor ovarian response (POR) [6]. This happens not only due to a decrease in the pool of follicles in the ovaries [7], but also due to a decrease in the sensitivity of the ovaries to follicle-stimulating hormone (FSH) [8] and a decrease in the level of androgen precursors (DHEAS, DHEA, A) and bioactive androgen, namely testosterone [9, 2].
It can also be assumed that bioavailability of androgens in the ovaries may be accompanied by activation of the ovarian response. However, a recent study where highly sensitive and specific methods of liquid chromatography-mass spectrometry were used showed that the levels of androgens in the blood or follicular fluid of the dominant follicle are not predictors of the results of assisted reproductive technology (ART) programs [10]. The latter initiates further discussion about the role of androgens.
It is difficult to assess androgen indicators due to a whole range of problems, including the method of studying biological media which is used for diagnosing various clinical conditions. Since the testosterone molecule is characterized by a low molecular weight, the use of routine methods of immunochemiluminescent assay (ICLA) does not always show the circulating testosterone levels in full; there is a difficulty of testing with various reagents and with the research methodology itself. Nowadays, the gold standard for determining steroids in various biological fluids is liquid chromatography – mass spectrometry [11–13].
Follicular fluid provides critical microenvironment for the development of oocytes; it is a transudate of serum components and secretions of theca cells and granulosa cells. Studying the key regulatory factors in the follicular fluid can predict the physiological status of the ripening oocyte which can be important for determining the quality of the oocyte and its subsequent potential to become a viable zygote supporting the development of the embryo.
Since the follicular fluid is directly involved in the metabolic processes of the follicular environment and is also associated with the quality of oocytes, it is relevant to study both hyperandrogenic and hypoandrogenic conditions to assess the relationship with the development of diminished ovarian reserve (DOR) and POR. The analysis of the correlation between the concentrations of androgens in serum and follicular fluid seems relevant especially in terms of studying DOR and POR. This correlation may help to identify the androgen profile in the biological environment and the diagnostic marker of a decrease in ovarian reserve.
Therefore, the aim of the study was to evaluate the correlation of androgens in blood serum and follicular fluid according to HPLC-MS/MS data in infertile women undergoing treatment with ART (IVF/ICSI) depending on the ovarian response.
Materials and methods
This was a cross-sectional study conducted in parallel groups. The study included 150 patients with infertility identified according to the ESHRE criteria (2011). These women underwent treatment with IVF/ICSI and had embryo transfer at the National Medical Research Center for Obstetrics, Gynecology and Perinatology in Moscow in the period from 2019 to 2021. The average age of women was 37.3 (2.4) years. All patients were examined according to the Order of the Ministry of Health of the Russian Federation No. 803n and signed an informed consent to participate in the study. The exclusion criteria from the study were surgical menopause, hysterectomy, adrenal insufficiency, hormone-producing tumors, obesity (BMI≥30 kg/m2) and body weight deficit (BMI≤18.5 kg/m2), HIV infection and other immunodeficiency disorders, immuno-inflammatory rheumatic diseases, immunomodulatory therapy, oncological diseases.
The patients were divided into groups depending on the ovarian reserve on the basis of the POSEIDON classification and ovarian response to the stimulation in accordance with the Bologna criteria: group 1 included 50 patients with DOR (AMH<1.2 ng/ml, AFC<5) and POR to stimulation (≤3 oocytes during transvaginal puncture); group 2 consisted of 100 patients with normal ovarian reserve (AMH≥1.2 ng/ml, AFC≥5), among them there were 50 patients with normal ovarian response (>9 oocytes) and 50 patients with reduced ovarian response (<9 oocytes).
The patients underwent ovarian stimulation in the ART program (IVF/ICSI) with recombinant FSH preparations (GONAL-f, solution for subcutaneous administration, Merck Serono S.P.A. (Italy)) and urinary gonadotropins (Menopur, lyophilisate for the solution for intramuscular and subcutaneous administration, “Ferring GmbH”). When the follicle diameter was at least 17–18 mm, an ovulation trigger was introduced 36 hours before oocyte retrieval. On the day of the transvaginal puncture of the ovaries, venous blood was taken from the ulnar vein. The obtained blood was centrifuged at the speed of 300g for 20min at 4°C. After centrifugation, supernatant liquid was sampled and centrifuged again at the speed of 12000 g for 10 min at room temperature.
Follicular fluid was taken from the follicles during transvaginal puncture of the ovaries. The obtained material was centrifuged for 10 min at the speed of 3000 g and -4°C. The biomaterial was stored at the temperature of -80°C.
Hormone concentrations were measured with tandem mass spectrometry using a set Steroid Hormones in Serum LC-MS/MS Analysis Kit (JASEM, Turkey). The kit contained 4 calibration mixtures of 16 steroid hormones in lyophilised form, 2 levels of quality control in lyophilised form and a mixture of internal standards (IS). There were mobile phases A (distilled milliQ water with 0.01% formic acid) and B (acetonitrile with 0.01% formic acid), as well as MTBE (≥99.5%, HPLC class, Fisher Chemical), MeOH (99.9%, HPLC BASIC, Scharlau), acetonitrile (99.9%, HPLC Gradient Grade, Fisher Chemical) and formic acid (98%, Sigma-Aldrich), which were used for phase preparation and sample preparation. Hormone separation was carried out on Poroshell 120 EC-C18 column with a length of 100 mm, an internal diameter of 2.1 mm and a grain size of 2.7 microns sorbent.
The samples were analyzed using the HPLC-MS/MS system, which included a triple quadrupole mass spectrometric detector ABSciexQTRAP 5500 with electrospray ionization source and Agilent 1260 Infinity liquid chromatograph (Agilent) with a high-pressure pump, a column thermostat and autosampler for 108 vials.
Statistical analysis
In order to perform statistical analysis, we used scripts written in the programming language R [R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/] в RStudio [RStudio Team (2016). RStudio: Integrated Development for R. RStudio, Inc., Boston, MA URL http://www.rstudio.com/].
Quantitative indicators were checked for normal distribution using the Kolmogorov–Smirnov test; the results are presented in the form of mean values and standard deviation M(SD), otherwise in the format of median and quartiles Me (Q1; Q3). Qualitative indicators are presented in the form of absolute and relative values. Spearman rank correlation (r) was used to determine the possible relationship between the variables.
Characteristics were compared in groups for quantitative variables using the parametric ANOVA criterion or the nonparametric Kruskal-Wallis test (when comparing three groups), pairwise comparison was carried out using the Student’s t-test or the nonparametric Mann–Whitney U test (for two groups).The value of p<0.05 was considered statistically significant; in case of applying the Bonferroni correction for multiple comparison of indicators between groups, the level of p<0.017 was considered significant.
Results
The study included reproductive-aged women with infertility who underwent treatment with IVF/ICSI at the National Medical Research Center for Obstetrics, Gynecology and Perinatology in Moscow. The average age of women was 37.3 (2.4) years. All patients had a regular menstrual cycle, the average duration of which was 27.4 (2.1) days. The age of menopause in the mothers of the patients in the group was 45.6 (2.6) years. All patients noted a high level of intelligence and social responsibility. The average body mass index (BMI) was 24.6 (5.4) kg/m2. All of the above indicators were comparable by groups (p>0.05, ANOVA criterion).
We compared the data on the concentrations of hormones obtained in blood serum and follicular fluid on the day of oocyte aspiration using HPLC-MS/MS methods. We revealed a statistically significant decrease in the concentration of total testosterone and androstenedione in the blood depending on the degree of a decrease in ovarian reserve and ovarian response to stimulation in the IVF program. Thus, women with reduced ovarian response showed a statistically significant decrease in serum parameters of total testosterone 0.438 (0.325; 0.553) and androstenedione 1.795 (1.443; 2.509) compared to women with normal ovarian reserve and normal ovarian response (total testosterone 0.654 (0.523; 0.945), androstenedione 2.624 (2.145; 3.273)). Similar, but statistically not significant changes in the concentration of total testosterone were found in the follicular fluid: 0.352 (0.117; 0.782) ng/ml in patients with normal ovarian response and normal ovarian reserve, 0.314 (0.106; 0.674) ng/ml in women with normal ovarian reserve and reduced ovarian response, 0.159 (0.097; 1.422) ng/ml in patients with diminished ovarian reserve and poor ovarian response, respectively. There was also a decrease in the concentration of DHEAS in the follicular fluid in women with a decrease in ovarian reserve and ovarian response. The analysis of androstenedione parameters in the follicular fluid showed an inverse but statistically not significant trend: 1. 795 (1.443; 2.509) ng/ml in the group of women with normal ovarian reserve and normal ovarian response compared to 2.238 (1.899; 2.914) ng/ml in the group of women with normal ovarian reserve and reduced ovarian response to stimulation and 2.553 (1.448; 2.965) ng/ml in women with diminished ovarian reserve (Table).
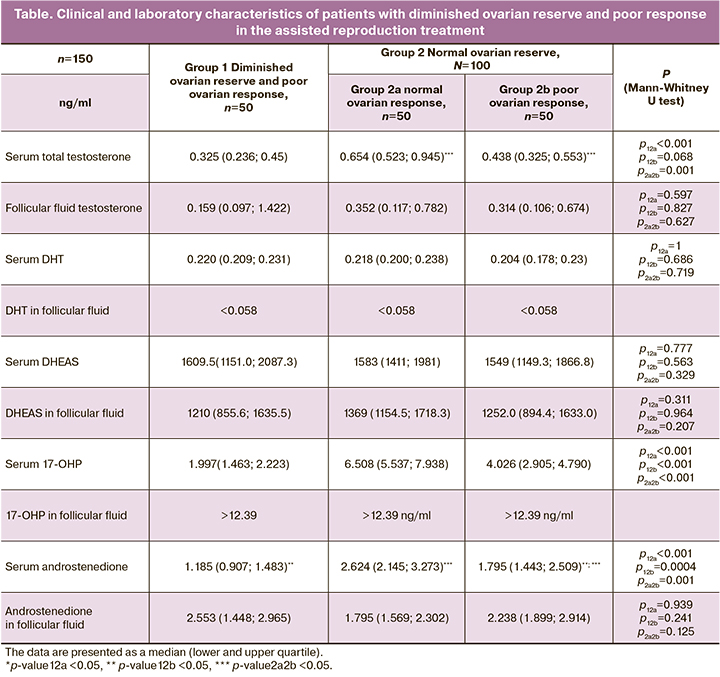
There was also a comparative correlation analysis of the concentrations of androgens obtained in blood plasma and follicular fluid during the study of the latter using HPLC–MS/MS on the day of oocyte aspiration in each of the groups (Fig.1–3).
The patients with diminished ovarian reserve and poor ovarian response had a strong correlation between the levels of androstenedione and follicular fluid testosterone (R=0.8, p<0.001), moderate correlation between concentrations of androstenedione and total testosterone in serum (R=0.69, p<0.001), androstenedione and 17-hydroxyprogesterone (17-OHP) in serum (R=0.65, p<0.001), DHEAS in serum and follicular fluid (R=0.61, p=0.002), androstenedione in serum and follicular fluid (R=0.54, p=0.007), androstenedione in serum and DHEAS in follicular fluid (R=0.51, p=0.009) and DHEAS in follicular fluid and total testosterone in serum (R=0.5, p=0.012) (Fig. 1).
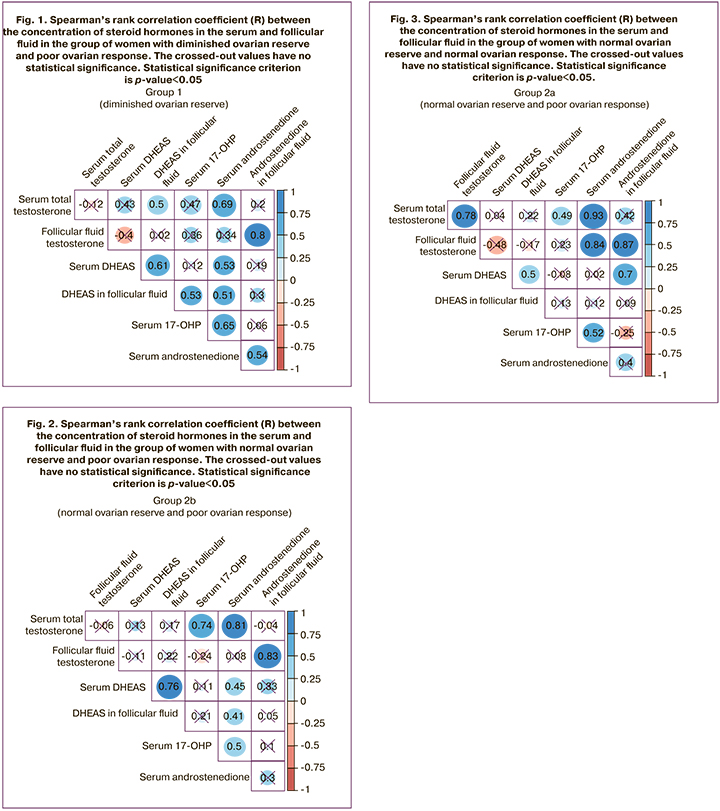
The patients with normal ovarian reserve and reduced ovarian response showed strong correlations between the levels of androstenedione and total testosterone in the follicular fluid (R=0.83, p<0.001), androstenedione and total testosterone in serum (R=0.76, p<0.001) and between 17-OHP and total testosterone in serum (R=0.74, p<0.001) (Fig. 2).
The patients with normal ovarian reserve and normal ovarian response showed strong correlations between the levels of androstenedione and total testosterone in serum (R=0.93, p<0.001), androstenedione and total testosterone in the follicular fluid (R=0.87, p<0.001), androstenedione in serum and total testosterone in follicular fluid (R=0.84, p<0.001), total testosterone in serum and follicular fluid (R=0.78, p<0.001), and moderate correlations between the levels of DHEAS in serum and androstenedione in follicular fluid (R=0.7, p<0.001), DHEAS in serum and follicular fluid (R=0.5, p=0.008) (Fig. 3).
Thus, the group of women with normal ovarian reserve and normal ovarian response demonstrated the strongest correlations between the parameters of androstenedione in serum, total testosterone in serum and androstenedione in serum and total testosterone in follicular fluid; it is possible to consider this group as a control group. However, the groups of patients with altered ovarian response such as patients with diminished ovarian reserve and poor ovarian response as well as patients with normal ovarian response and diminished ovarian reserve did not demonstrate strong correlation unlike the control group.
Discussion
Studies on the processes of folliculogenesis indicate the involvement of androgens in the bioregulation of the ovarian reserve which leads to specific characteristics of oogenesis and embryogenesis; in some cases, it can be accompanied by disorders in oocyte-cumulus complexes affecting the quality of embryos and the frequency of fertilization. A comparative assessment of the androgen profile in infertile patients using mass spectrometry was carried out for the first time in this study.
It is natural that androgen deficiency in women is studied in the postmenopausal period and it does not have clear clinical manifestations. The assessment of its manifestations in the earlier period in patients of reproductive age with the certain rhythm of menstruation is quite justified. The women after 35 years of age are known to have a gradual decrease in AFC and AMH levels regardless of their baseline indicators; such a decrease is also typical for patients with hyperandrogenism and PCOS and may contribute to the problem of infertility.
Numerous modern studies prove that there is a great interest in the research of androgens as factors determining sexual differentiation and behavioral reactions that ensure and maintain the quality of life not only in men, but also in women.
Adequate levels of androgens are necessary for the continuous growth and development of follicles and prevention of follicular atresia. The ovarian response to ovarian stimulation certainly depends on the ovarian reserve and the age of the woman [14, 15] and it has a low variability [16]. Predicting the ovarian response prior to initiating an in vitro fertilization (IVF) program can help determine management tactics and predict its effectiveness [17]. The concentrations of hormones in the blood serum decrease in women with the years, along with a decrease in reproductive potential; similar data can be observed in patients with primary ovarian insufficiency and diminished ovarian reserve [18, 19].
After the natural cessation of estrogen synthesis, granulosa cells have been found to retain the ability to secrete androgens in the presence of an androgenic substrate [20], therefore, the regulatory system may not be at the level of aromatase activity (i.e., the conversion of testosterone into estradiol). A decrease in the concentration of androgens leads to a gradual decrease in the synthesis of estradiol by the follicle; this is preceded by a depression in the concentration of androgens as an earlier physiological event, as it is carried out by theca cells, not granulosa cells [21].
The results of our study confirm the role and involvement of androgens in the processes of folliculogenesis. Statistically significant changes in the concentration of one of the possible markers of folliculogenesis, namely androstenedione, in the follicular fluid and blood indicate different biochemical reactions of granulosa cells to exogenous gonadotropins depending on the ovarian reserve. We revealed in our study that women with normal ovarian response and normal ovarian reserve had the highest concentration of androstenedione in the blood and the lowest concentration in the follicular fluid; whereas women with poor ovarian response, normal ovarian reserve and reduced ovarian reserve showed statistically significantly different androstenedione levels in blood serum and follicular fluid in the groups. Higher levels of androstenedione in serum and follicular fluid were noted in patients with normal ovarian reserve and poor ovarian response, and, on the contrary, the highest level of androstenedione in follicular fluid and its low concentration in serum was noted in women with the lowest AMH parameters, in women with diminished ovarian reserve. The results of our study show that androgens play a dominant role in the processes of folliculogenesis and ovarian response, as well as numerous changes in the androgen profile depending on the ovarian response; this fact confirms the activity of the enzyme systems involved in the synthesis of ovarian androgens.
There was also a decrease in testosterone levels depending on the ovarian response with the lowest concentrations in serum and follicular fluid in women with diminished ovarian reserve and poor ovarian response, despite the lack of statistical significance. However, women with normal ovarian response and normal ovarian reserve had the highest levels of total testosterone in both blood serum and follicular fluid.
Most of the studies on the involvement of androgens in the process of folliculogenesis confirm their stimulating role at the stages of early follicle growth, their supporting role in the dynamics of the development of the follicle and their participation in the initiation of follicle development at a late stage. These findings explain the concept of the possibility of preliminary treatment with androgens to enhance the follicular response to FSH in women who previously had a poor ovarian response in the IVF program; though this concept has not been proven yet, it has been accepted by researchers. Therefore, it is relevant to discuss the role of both hyperandrogenism and hypoandrogenism in the development of oocytes, for example, in PCOS [22]. Moreover, the presence of various androgens in the follicular fluid [23], strong expression of androgen receptors in cumulus cells of preovulatory follicles [24] and enlargement of cumulus and impaired viability of oocytes/embryos due to loss of signal transmission by androgen granulosa receptors [25] suggest that the potential use of androgens in cultivation systems in vitro is an area that requires additional research.
Basic studies on the concentration of testosterone in blood serum and follicular fluid are quite contradictory. Meldrum D.R. detected neither a decrease in serum testosterone levels in women with the years, nor a decrease in its concentration in the follicular fluid of patients with poor response in the natural cycle [26]. Therefore, it is necessary to study the endocrine relationship between serum and follicular fluid in order to answer the question how the concentration of androgens in serum is closely interrelated with folliculogenesis and to assess the effect of androgens in patients with infertility and diminished ovarian reserve.
The study conducted by Fuentes A. et al. showed that the level of circulating androgens under the conditions of controlled ovarian stimulation in patients without diminished ovarian reserve is higher than the level of androgens in natural cycles in the serum and follicular fluid of women with poor ovarian response in the IVF program. The parameters of total testosterone, androstenedione and DHEAS did not differ in the POSEIDON 1 group, but significantly decreased in the POSEIDON 3 group. In addition, the parameters of DHEAS in the follicular fluid were also significantly reduced in the POSEIDON 3 group, compared with the control. Serum testosterone levels were also reduced in POSEIDON groups 2 and 4, compared with the control [27].
One can suggest that a lower concentration of peripheral androgens in women with poor response, which decreases in patients with the years, may not be sufficient to block follicle apoptosis. However, this can be compensated by their increased sensitivity to FSH for determining their growth and development. According to Klein et al., low saturation of peripheral androgen concentrations in blood serum is not associated with the concentration of androgens in the follicular fluid [28]. As some researchers believe, after endogenous release or injection of hCG, the concentration of androstenedione decreases to different parameters, however, it is assumed that the level of reduction should vary depending on the ovarian response [29, 30].
There is also another point of view about the ability of the follicular environment to compensate for an increase or decrease in the concentration of androgens in the blood serum; according to the results of our study, it has not been confirmed, otherwise the obtained data would have been presented in the form of identical values. Moreover, there was a change in the concentration of androgens in the follicular fluid depending on the ovarian reserve and response. Thus, our study confirms the concept of the relationship between the concentrations of androgens in the follicular fluid and blood serum depending on the ovarian response. These data were confirmed in the study on the assessment of follicular concentrations of DHEAS in the ART program. After oral administration of DHEA, higher follicular concentrations of DHEAS were observed in patients with infertility after androgen priming due to the activity of follicular sulfatase which converts DHEA into DHEAS.
According to our data, higher concentrations of total testosterone in the follicular fluid and in blood, as well as androstenedione in blood and low concentrations in the follicular fluid are associated with better indicators of oogenesis [31, 32]. Changes in the concentration of follicular hormones during ovulation are necessary for the final oocyte maturation and reproductive success.
Our study was the first to show that the decrease in the concentration of androgens has a multidirectional character, and the development of its insufficiency or deficiency is associated with a decrease in ovarian reserve and infertility; the clear correlation dynamics of a decrease in androstenedione depending on the ovarian reserve makes it possible to consider it as a biochemical marker not only of hyperandrogenic conditions, but also of hypoandrogenic ones, including androgen deficiency in young women.
Conclusion
Thus, our study confirms the hypothesis about the important role of the influence of androgens on the processes of folliculogenesis and ovarian response. However, the physiology of ovarian processes is quite complex and it is necessary to conduct additional research to study the decrease in ovarian reserve and the results of ART treatment. One can assume that the results of our work will allow us to identify and explain from the pathogenetic point of view the necessity of conducting studies on the effect of hormonal priming in women with infertility and diminished ovarian reserve as a factor that blocks follicle apoptosis and increases sensitivity to FSH.
References
- Simpson E.R., Clyne C., Rubin G., Boon W.C., Robertson K., Britt K. et. al. Aromatase – a brief overview. Annu. Rev. Physiol. 2002; 64: 93-127.https://dx.doi.org/10.1146/annurev.physiol.64.081601.142703.
- Davison S.L., Davis S.R. Androgens in women. J. Steroid Biochem. Mol. Biol. 2003; 5(2-5): 363-6. https://dx.doi.org/10.1016/s0960-0760(03)00204-8.
- Quigley C.A., De Bellis A., Marschke K.B., El-Awady M.K., Wilson E.M., French F.S. Androgen receptor defects: historical, clinical, and molecular perspectives. Endocr. Rev. 1995; 16(3): 271-321. https://dx.doi.org/10.1210/edrv-16-3-271.
- Burger H.G. Androgen production in women. Fertil. Steril. 2002; 77(Suppl. 4): S3-5. https://dx.doi.org/10.1016/s0015-0282(02)02985-0.
- Homan G.F., Davies M., Norman R. The impact of lifestyle factors on reproductive performance in the general population and those undergoing infertility treatment: a review. Hum. Reprod. Update. 2007; 13(3): 209-23. https://dx.doi.org/10.1093/humupd/dml056.
- Ferraretti A.P., LaMarca A., Fauser B.C., Tarlatzis B., Nargund G., Gianaroli L.; ESHRE Working Group on Poor Ovarian Response Definition. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum. Reprod. 2011; 6(7): 1616-24.https://dx.doi.org/10.1093/humrep/der092.
- Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr. Rev. 1996; 17(2): 121-55. https://dx.doi.org/10.1210/edrv-17-2-121.
- Goverde A.J., Mcdonnell J., Schats R., Vermeiden J.P., Homburg R., Lambalk C.B. Ovarian response to standard gonadotrophin stimulation for IVF is decreased not only in older but also in younger women in couples with idiopathic and male subfertility. Hum. Reprod. 2005; 20(6): 1573-7. https://dx.doi.org/10.1093/humrep/deh827.
- Zumoff B., Strain G.W., Miller L.K., Rosner W. Twenty-four-hour mean plasma testosterone concentration declines with age in normal premenopausal women. J. Clin. Endocrinol. Metab. 1995; 80(4): 1429-30. https://dx.doi.org/ 10.1210/jcem.80.4.7714119.
- Walters K.A., Eid S., Edwards M.C., Thuis-Watson R., Desai R., Bowman M. et al. Steroid profiles by liquid chromatography-mass spectrometry of matched serum and single dominant ovarian follicular fluid from women undergoing IVF. Reprod. Biomed. Online. 2019; 38 (1): 30-7. https://dx.doi.org/10.1016/j.rbmo.2018.10.006.
- Wang C., Catlin D.H., Demers L.M., Starcevic B., Swerdloff R.S. Measurement of total serum testosterone in adult men: comparison of current laboratory methods versus liquid chromatography-tandem mass spectrometry. J. Clin. Endocrinol. Metab. 2004; 89(2): 534-43. https://dx.doi.org/10.1210/jc.2003-031287.
- Taieb J., Mathian B., Millot F., Patricot M.C., Mathieu E., Queyrel N. et al. Testosterone measured by 10 immunoassays and by isotope- dilution gas chromatography-mass spectrometry in sera from 116 men, women, and children. Clin. Chem. 2003; 49(8): 1381-95. https://dx.doi.org/10.1373/49.8.1381.
- Sikaris K., McLachlan R.I., Kazlauskas R., de Kretser D., Holden C.A., Handelsman D.J. Reproductive hormone reference intervals for healthy fertile young men: evaluation of automated platform assays. J. Clin. Endocrinol. Metab. 2005; 90(11): 5928-36. https://dx.doi.org/10.1210/jc.2005-0962.
- Fauser B.C., Diedrich K., Devroey P.; Evian Annual Reproduction Workshop Group 2007. Predictors of ovarian response: progress towards individualized treatment in ovulation induction and ovarian stimulation. Hum. Reprod. Update. 2008; 14(1):1-14. https://dx.doi.org/10.1093/humupd/dmm034.
- de Boer E.J., den Tonkelaar I., te Velde E.R., Burger C.W., Klip H., van Leeuwen F.E.; OMEGA-Project Group. A low number of retrieved oocytes at in vitro fertilization treatment is predictive of early menopause. Fertil. Steril. 2002; 77(5): 978-85. https://dx.doi.org/10.1016/s0015-0282(02)02972-2.
- te Velde E.R., Pearson P.L. The variability of female reproductive ageing, Hum. Reprod. Update. 2002; 8(2): 141-54. https:/dx.doi.org/10.1093/humupd/8.2.141.
- Klinkert E.R., Broekmans F.J., Looman C.W., te Velde E.R. A poor response in the first in vitro fertilization cycle is not necessarily related to a poor prognosis in subsequent cycles. Fertil. Steril. 2004; 81(5): 1247-53.https://dx.doi.org/10.1016/j.fertnstert.2003.10.030.
- van der Stege J.G., Groen H., van Zadelhoff S.J., Lambalk C.B., Braat D.D., van Kasteren Y.M. et al. Decreased androgen concentrations and diminished general and sexual well-being in women with premature ovarian failure. Menopause. 2008; 15(1): 23-31. https://dx.doi.org/10.1097/gme.0b013e3180f6108c.
- Gleicher N., Kim A., Weghofer A., Kushnir V.A., Shohat-Tal A., Lazzaroni E. et al. Hypoandrogenism in association with diminished functional ovarian reserve. Hum. Reprod. 2013; 28(4): 1084-91. https://dx.doi.org/10.1093/humrep/det033.
- Hillier S.G. Regulation of follicular oestrogen biosynthesis: a survey of current concepts. J. Endocrinol. 1981; 89(Suppl.): 3P-18P.
- Dieleman S.J., Kruip T.A., Fontijne P., de Jong W.H., van der Weyden G.C. Changes in oestradiol, progesterone and testosterone concentrations in follicular fluid and in the micromorphology of preovulatory bovine follicles relative to the peak of luteinizing hormone. J. Endocrinol. 1983; 97(1): 31-42.https://dx.doi.org/10.1677/joe.0.0970031.
- Palomba S., Daolio J., La Sala G.B. Oocyte competence in women with polycystic ovary syndrome. Trends Endocrinol. Metab. 2017; 28(3): 186-98. https://dx.doi.org/10.1016/j.tem.2016.11.008.
- Kushnir M.M., Naessen T., Wanggren K., Hreinsson J., Rockwood A.L., Meikle A.W., Bergquist J. Exploratory study of the association of steroid profiles in stimulated ovarian follicular fluid with outcomes of IVF treatment. J. Steroid Biochem. Mol. Biol. 2016; 162: 126-33. https://dx.doi.org/10.1016/j.jsbmb.2015.09.015.
- Lenie S., Smitz J. Functional AR signaling is evident in an in vitro mouse follicle culture bioassay that encompasses most stages of folliculogenesis. Biol. Reprod. 2009; 80(4): 685-95. https://dx.doi.org/10.1095/biolreprod.107.067280.
- Walters K.A., Allan C.M., Handelsman D.J. Rodent models for human polycystic ovary syndrome. Biol. Reprod. 2012; 86(5): 1-12. https://dx.doi.org/10.1095/biolreprod.111.097808.
- Meldrum D.R., Chang R.J., Giudice L.C., Balasch J., Barbieri R.L. Role of decreased androgens in the ovarian response to stimulation in older women. Fertil. Steril. 2013; 99(1): 5-11. https://dx.doi.org/10.1016/j.fertnstert.2012.10.011.
- Fuentes A., Sequeira K., Tapia-Pizarro A., Muñoz A., Salinas A., Céspedes P. et al. Androgens profile in blood serum and follicular fluid of women with poor ovarian response during controlled ovarian stimulation reveals differences amongst POSEIDON stratification groups: A Pilot Study. Front. Endocrinol. (Lausanne). 2019; 10: 458. https://dx.doi.org/ 10.3389/fendo.2019.00458.
- Klein N.A., Harper A.J., Houmard B.S., Sluss P.M., Soules M.R. Is the short follicular phase in older women secondary to advanced or accelerated dominant follicle development? J. Clin. Endocrinol. Metab. 2002; 87(12): 5746-50. https://dx.doi.org/10.1210/jc.2002-020622.
- Templeton A.A. Ovulation timing and in vitro fertilisation. In: Thompson W., Joyce D.N., Newton J.R., eds. In vitro fertilisation and donor ituerninution. London: Royal College of Obstetricians and Gynaccologists, 1985: 241-51.
- Edwards R.G., Steptoe P.C., Fowler R.E., Baillie J. Observations on preovulatory human ovarian follicles and their aspirates. Br. J. Obstet. Gynaecol. 1980; 87(9): 769-79. https://dx.doi.org/10.1111/j.1471-0528.1980.tb04612.x.
- Li J., Yuan H., Chen Y., Wu H., Wu H., Li L. A meta-analysis of dehydroepiandrosterone supplementation among women with diminished ovarian reserve undergoing in vitro fertilization or intracytoplasmic sperm injection. Int. J. Gynaecol. Obstet. 2015; 131(3): 240-5. https://dx.doi.org/10.1016/j.ijgo.2015.06.028.
- Hu Q., Hong L., Nie M., Wang Q., Fang Y., Dai Y. et al The effect of dehydroepiandrosterone supplementation on ovarian response is associated with androgen receptor in diminished ovarian reserve women. J. Ovarian Res. 2017; 10(1): 32. https://dx.doi.org/10.1186/s13048-017-0326-3.
Received 12.10.2022
Accepted 19.10.2022
About the Authors
Alla A. Gavisova, PhD, Senior Researcher at the 1st Gynecological Department, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(916)829-05-90,gavialla@yandex.ru, 117997, Russia, Moscow, Akademika Oparina str., 4.
Svetlana V. Kindysheva, PhD, Senior Researcher at the Laboratory of Proteomics and Metabolomics of Human Reproduction, Department of Systems Biology
in Reproduction, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)438-07-88, s_kindysheva@oparina4.ru, 117997, Russia, Moscow,
Academician Oparin str., 4.
Natalia L. Starodubtseva, PhD, Head of the laboratory of Clinical Proteomics of the Department of Systems Biology in Reproductive Medicine of the Institute of Translational Medicine, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, aurum19@mail.ru, https://orcid.org/0000-0001-6650-5915, 117997, Russia, Moscow, Academician Oparin str., 4.
Vladimir E. Frankevich, Dr. Sci. (Physics and Mathematics), Deputy Director for Research, Head of the Department of Systems Biology in Reproduction, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, v_frankevich@oparina4.ru, https://orcid.org/0000-0002-9780-4579, 117997, Russia, Moscow, Academician Oparin str., 4.
Nataliya V. Dolgushina, Dr. Med. Sci., Professor, Deputy Director – Head of the Department for Scientific Projects Organization, V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia, n_dolgushina@oparina4.ru, https://orcid.org/0000-0003-1116-138X, 117997, Russia, Moscow, Academician Oparin str., 4.
Authors’ contributions: Gavisova A.A. – developing the concept and design of the study, review on the subject of publication, collecting the material, writing the article, final approval of the version for publication; Kindysheva S.V. – processing the material, statistical analysis of the results; Starodubtseva N.L. – processing the material, review; Frankevich V.E. – review; Dolgushina N.V. – developing the concept and design of the study, review.
Conflicts of interest:. The authors declare that there are no conflicts of interest.
Funding: The study was carried out without sponsorship.
Ethical Approval: The study was approved by the Ethical Review Board of Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia
Patient Consent for Publication: All patients participating in the study provided an informed consent for the publication of their data.
Authors’ Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Gavisova A.A., Kindysheva S.V., Starodubtseva N.L.,
Frankevich V.E., Dolgushina N.V. Androgen profile in infertile women undergoing treatment
with assisted reproductive technologies depending on the ovarian response.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 10: 129-137 (in Russian)
https://dx.doi.org/10.18565/aig.2022.10.129-137



