Активное внедрение вспомогательных репродуктивных технологий (ВРТ) в медицину обусловлено широким распространением проблемы бесплодного брака по всему миру.
Современные методы интрацитоплазматической инъекции сперматозоида в цитоплазму ооцита (ИКСИ), дополнительный отбор зрелого сперматозоида по связыванию с гиалуроновой кислотой (ПИКСИ), преимплантационный генетический скрининг (ПГС), позволяющий провести анализ полного хромосомного набора эмбриона до его переноса в полость матки, повышают эффективность лечения в программах экстракорпорального оплодотворения (ЭКО) [1].
Несмотря на внедрение и использование новых методик, проблема негативных исходов программ ВРТ остается крайне актуальной, поскольку частота наступления беременности не превышает 50%, даже при наличии нормальных ультразвуковых параметров прегравидарного эндометрия и эуплоидного хромосомного набора переносимого эмбриона [2].
Точность морфологической системы классификации эмбрионов недостаточна [3], так как имеет ограниченную прогностическую ценность для определения вероятности наступления и прогрессирования беременности [4], о чем свидетельствует тот факт, что не все эмбрионы «хорошего» морфологического качества успешно имплантируются [5].
Вспомогательным методом селекции эмбриона в последнее время является проведение ПГС, который используется уже более десяти лет, показывая превосходство над отбором только по морфологическим критериям [5]. Основными показаниями для проведения ПГС являются наличие генетических отклонений (наследственных заболеваний) у одного или обоих супругов, наличие наследственных заболеваний в семье (в том числе проявляющихся в зрелом возрасте), наличие наследственных заболеваний у детей, неоднократные неудачные попытки ЭКО, наличие у мужчины тяжелых нарушений сперматогенеза (единичные сперматозоиды, высокий процент аномальных сперматозоидов и др.), привычное невынашивание беременности в анамнезе (особенно в случаях прерывания беременности на ранних сроках), возраст супругов старше 35 лет.
ПГС численных или структурных хромосомных аномалий – это инвазивный способ тестирования эмбриона, направленный на выявление эуплоидных эмбрионов в когорте полученных во время цикла ЭКО. Наличие некоторых ограничений этого метода, а именно воздействие на эмбрион [6], отсутствие учета мозаицизма при интерпретации результатов [7], возможность самокоррекции и селективного апоптоза эмбрионов раннего развития [8], поднимает вопрос о точности изображения хромосомного набора зародыша в целом, а высокая стоимость исследования приводит к ограничению использования данного метода в широкой клинической практике.
В связи с этим возникает необходимость развития и внедрения в практику клиницистов новых методов повышения результативности лечения в программе ЭКО путем селективного переноса в полость матки эмбриона с высоким потенциалом к имплантации [4].
Одним из перспективных подходов в селекции эмбрионов является анализ состава питательных сред после инкубации эмбрионов, с целью выявления отличий в содержании отдельных метаболитов или их профилей. В 1980 году J.P. Renard с соавт. показали, что уровень потребления глюкозы был выше в средах культивирования перспективных бластоцист крупного рогатого скота, полученных через 10 дней после эструса [9]. Подобные работы проводились на эмбрионах разных биологических видов [10], однако количество исследований на эмбрионах человека остается до сих пор незначительным.
Используя ультрамикрофлуоресцентное определение скорости потребления глюкозы 4-дневными эмбрионами мышей до их переноса D.K. Gardner и H.J. Leese показали, что уровень потребления глюкозы был значительно выше у развивающихся бластоцист [11]. Полученные авторами данные также подтвердила научная группа, возглавляемая M. Lane [12]. Помимо глюкозы, внимание ученых было также сосредоточено на изучении метаболизма других важных субстратов: аминокислот, лактата и пирувата [13].
 D.К. Gardner с соавт. наблюдал более высокое потребление пирувата и глюкозы у эмбрионов, развившихся до стадии бластоцисты. Кроме того, скорость потребления глюкозы была выше у бластоцист отличного качества, в отличие от поглощения пирувата [14]. В другой работе D.К. Gardner с соавт. продемонстрировали взаимосвязь между потреблением компонентов питательных сред культивирования человеческим эмбрионом, его потенциалом к имплантации и дальнейшему развитию после переноса в полость матки [15].
D.К. Gardner с соавт. наблюдал более высокое потребление пирувата и глюкозы у эмбрионов, развившихся до стадии бластоцисты. Кроме того, скорость потребления глюкозы была выше у бластоцист отличного качества, в отличие от поглощения пирувата [14]. В другой работе D.К. Gardner с соавт. продемонстрировали взаимосвязь между потреблением компонентов питательных сред культивирования человеческим эмбрионом, его потенциалом к имплантации и дальнейшему развитию после переноса в полость матки [15].
Исследование потребления глюкозы проводилось методом ультрамикрофлуориметрии с использованием флуоресцентного микроскопа в образцах объемом несколько нанолитров, отбираемых из питательных сред через определенные промежутки времени на 4-й день культивирования. Было показало, что уровень потребления глюкозы был выше в культуральных средах эмбрионов, после переноса которых развилась беременность по сравнению с теми эмбрионами, после переноса которых беременность не наступила.
Еще одним перспективным подходом в данном направлении является профилирование совокупности метаболитов питательных сред эмбрионов. В работе E. Seli и соавт. было показано, что профилирование метаболитов культуральных сред эмбрионов 3 суток развития при помощи спектроскопии ядерного магнитного резонанса может определить эмбрионы с высоким репродуктивным потенциалом [3]. В частности, было установлено, что содержание глутамата, потенциального энергетического субстрата, было значительно выше в культуральных средах имплантировавшихся эмбрионов. Авторы также показали, что индекс жизнеспособности, рассчитанный по результатам спектроскопии ядерного магнитного резонанса с использованием показателей глутамата и аланин/лактата, был выше для эмбрионов, после переноса которых развилась беременность.
Важно отметить, что в цитируемых выше работах оценка состава метаболитов проводилась на дорогостоящем оборудовании, требующем определенной квалификации персонала. Кроме того, использованные методики не являются оптимальными для рутинного применения. В этой связи нами предложено использовать имеющиеся биохимические подходы, основанные на флуориметрическом детектировании специфических продуктов реакций глюкозы и глутамата, как более простого и удобного метода оценки потребления этих субстратов, для выявления возможной ассоциации исследуемых показателей с качеством и плоидностью эмбрионов пятых суток развития.
Цель исследования: оценить изменение содержания глюкозы и глутамата в культуральных средах эмбрионов 5 суток развития методом флуориметрической детекции и определить ассоциацию исследуемых показателей с качеством и плоидностью эмбрионов.
Материал и методы исследования
На базе отделения вспомогательных технологий в лечении бесплодия (руководитель отделения д.м.н., доцент Е.А. Калинина) и лаборатории молекулярной патофизиологии (руководитель лаборатории к.х.н. М.Ю. Бобров) ФГБУ НМИЦ АГП им. В.И. Кулакова МЗ РФ проведено одномоментное исследование в параллельных группах.
В исследование была включена 21 пациентка, проходящая программу ЭКО (ИКСИ) по стандартному протоколу стимуляции функции яичников с последующим проведением ПГС методом сравнительной геномной гибридизации по показаниям (наличие наследственных заболеваний в семье, наличие наследственных заболеваний у детей, неоднократные неудачные попытки ЭКО, привычное невынашивание беременности в анамнезе, возраст супругов старше 35 лет).
Все пациентки соответствовали критериям включения (нормальный кариотип супругов, возраст пациентки от 18 до 40 лет, сохраненный овариальный резерв, фертильная/субфертильная сперма партнера, трубно-перитонеальный фактор бесплодия) и невключения (противопоказания для проведения ЭКО, в том числе экстрагенитальная патология и онкологические заболевания, подтвержденный лапароскопически и/или ультрасонографически генитальный эндометриоз III–IV степени, интерстициальная и/или субсерозная миома матки более 4 см, субмукозная миома, деформирующая полость матки, патология эндометрия, пороки развития половых органов, тяжелая патозооспермия).
Перед включением в протокол ЭКО все женщины были обследованы согласно Приказу Минздрава России от 30.08.12 № 107н «О порядке использования вспомогательных репродуктивных технологий (ВРТ), противопоказаниях и показаниях к их применению» [16].
Стимуляция функции яичников проводилась с использованием препаратов гонадотропинов и антагонистов гонадотропин-рилизинг-гормона. Триггер овуляции вводился при диаметре лидирующих фолликулов 18 мм и более. В качестве триггера использовался препарат человеческого хорионического гонадотропина (ЧХГ) в стандартной дозе. Трансвагинальную пункцию яичников осуществляли через 35 часов после введения триггера овуляции. Через 24 часа после пункции назначали препарат микронизированного прогестерона в дозе 200–300 мкг/сут. в течение 10 дней для формирования двухфазного менструального цикла.

Культивирование эмбрионов проводилось в индивидуальных каплях сред «CSC Irvine» одинакового объема 35 µl. На 5-е сутки культивирования всем эмбрионам проводилась морфологическая оценка качества согласно классификации, принятой Istanbul consensus workshop on embryo assessment (ESHRE, 2011) («модифицированная» классификация D. Gardner) [17].
В частности проводилась количественная оценка внутриклеточной массы по следующим параметрам: А – большое количество плотно упакованных клеток; В – среднее количество клеток, менее компактная группировка; С – малое количество клеток. Также оценивалось количество клеток трофэктодермы: А – большое количество клеток, формирующих плотный эпителий; В – незначительное количество формирующих рыхлый эпителий клеток; С – малое количество клеток.
Эмбрионам «отличного» и «хорошего» качества проводилась биопсия трофоэктодермы бластоцисты с целью проведения ПГС методом сравнительной геномной гибридизации для выявления хромосомных нарушений, после чего эмбрионы были криоконсервированы методом витрификации. Через 2–3 менструальных цикла по результатам ПГС проводился перенос пригодных криоконсервированных/размороженным эмбрионов. Всего в исследование было включено 68 эмбрионов. Образцы отработанных культуральных сред были отобраны в равных объемах по 25 µl для исследуемых групп, промаркированы, заморожены и впоследствии исследованы для определения потребления глюкозы и глутамата методом флуоресцентной фотометрии. В качестве контроля использовали культуральные среды, инкубированные в отсутствии эмбрионов.
Определение потребления питательных компнентов из сред культивирования проводился на флуориметре Qubit 3.0 (Life technologies). Измерения проводились в соответствии с инструкцией производителя коммерческих наборов реактивов Amplex Red Glucose Assay kit и Amplex Red glutamic Acid Assay kit (Molecular Probes Life technologies). Принцип метода основан на сопряжении двух ферментативных реакций: окисления глюкозы глюкозаоксидазой с эквимолярным образованием одного из продуктов – перекиси водорода, которая используется другим ферментом пероксидазой для превращения реагента AmplexRed (10-ацетил-3,7-дигидроксифеноксазин) во флуоресцирующее производное резоруфин. Соотношение образующегося резоруфина и перикиси равно 1:1. Таким образом, снижение флуоресценции резоруфина соответствует снижению концентрации глюкозы в исследуемом образце.
Статистическая обработка данных выполнена на индивидуальном компьютере с помощью электронных таблиц Microsoft Excel и пакета прикладных программ SPSS Statistics 17.0, Statistica for Windows v. 7.0. Все полученные количественные данные обработаны методом вариационной статистики.
Исследование было одобрено комиссией по этике ФГБУ НМИЦ АГП им. академика В.И. Кулакова Минздрава России.
Результаты исследования и обсуждение
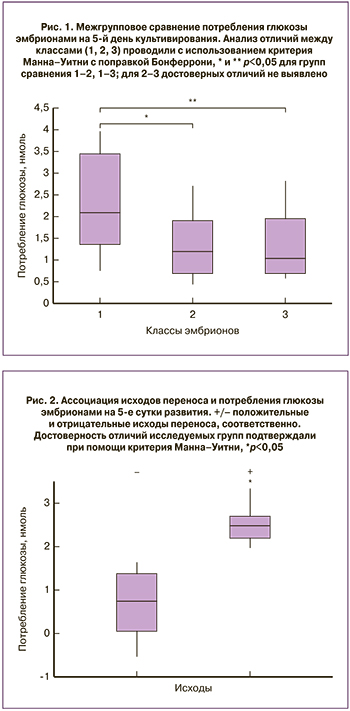
В результате морфологической оценки эмбрионы были разделены на индивидуальные классы на основе количественных показателей клеток: в 1-й класс вошли эмбрионы морфологических групп: 6АА, 4АА, 4АВ (18 образцов); во 2-й класс – эмбрионы морфологической группы 3АА (28 образцов); в 3-й класс – эмбрионы морфологических групп 3АВ, 3ВА, 3ВВ (22 образца). Выбор указанных морфологических групп был также обусловлен их более предпочтительным использованием для переноса. Проведенный анализ ПГС показал, что среди полученных эмбрионов насчитывалось в среднем 50% анэуплоидных кариотипов с изменениями по одной и более парам хромосом (Таблица). Сходное распределение наблюдалось и по полу, за исключением 2-го класса, где эмбрионов XY было в два раза больше (таблица). Из отобранных эмбрионов 12 были использованы для переноса. В качестве критериев имплантации использовали данные анализа ХГЧ и ультразвукового исследования.
Оценка потребления глюкозы выявила в каждом классе эмбрионы, в питательных средах которых значения содержания глюкозы по данным флуориметрии не отличались от контрольных (максимальное падение не более 1%) или были выше таковых. В каждом классе насчитывалось порядка 30% подобных эмбрионов, и они были исключены из сравнительных расчетов потребления глюкозы, как условно «метаболически неактивные» (таблица, н/а). В данной работе был также проведен анализ изменений потребления глутамата, однако достоверных отличий в исследуемых и контрольных группах зафиксировано не было, что может быть связано с его относительно низкой концентрацией (46 мкМ) в питательной среде и недостаточной чувствительностью используемого метода регистрации.
На основании морфологических и генетических критериев были проведены межгрупповые и внутригрупповые сравнения потребления глюкозы. Внутригрупповой анализ не выявил достоверных отличий между эуплоидными и ануэплоидными эмбрионами, а также эмбрионами разного пола (данные не приведены).
Межгрупповые сравнения выявили достоверное увеличение потребления глюкозы у эмбрионов 1-го класса по сравнению со 2-м и 3-м классами. Отличий по данному параметру между 2-м и 3-м классом не наблюдалось (рис. 1). Повышение потребления глюкозы эмбрионами 1-го класса может быть вызвано увеличением объема клеточной массы и ускорением энергетического обмена на данной стадии развития.
Оценка содержания глюкозы и исходов имплантации показала, что критерием удачной имплантации может служить повышенный уровень потребления глюкозы (рис. 2). При этом в группы сравнения с удачными и неудачными исходами попали эмбрионы из всех исследуемых классов (по 6 эмбрионов в каждой группе). Обращает на себя внимание тот факт, что половина эмбрионов с неудачным исходом имплантации попали в группу «метаболически неактивных» (таблица).
Отсутствие потребления глюкозы в таких эмбрионах может быть связано с нарушением переключения метаболизма карбоновых кислот, преобладающего на ранних стадиях развития эмбриона, на использование глюкозы в качестве энергетического субстрата [18, 19]. Известно, что по мере развития эмбриона, начиная со стадии бластоцисты, потребление глюкозы неуклонно возрастает. Это может быть связано с тем, что усиление анаэробного и аэробного расщепления глюкозы стимулирует продукцию АТФ, обеспечивая растущие потребности развивающегося эмбриона. Данное явление наблюдалось в ряде работ при исследовании этапов развития эмбрионов млекопитающих [19, 20].
Наблюдаемое в некоторых образцах повышение флуоресценции, свидетельствующее о возможном повышении содержания глюкозы, может быть связано с выделением эндогенной глюкозы, образуемой, например, при избыточном распаде гликогена. Кроме этого, используемый метод регистрации глюкозы чувствителен к перекиси водорода (см. «Материалы и методы»), поэтому повышение продукции эмбрионами перекиси водорода, в частности, в результате развития оксидативного стресса, может вносить искажения в регистрируемые параметры. Оба указанных фактора могут служить маркерами нарушений энергетическгого обмена и невозможности реализовать дальнейшую программу развития. Проверка маркеров окислительного стресса в питательных средах более специфичными методами может быть еще одним перспективным подходом для селекции эмбрионов.
Заключение
Таким образом, можно заключить, что при отсутствии выраженного снижения содержания глюкозы, либо повышении значений флуоресценции относительно контроля, перенос эмбрионов является нежелательным. На основании полученных данных, в перспективе можно рекомендовать предложенный метод измерения концентрации глюкозы в качестве дополнительного неинвазивного исследования для селективного переноса эмбрионов со сходными морфологическими параметрами.



