A prospective randomized controlled study on the effectiveness of neodymium (Nd:YAG) laser therapy for vulvovaginal atrophy in postmenopausal women
Ramazanova M.O., Apolikhina I.A., Shatilova K.V., Khlopkov A.D., Sencha L.M.
Objective: To evaluate the effectiveness and safety of non-ablative neodymium laser for the treatment of vulvovaginal atrophy (VVA) symptoms in postmenopausal women.
Materials and methods: The study included 120 patients aged 50 to 85 years with symptoms of postmenopausal VVA. According to the standard regimen, all patients underwent treatment with topical estriol (n=40), or three sessions of non-ablative neodymium laser therapy with an interval of 4–6 weeks between sessions (n=40), or a combination of laser treatment and topical estriol (n=40) between 2021 and 2023. The effectiveness of the therapy was assessed using the vaginal health index (VHI) and validated questionnaires: the Female Sexual Function Index (FSFI), the Vulvovaginal Symptom Questionnaire (VSQ), the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12), and the visual analogue scale (VAS). The observation period accounted for 9 months from the onset of treatment.
Results: Statistically significant differences were observed between pre-treatment and follow-up visits, and between groups at follow-up visits. It was shown that in the laser and combination therapy groups, compared with the hormonal treatment group, after 3 and 9 months from the start of therapy, the average score on the PISQ-12 questionnaire significantly increased, and the severity of VVA symptoms on the VAS scale decreased (up to complete elimination). According to the VHI data, a statistically significant difference was noted after 3 and 9 months from the start of therapy in the laser and combination treatment groups compared to the hormonal treatment group. Analysis of the changes in the VSQ questionnaire scores revealed a significantly more pronounced symptoms reduction in the laser and combination therapy groups in comparison with the hormonal treatment. However, no statistically significant differences were found in the effectiveness of laser monotherapy and combination therapy. When assessing the FSFI questionnaire scores 3 and 9 months after the start of therapy, a statistically significant increase in scores was observed in all groups. A statistically significant difference was noted after 3 and 9 months from the start of therapy in the laser and combination treatment groups compared to the hormonal treatment group, while the highest changes in subjectively assessed indicators of the quality of sexual life were detected in the laser treatment group without the use of hormonal therapy.
Conclusion: Localized neodymium laser therapy is an effective treatment for VVA symptoms and has a positive effect on the quality of women's sexual life.
Authors’ contributions: Ramazanova M.O. – study design, collection of clinical material, text writing and editing;
Apolikhina I.A. – study design, collection of clinical material, text editing; Shatilova K.V. – participation in the study planning, data analysis, and text editing; Khlopkov A.D. — data collection and analysis; Sencha L.M. — statistical analysis and text editing.
Conflicts of interests: Melsytech LLC was the sponsor of this study. Shatilova K.V., Khlopkov A.D., and Sencha L.M. are employees of Melsytech LLC.
Funding: Melsytech LLC was the sponsor of this study.
Ethical Approval: The study protocol was approved by the Ethics Committee for Biomedical Research at the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request after approval from the principal investigator.
For citation: Ramazanova M.O., Apolikhina I.A., Shatilova K.V., Khlopkov A.D., Sencha L.M. A prospective randomized controlled study on the effectiveness of neodymium laser therapy for vulvovaginal atrophy in postmenopausal women.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (11): 102-118 (in Russian)
https://dx.doi.org/10.18565/aig.2025.149
Keywords
Vulvovaginal atrophy (VVA) is a chronic, progressive disorder featuring a set of symptoms resulting from a natural decline in estrogen levels [1]. Given the high prevalence rate of VVA and the expected development of this condition due to the increasing average age of the female population, its significant social impact is quite clear [2].
Before the onset of menopause, given the normal levels of circulating endogenous estrogens, the vaginal mucosa demonstrates a thickened, well-perfused, non-keratinized epithelial layer. Estrogen deficiency during menopause leads to a decrease and loss of elasticity of the vaginal wall, along with lowering discharge and thinning of the vaginal epithelium [3]. The epithelial surface becomes pale and loose; petechiae, irritation, and bleeding appear, which can occur after minimal trauma [1, 4, 5]. VVA often causes a decrease in the diameter and elasticity of the vaginal opening, thinning of the vaginal tissues and loss of natural lubrication, which results in dryness, itching, burning, dyspareunia and sexual dysfunction. This condition requires long-term treatment, upon discontinuation of which the symptoms usually return [2].
According to a number of researchers, no more than 25% of women receive adequate therapy for VVA even in developed countries [6].
In mild VVA symptoms the treatment of choice are such conservative methods as lifestyle change, local application of non-hormonal lubricants and moisturizers [7].
In severe cases of VVA the “gold therapy standard” is considered to be estrogen-based hormonal therapy (systemic, local or combined) [8].
According to the latest guidelines from the North American Menopause Society, local low-dose estrogen therapy is recommended for women with VVA symptoms who do not have indications for systemic hormone therapy [5].
Nowadays we know that VVA has a chronic course with a trend towards symptoms progression when no maintenance therapy is used [1]. The European Menopause and Andropause Society (EMAS) guidelines state that VVA symptoms recur within 1–3 months after discontinuation of local estrogen therapy, and local estrogen use for the treatment of postmenopausal VVA should be continued until the patient experiences a positive effect [9].
Estrogen therapy has limitations among patients who have undergone breast cancer treatment, who very often experience symptoms of iatrogenic VVA. Furthermore, many women are often concerned about the consequences associated with the risk of cancer while undergoing hormone therapy.
Therefore, we need to select alternative hormonal treatment methods that can provide long-term relief of VVA symptoms.
Instrumental methods have proven their effectiveness in treating patients who cannot receive hormonal therapy, those suffering from or with a history of gynecological cancers [10]. One of the advantages of the latest minimally invasive energy-based techniques is that they may become a high-potential solution for treating VVA symptoms, having a good effect on the processes of neoangiogenesis and neocollagenesis.
Currently, there are the following types of medical lasers with different wavelengths and application points: gas (carbon dioxide laser; copper vapor laser), liquid (fluorescent medium – rhodamine), semiconductor (diode laser), solid-state (Er:YAG, ruby crystals represented by aluminum oxide doped with chromium; neodymium Nd:YAG laser).
Neodymium lasers are used in cosmetology, surgery, dentistry, urology, and now in gynecology. Russia became the first country in the world where the neodymium laser was applicated to treat diseases of the lower urogenital tract. The neodymium laser belongs to a group of solid-state medical lasers that generate optical radiation due to quantum transitions between the energy levels of the trivalent neodymium ion Nd3+ placed in a matrix [11]. Radiation generation occurs at a wavelength of 1064 nm. The main chromophores for a wavelength of 1064 nm are oxy- and deoxyhemoglobin of the microcirculation, as well as protein structures of the vaginal wall (elastin and collagen) [12, 13]. The probable mechanism of action of the neodymium laser is associated with the photothermal effect, which results in heat accumulation and thermal diffusion in tissues, that activate the expression of heat shock proteins [14]. The total changes in tissues caused by the influence of spreading heat are commonly referred to as photothermal reconstruction [15].
Laser exposure results in aseptic inflammation, which results in stimulation of neocollagenesis and neoangiogenesis [14, 16]. Secondary effects are connected with the partial destruction of the microcirculatory bed in the tissues, which leads to the opening of reserve capillaries and a significant increase in microcirculation of the vaginal wall. As a result of the above-described effects, reconstruction of the vaginal walls and reorganization of the connective tissue occur, during which a dense network of thickened collagen bundles is formed [15], and blood supply in the genital area improves, which leads to the elimination of VVA events.
Relevant studies confirm the effectiveness and safety of laser tachnique to relieve symptoms of VVA [17, 18]. However, there is a need for further studies on different patient populations, and also for a comparative analysis of laser treatment and other types of therapy.
To sum up, the aim of our study is to conduct a comparative assessment of the efficacy and safety of VVA treatment methods, including local estrogen therapy, the use of a non-ablative neodymium laser and their combination.
Materials and methods
A single-center prospective randomized clinical trial was conducted at the Department of Aesthetic Gynecology and Rehabilitation of the V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. The study was registered in the international ClinicalTrials.gov database, ID: NCT04735549. The study protocol was approved by the Biomedical Research Ethics Committee. During the period of 2021–2023, 120 women aged 50–85 with VVA were examined and treated according to the inclusion and exclusion criteria (Table 1).

The patients were randomly assigned to three groups: Group 1 included 40 patients who received non-ablative neodymium laser treatment three times with an interval of 30–40 days between sessions (laser treatment group); Group 2 included 40 patients who applied 1% vaginal cream with estriol for 9 months (0.5 g daily for 2 weeks, then 0.5 g twice a week; hormonal treatment group); Group 3 included 40 patients who underwent laser treatment according to the same regimen as Group 1, plus vaginal cream with estriol according to the same regimen as Group 2 (combination treatment group). Randomization was performed as follows: upon patient inclusion in the study, a random number from 1 to 3 was generated (https:// рандомайзер.рф). The patient was assigned to a treatment group based on this number, where 1 stands for laser treatment, 2 means hormonal treatment, and 3 – for combination treatment.
Blinding of patients and the investigator was not possible due to the method of administration of the investigational medical device and study design.
The laser treatment parameters for each patient were individual and depended on the thickness of the vaginal and vulvar mucosa, the state of the microcirculatory bed, etc. To assess the adequacy of the procedure we analyzed the patient’s ability for heat tolerance.
To perform the procedures, we used a neodymium laser Magic Gyno (OOO Melsytech, Russia).
The following instruments were used for the laser therapy: a scanner – for the proportional energy distribution onto the vaginal mucosa, a vaginal dilator (retractor), a handle with a conical mirror, and a handle with an angular mirror (Fig. 1).

The laser treatment consisted of three stages.
In the first stage we performed circular processing of the vaginal walls using a conical mirror nozzle (Fig. 1b). The conical mirror nozzle distributes energy across a 360° field. The treatment width is 10 mm, and the circumference is 9 cm. The treated mucosal area reaches 9 cm². The nozzle can be adjusted to 20 treatment positions with 5 mm pace.
The first stage of laser exposure was carried out with the following parameters: 14–18 W, 3–4 cycles in the upper third; 12–18 W, 3 cycles in the middle third; 11–16 W, 2 cycles in the lower third of the vagina, the duration of one cycle was 0.8–1.4 s. Also laser exposure was performed over the entire depth of the vagina for 1–3 times.
To ensure proportionate treatment of the vaginal walls, it is necessary to change the position of the retractor (as part of the wall is covered by the dilator).
In the second stage the vaginal walls were treated using a nozzle with an "angle mirror" (Fig. 1a), which enables to treat a circular segment forming 30° angle, with a treatment area of 0.8 cm².
At this stage targeted step-by-step treatment of the problem area was performed: a segment of the anterior, posterior, or lateral vaginal walls equal to 30% of the circumference, corresponding to 1 o'clock position. This stage is especially important for patients with urinary incontinence and pelvic organ prolapse. The therapy is carried out at 12, 3, 6, and 9 o'clock positions. In the presence of a severe degree of prolapse, additional treatment along lines 1, 4, 8, and 11 is possible. In this study, we performed treatment only at 12 and 6 o'clock positions.
Also the following parameters were used at the second stage: power 12–16 W, 2-3 cycles in the upper third; 11–14 W, 2–3 cycles in the middle third, 11–12 W, 2–3 cycles in the lower third of the vagina, the duration of one cycle is 0.8–1.2 s. When using the angle mirror nozzle no more than 1 laser pass was carried out on one wall of the vagina.
The third stage consisted of the vulva, vaginal vestibule and periurethral area processing using a Zoom-nozzle (Fig. 2) with an adjustable spot size of 1–6 mm.

Laser exposure was carried out with the following parameters: spot diameter – 6 mm, power – 15–18 W, pulse duration – 100 ms, pause between pulses – 50 ms, exposure time on an area of 1 cm² – 2–3 s (until the patient feels pronounced warmth), with 2–6 laser passes.
All three stages of laser treatment were painless and required no anesthesia. Patients felt comfortable warmth during the procedure.
Before the procedures all patients signed informed consent and completed questionnaires: Female Sexual Function Index (FSFI), Vulvovaginal Symptom Questionnaire (VSQ), and Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ12). A vaginal health index (VHI) assessment as well as a visual analog scale (VAS) score were carried out before treatment and 1, 3, and 9 months after the start of treatment.
The primary endpoint of the study was the change in the VHI score relative to the initial state, and the secondary endpoints described the change in the scores of the validated questionnaires. To assess the degree of atrophic changes in the vagina, we used the VHI score (Bachmann G., 1994), which allows to assess the intensity of manifestations of VVA and includes the following indicators: the volume and quality of vaginal discharge, the pH level of vaginal fluid and the condition of the vaginal epithelium – its moisture, elasticity, thinness and, accordingly, susceptibility to trauma. Each parameter was assessed on a 5-point scale, with 1 point referring to “the worst condition” and 5 points meaning “the best condition”. The scores were then summed. The maximum score accounts for 25 points. A vaginal epithelial condition of 20 points or less corresponds to VVA. A statistically significant increase in VHI scores after treatment relative to baseline was used as the criterion for treatment effectiveness.
To assess sexual life quality, we used the FSFI questionnaire which consists of 19 questions divided into six categories: desire, arousal, lubrication, orgasm, satisfaction, and pain [19]. Each parameter is measured on a 5-point scale. The overall score represents the sum of the scores for each area. The maximum score accounts for 36 points (high sexual function), and the minimum is 2 points (low sexual function).
The VSQ questionnaire was developed in 2013 by Erekson E.A. et al. at Yale University, USA [20]. It has got 21 questions assessing vulvovaginal symptoms (itching, pain, burning, dryness), the impact of these symptoms on a woman's emotional state, quality of life, and sexual relations. The maximum score on the VSQ is 20, which corresponds to a pronounced atrophic process that has a direct impact on the patient's quality of life.
The PISQ-12 questionnaire assesses sexual function in women with pelvic floor dysfunction. The questionnaire includes 12 questions, divided into three areas: behavioral-emotional (items 1–4), physical (items 5–9), and partner-related (items 10–12). Responses are rated on a 5-point Likert scale ranging from 0 (always) to 4 (never). Up to two missing answers are allowed. The total score is calculated by multiplying the number of items by the mean of the item responses. The maximum score is 48. Higher scores indicate better sexual function, and vice versa [21].
The Visual Analogue Scale (VAS) was proposed by Huskisson in 1974. The VAS is a 10 cm long straight line segment. Its beginning corresponds to the absence of pain – “no pain”, and the end point reflects excruciating “unbearable pain”. The patient puts a mark on the line corresponding to the intensity of current pain degree. The distance between the beginning of the line (“no pain”) and the mark is measured in cm and rounded up to the nearest whole number. Each centimeter on the line corresponds to 1 point. A mark of up to 2 cm is classified as mild, 2 to 4 cm – as moderate, 4 to 6 cm – as severe, 6 to 8 cm – as severe, and a line up to 10 cm means unbearable pain.
The results obtained from the questionnaires were also taken into account when assessing treatment effectiveness. In this case the effectiveness criteria comprised a statistically significant increase in FSFI scores, an increase in PISQ-12 scores, a decrease in VSQ scores, and a decrease in VAS scores after treatment relative to baseline.
Statistical analysis
In this prospective randomized study the following null (H₀) and alternative (H₁) hypotheses were formulated:
- null hypothesis (H₀): there are no statistically significant differences in the dynamics of key efficacy indicators (VHI, FSFI, VAS, VSQ questionnaire, PISQ-12 questionnaire) between the laser treatment, hormonal treatment and combination treatment groups 3 and 9 months after the start of therapy;
- alternative hypothesis (H₁): there is a statistically significant difference in the dynamics of key efficacy indicators between at least two treatment groups, with the laser and combination treatment groups expected to outperform the hormonal therapy group.
Prior to conducting statistical analysis, we assessed the distribution of quantitative data using the D'Agostino–Pearson test. Since the distribution in most samples deviated significantly from normal, nonparametric methods were taken for subsequent analysis. Data are presented as medians and quartiles – Me (Q1; Q3).
Comparison of baseline characteristics between the three groups (age, menopause duration, questionnaire scores, etc.) at the stage of inclusion into the study, as well as comparison of groups at each time point, was performed using the Kruskal–Wallis test. If statistically significant differences (p<0.05) were detected, Dunn's post-hoc test with correction for multiple comparisons was used for pairwise comparison of the groups.
Within-group changes in the quantitative indicator over time were assessed using the Friedman test. If statistically significant differences (p<0.05) were detected, further pairwise analysis between time points was performed using Dunn's post-hoc test with correction for multiple comparisons. Statistical significance was set at p<0.05.
The primary analysis of treatment efficacy included comparisons of absolute changes from baseline (Δ) between groups at each time point. Comparisons of Δ values between the three groups were performed using the Kruskal–Wallis test followed by Dunn's post-hoc test with correction for multiple comparisons. Differences were considered statistically significant at p<0.05.
To describe the magnitude of the clinical effect, we calculated the median difference (Δ) between groups with 95% confidence intervals (CI). CI was analyzed using a nonparametric bootstrap method involving 5,000 random samples with replacement from the original data. CI limits were set as the 2.5th and 97.5th percentiles of the bootstrap scores distribution.
Statistical analysis was performed using Excel 2021 (Microsoft Corp., USA), Prism 10 (GraphPad Software, USA), and the scipy (version 1.16.2) and scikit-posthocs (version 0.11.4) libraries for Python (version 3.8.19).
Results
113/120 patients, included in the study, completed treatment according to the study schedule and participated in follow-up at 1, 2, 3, and 9 months after the start of therapy. Results for patients who did not complete the full course of laser treatments in the combination treatment group (4 patients excluded from the study) were not included in the statistical evaluation: an intention-to-treat analysis was not performed due to the small sample size. Three patients from the combination treatment group who completed the course of therapy and participated in the follow-up visits at 1, 2, and 3 months after its start, but did not complete the 9-month follow-up visit, were included in the analysis. It should be noted that patients who were not sexually active were excluded from the FSFI and PISQ-12 assessment. The CONSORT flowchart is shown in Figure 3.

The characteristics of the groups before the start of therapy is presented in Table 2.
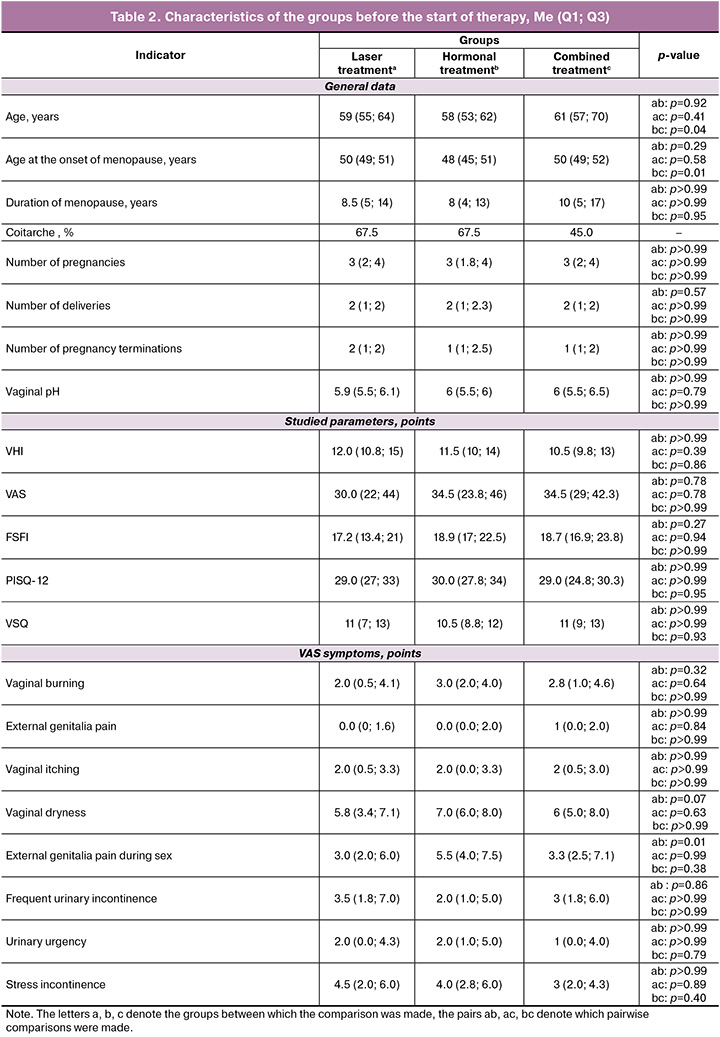
Analysis of baseline demographic and clinical characteristics of patients in all three treatment groups demonstrated comparability across most significant parameters. Although statistical analysis revealed some differences in age and menopause onset age between the groups, the range of these differences (not exceeding 3 years) does not appear to be clinically significant in the context of the studied pathology. The most important indicators for assessing VVA severity (baseline values of the VHI, VAS, and quality-of-life questionnaires such as FSFI, PISQ-12, VSQ) did not show statistically significant differences between the groups. We observed a similar pattern while analyzing individual symptoms, where only one indicator showed a statistical difference, with no clinically significant difference in the medians. Thus, we conclude that the groups were representative and comparable for further comparative analysis of the effectiveness of different treatment methods, and minimal differences in baseline characteristics do not have a significant impact on the interpretation of the results obtained.
Before the start of treatment, the VHI value did not differ significantly and accounted for 12 (10.8; 15) points in the laser treatment group, 11.5 (10; 14) points in the hormonal treatment group, and 10.5 (9.8; 13) points in the combination treatment group. Statistically significant changes in the VHI value were observed in all groups, as we compare the scores at 2 months after the onset of therapy with the scores at the start of treatment. Statistically significant changes were also observed in all groups after 3 and 9 months from the start of the therapy (Table 3).

9 months after the start of treatment the VHI index increased, if compared to the time of the onset of treatment, by 9 (8; 11) points (p<0.001) in the laser treatment group, by 7 (5; 8) points (p<0.001) in the hormonal treatment group, and by 11 (9; 12) points (p<0.001) in the combined treatment group. Also, the difference in VHI score change (Δ) between the combined and hormonal treatment groups was 4 points (95% CI: 2.5; 5.0; p<0.001). Compared with the laser treatment group, the VHI value in the hormonal treatment group was also significantly lower, as the difference in VHI score change (Δ) accounted for 2 points (95% CI: 1.0; 4.0; p<0.001) (Table 4).
The statistically significant differences in the VVA score change have important clinical value. Thus, the 4-point difference between the combined and hormonal treatment groups may directly refer to a higher quality of life for patients. The 2-point difference by which the laser treatment group outperformed the hormonal treatment group may be associated with better control of certain VVA manifestations. Therefore, the showcased superiority of the new methods is not only statistically justified but also a compelling argument when looking for patient management strategies.
The statistically significant improvement in the urinary tract infection rate indicates a decrease in the intensity of urogenital disorders, thickening of the stratified squamous epithelium, and an increase in its elasticity and hydration (Fig. 4).

The analysis of patients' assessment of the quality of their sexual life using the FSFI questionnaire also revealed a number of important trends. The FSFI questionnaire scores did not statistically differ prior to the onset of treatment, and accounted for 17.2 (13.4; 21) points in the laser treatment group, 18.9 (17; 22.5) points in the hormonal treatment group, and 18.7 (16.9; 23.8) points in the combination treatment group. Statistically significant changes in this indicator were observed in the laser and hormonal treatment groups 2 months after the start of therapy (p<0.001) and in the combination treatment group 3 months after the start of therapy compared with baseline values (p<0.001).
At 3 and 9 months after the onset of treatment, we noted statistically significant changes in all studied groups (Table 5). 9 months later the ΔFSFI value increased comparing to the onset of treatment by 10.7 (8.4; 16.6) points (p<0.001) in the laser treatment group; by 4.3 (2.4; 4.8) points (p<0.001) in the hormonal treatment group; and by 8.3 (6.3; 10.6) points (p<0.001) in the combined treatment group. After 9 months of therapy the ΔFSFI index in the hormonal treatment group was significantly lower than in the combined (p=0.001) and laser treatment (p<0.001) groups (Table 6). The difference in ΔFSFI between the hormonal and combination treatment groups was 4.0 points (95% CI: 1.9; 7.2; p=0.001). The difference in ΔFSFI was 6.5 points in favor of laser treatment (95% CI: 4.6; 9.1; p<0.001), compared with the hormonal treatment group, which was also statistically significantly lower. At the same time, there was no statistically significant difference between the laser and combination treatment groups (Δ=-2.5 points, 95% CI: -6.2; 1.2; p>0.99).
The observed intergroup changes in the FSFI score are not only statistically significant but also clinically valuable (Figure 5). The difference in scores outgrowth accounted for 4.0 and 6.5 points in combined and laser treatment compared to hormonal therapy is paramount in the context of the overall questionnaire scale. This allows us to conclude that both laser and combined treatments provide a qualitatively higher and clinically significant improvement in sexual function parameters in patients with VVA compared to standard hormonal therapy.

The analysis of patients' assessment of VVA symptoms using the VSQ questionnaire also revealed significant trends. Given the period prior to the start of treatment, the VSQ questionnaire results did not statistically differ and were 11 (7; 13) points in the laser treatment group, 10.5 (8.8; 12) points in the hormonal treatment group, and 11 (9; 13) points in the combination treatment group (Table 7). Statistically significant changes in this indicator were noted in all treatment groups 2 months after the start of therapy compared with baseline values.
At 3 and 9 months after the onset of therapy, we identified statistically significant changes in all groups (Table 7). 9 months later the ΔVSQ index decreased compared to the start of treatment by 7.5 (4; 9.3) points (p<0.001) in the laser treatment group, by 4 (2; 5) points (p<0.001) in the hormonal treatment group, and by 9 (6; 10) points (p<0.001) in the combination treatment group (Table 8). 9 months after the start of therapy the VSQ score in the hormonal treatment group was statistically higher (i.e., the decrease was less marked) than in the combined (p<0.001) and laser treatment (p<0.001) groups. The difference in ΔVSQ change between the hormonal and combined treatment groups comprised 5.0 points (95% CI: 3.0; 6.0; p<0.001). Compared with the laser treatment group, the score in the hormonal treatment group was also statistically significantly higher as the difference in ΔVSQ change accounted for 3.5 points (95% CI: 2.0; 5.5; p<0.001). However, no statistically significant difference was found between the laser and combined treatment groups (Δ=1.5 points, 95% CI: -1.5; 3.0; p=0.55) (Table 8).
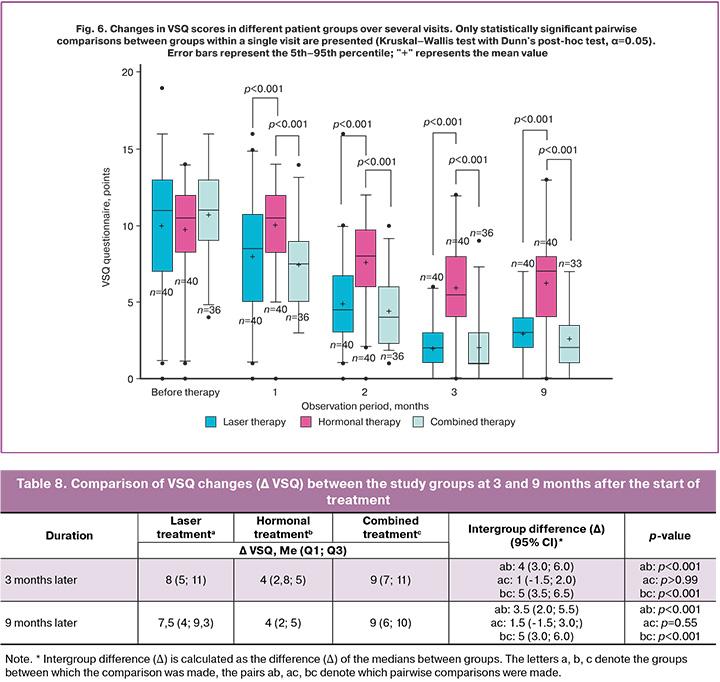
The statistically significant changes in VSQ scores at 3 and 9 months after the start of treatment, compared with baseline data, confirm that neodymium laser therapy significantly improves vaginal condition and reduces the symptoms of vaginal atrophy in postmenopausal women (Figure 6). The greatest symptomatic regression was observed in the combination and laser treatment groups, indicating the greater effectiveness of these methods compared to hormonal monotherapy.
Particular interest for the study was aimed at the VAS questionnaire, as it assesses symptoms that reduce quality of life. It is easier for patients to evaluate the elimination of these symptoms, than to find improvements in other parameters. Furthermore, the use of a visual scale makes it more comfortable to answer the questions. The following results were obtained. Before the start of the therapy, the results of the VAS questionnaire did not statistically differ and referred to 30 (22; 44) points in the laser treatment group, 34.5 (23.8; 46) points in the hormonal treatment group and 34.5 (29; 42.3) points in the combination treatment group. Statistically significant improvements in the indicator were observed in all groups 2 months after the start of therapy compared with the baseline data.
After 3 and 9 months from the start of treatment, statistically significant changes were demonstrated in all groups (Table 9). 9 months later the VAS score in the laser treatment group decreased by 20.5 (14.8; 29.5) points, p<0.001, and by 25 (21; 36) points in the combination treatment group, p<0.001, as well as by 10.5 (8; 15) points in the hormonal treatment group, p<0.001. At 9 months from the start of therapy, the VAS score in the hormonal treatment group was statistically higher (i.e., the decrease was less marked) than in the laser group. The difference in the ΔVAS between these groups was 10 points (95% CI: 6.0; 16.0; p<0.001). In comparison with the combination treatment group, the indicator in the hormonal treatment group was also higher – the difference in the change in ΔVAS was 14.5 points (95% CI: 11.0; 21.5; p<0.001). At the same time, we detected no statistically significant difference between the laser and combination treatment groups (Δ=4.5 points, 95% CI: -1.0; 12.0; p=0.35) (Table 10).
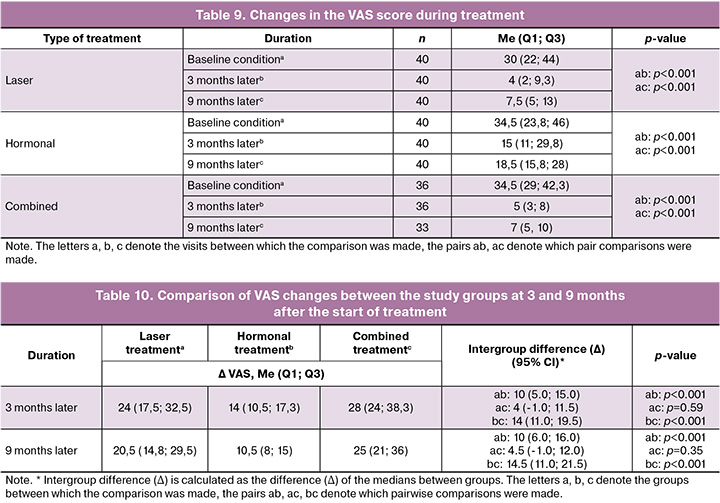
The analysis of the VAS score changes demonstrates not only statistical significance but also the pronounced clinical value of the obtained results. The difference in VAS score reduction between the hormonal treatment group and the laser treatment group comprised 10 points, while between the hormonal treatment group and the combination treatment group it was 14.5 points (Fig. 7). This range of difference is paramount for clinical practice and indicates a significantly more pronounced symptom reduction with laser treatments. It is noteworthy that no statistically significant differences for the therapy effectiveness were found between the laser and combination treatment groups, confirming the high therapeutic value of laser therapy as a standalone treatment for VVA.
More obvious trend was observed in such units of VAS questionnaire as vaginal pain, burning, and itching. The absence of symptoms before treatment and at follow-up visits was assessed as ≤1, and complete absence of symptoms was referred to as ≤1 in all three items simultaneously.
Three symptoms were completely absent in 8/40 (20%) patients in the laser treatment group, 5/40 (13%) patients in the hormonal treatment group, and 5/33 (15%) patients in the combination treatment group before the start of the therapy. Moreover, 3 and 9 months after the start of therapy, 34/40 (85%) patients in the laser treatment group and 32/33 (97%) and 31/33 (94%) patients in the combination treatment group reported no pain, itching, or burning in the vagina, compared with 23/40 (58%) and 18/40 (45%) in the traditional hormonal treatment group. Therefore, 3 and 9 months after the onset of therapy, there was complete elimination of symptoms in 26/40 (65%) patients in the laser treatment group, 27/33 (82%) and 26/33 (79%) patients in the combination treatment group, compared with 18/40 (45%) and 13/40 (33%) in the hormonal treatment group.
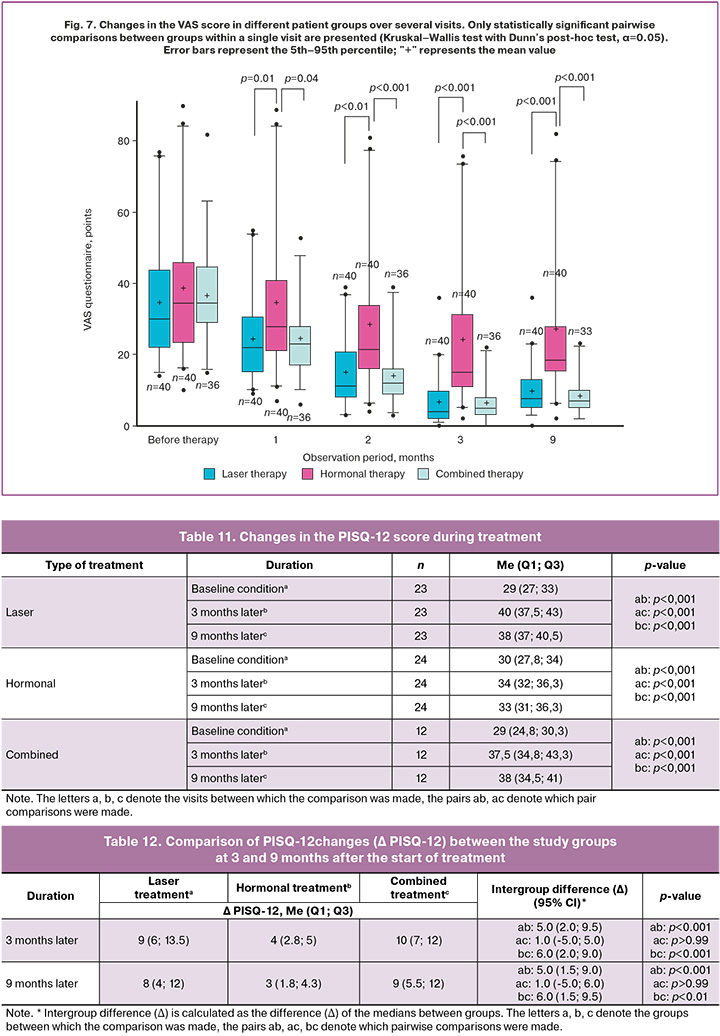
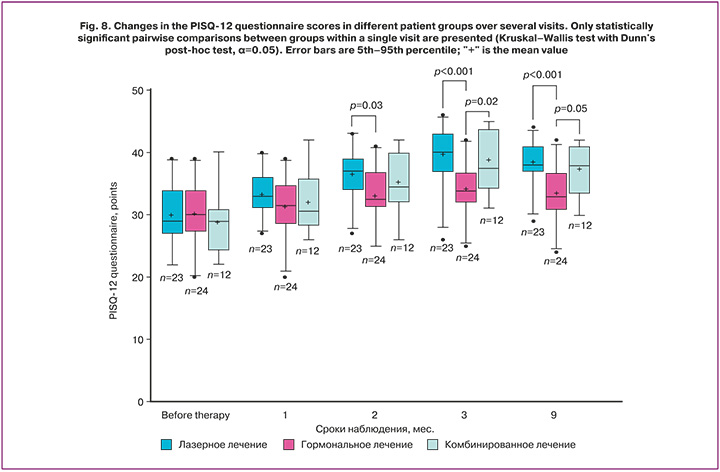
The results of the PISQ-12 questionnaire also showed statistically significant differences between the groups after therapy, which we did not observe before treatment.
Prior to the start of treatment, the results of the PISQ-12 questionnaire did not differ significantly and accounted for 29 (27; 23) points in the laser treatment group, 30 (27.8; 34) points in the hormonal treatment group, and 29 (24.8; 30.3) points in the combination treatment group. Statistically significant changes for this questionnaire were observed in all study groups 2 months after the start of therapy compared with the baseline data.
After 3 and 9 months from the start of treatment, statistically significant changes were observed in all study groups (Table 11). 9 months later we noted 8 (4; 12) points (p<0.001) and 9 (5.5; 12) growth in the laser and combination treatment groups (p<0.99), respectively (Table 12).
The analysis of the changes in the PISQ12 questionnaire scores confirmed the presence of not only statistically significant but also clinically important differences between the groups (Fig. 8). We detected marked difference in score outgrowth of 5.0 and 6.0 points in favor of the laser and combination treatment groups compared to the hormonal treatment group. This points to a more pronounced improvement in various aspects of sexual function in patients treated with laser technologies. The lack of significant difference between the laser and combination treatment groups makes laser monotherapy a highly effective and clinically valuable alternative for the treatment of sexual dysfunction associated with VVA.
No serious treatment-related adverse events were observed in all study groups. In Groups 1 and 3 neodymium laser treatments were well tolerated by patients; no side effects or adverse events were reported during or after treatment.
Accordungly, statistically significant differences were recorded between pre-treatment and follow-up visits, as well as between groups at follow-up visits. It was shown that in the laser and combination treatment groups, when compared with the hormonal treatment group, the mean PISQ-12 score significantly increased at 3 and 9 months after the start of therapy, and the severity of VVA symptoms on the VAS scale decreased (up to complete elimination). According to the VHI data, statistically significant difference was noted 9 months after the onset of therapy in the laser and combination treatment groups in comparison to the hormonal treatment group. When assessing the FSFI questionnaire scores, statistically significant increase in scores was observed in the laser treatment groups (an increase of 10.7 (8.4; 16.6) points, p<0.001) and combined treatment (an increase of 8.3 (6.3; 10.6) points, p<0.001), while in the hormonal treatment group it was only 4.3 (2.4; 4.8) points (p<0.001) 9 months after the start of therapy. Moreover, the greatest change in the score evaluating the quality of sexual life was observed in the laser treatment group without using the hormonal therapy.
Discussion
VVA symptoms progress rapidly with advancing age, having a strong impact on women's quality of life. The primary goal of treatment is to restore the normal physiological condition of the vulvar and vaginal mucosa and relieve VVA symptoms.
The VVA symptoms develop as a result of a decrease in estrogen levels during menopause. At histopathological examination these processes manifest as structural changes in the vaginal and vulvar walls (thinning and thinning of the epithelium), and at physical examination patients usually report dryness, increased vaginal discharge pH, dyspareunia, irritation, itching, and burning in the vulva and vagina, tissue pallor, and loss of elasticity. VVA symptoms can be alleviated by local increase in estrogen levels or by enhancing the blood flow to the vulvovaginal area.
It is well known that local estrogen therapy in the form of estradiol, estriol, or conjugated estrogens restores pH, increases epithelial thickness, and increases vaginal lubrication [5]. Local estrogens have also been shown to induce collagen synthesis and reorganization in both animal models and clinical practice [22, 23]. However, it is known that topical estrogen therapy does not have a significant effect on the angiogenesis process. In our study, we used estriol to compare it with laser therapy. Estriol (E3) is a lower potency estrogen that reduces the severity of VVA symptoms [24]. Local estrogens act primarily on the surface of the vaginal mucosa, and the improvement lasts only as long as therapy is administered [25].
The first results of the use of laser therapy for the treatment of VVA symptoms were described in one of the studies by Gaspar A. et al. [26]. The authors reported a beneficial effect of carbon dioxide laser on the vaginal mucosa and sexual function.
Subsequent studies have demonstrated the effectiveness of laser photothermolysis for the treatment of VVA symptoms. In 2015, Zerbinati N. et al. confirmed morphological changes in the vaginal mucosa after a course of CO2 laser treatment, detecting an increase in glycogen content in the vaginal epithelium [27]. A similar result was obtained by Salvatore S. et al., who described a significant improvement in sexual health and quality of life in women suffering from VVA during a 12-week follow-up [28].
The major defining feature between the CO2 laser and the neodymium laser used in this study is the ablative nature of the CO2 laser's radiation, which works by vaporizing tissue areas. Vaporization of the tissue on the epithelial surface is necessary to expose the deeper underlying connective tissue, which is more saturated with water, to the thermal action of the laser pulse in order to achieve the desired photobiomodulatory effect [27].
Di Donato V. et al. conducted a prospective observational study on the safety of laser treatment. The researchers came to a conclusion that fractional CO2 laser is a safe treatment method for menopausal women, and no serious complications were observed in 53 studied women [29].
The use of a non-ablative neodymium laser is expected to improve blood flow in vaginal and vulvar tissues. The 1064 nm wavelength allows laser energy to penetrate to a depth of several millimeters, affecting the entire thickness of the vaginal wall, which is deeper than fractional erbium and CO2 lasers. Therefore, it becomes possible to target deeper layers up to the muscular layer, while the use of low-energy nanosecond pulses eliminates the possibility of damaging the vaginal mucosa. Consequently, the risk of infection, necrosis, scarring, and other side effects is minimized compared to ablative lasers. Exposure to Nd:YAG laser improves the condition of vaginal and vulvar tissues due to the photothermal effect, as well as stimulation of neoangiogenesis, neocollagenesis, and regenerative processes in vaginal and vulvar tissues, which is confirmed by research data and the results of our study [18, 30].
Conclusion
Thus, the results of this study demonstrate clinical efficacy of using a neodymium laser to treat VVA symptoms, as well as a significant improvement in women's quality of life. Neodymium laser is believed to be an innovative and promising treatment method in gynecological practice. We are currently continuing to follow-up this group of patients.
References
- Salvatore S., Ruffolo A.F., Phillips C., Athanasiou S., Cardozo L., Serati M. Vaginal laser therapy for GSM/VVA: where we stand now - a review by the EUGA Working group on laser. Climacteric. 2023; 26(4): 336-52. https://dx.doi.org/10.1080/13697137.2023.2225766
- Benini V., Ruffolo A.F., Casiraghi A., Degliuomini R.S., Frigerio M., Braga A. et al. New innovations for the treatment of vulvovaginal atrophy: an up-to-date review. Medicina (Kaunas). 2022; 58(6): 770. https://dx.doi.org/10.3390/medicina58060770
- Тихомирова Е.В., Балан В.Е., Фомина-Нилова О.С. Методы лечения генитоуринарного синдрома на современном этапе. Медицинский cовет. 2020; (13): 91-96. [Tikhomirova E.V., Balan V.E., Fomina-Nilova O.S. Current treatment options for genitourinary syndrome. Medical Council. 2020; (13): 91-6 (in Russian)]. https://dx.doi.org/10.21518/2079-701X-2020-13-91-96
- Gaspar A., Brandi H., Gomez V., Luque D. Efficacy of Erbium:YAG laser treatment compared to topical estriol treatment for symptoms of genitourinary syndrome of menopause. Lasers Surg. Med. 2017; 49(2): 160-8. https://dx.doi.org/10.1002/lsm.22569
- “The 2022 Hormone Therapy Position Statement of The North American Menopause Society” Advisory Panel. The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022; 29(7): 767-94. doi: 10.1097/GME.0000000000002028.
- Turcan N., Grădinaru-Fometescu D., Baros A., Coravu V., Turcan G., Cirstoiu M.M. Vulvovaginal atrophy – the impact on the quality of life and self-regard. Review of literature. Rom. J. Military Med. 2022; 125 (1): 50-5.
- Angelou K., Grigoriadis T., Diakosavvas M., Zacharakis D., Athanasiou S. The genitourinary syndrome of menopause: an overview of the recent data. Cureus. 2020; 12(4): e7586. https://dx.doi.org/10.7759/cureus.7586
- Briggs P. Genitourinary syndrome of menopause. Post Reprod. Health. 2020: 26(2): 111-4. https://dx.doi.org/10.1177/2053369119884144
- Hirschberg A.L., Bitzer J., Cano A., Ceausu I., Chedraui P., Durmusoglu F. et al. Topical estrogens and non-hormonal preparations for postmenopausal vulvovaginal atrophy: an EMAS clinical guide. Maturitas. 2021; 148: 55-61. https://dx.doi.org/10.1016/j.maturitas.2021.04.005
- D'Oria O., Giannini A., Buzzaccarini G., Tinelli A., Corrado G., Frega A. et al. Fractional Co2 laser for vulvo-vaginal atrophy in gynecologic cancer patients: a valid therapeutic choice? A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022; 277: 84-89. https://dx.doi.org/10.1016/j.ejogrb.2022.08.012
- Звелто О. Принципы лазеров. 4-е изд. СПб; 2008. 720 с. [Svelto O. Principles of lasers. 4th ed. St. Petersburg; 2008. 720 p. (in Russian)].
- Jacques S.L. Optical properties of biological tissues: a review. Phys. Med. Biol. 2013; 58(11): R37-61. https://dx.doi.org/10.1088/0031-9155/58/11/R37
- Sekar S.K.V., Beh J.S., Farina A., Dalla Mora A., Pifferi A., Taroni P. Broadband diffuse optical characterization of elastin for biomedical applications. Biophys. Chem. 2017; 229: 130-4. https://dx.doi.org/10.1016/j.bpc.2017.07.004
- Sajjadi A.Y., Mitra K., Grace M. Expression of heat shock proteins 70 and 47 in tissues following short-pulse laser irradiation: assessment of thermal damage and healing. Med. Eng. Phys. 2013; 35(10): 1406-14. https://dx.doi.org/10.1016/j.medengphy.2013.03.011
- Potapov A., Moiseev A., Kiseleva E., Krupinova D., Shatilova K., Karabut M. et al. Depth-resolved attenuation mapping of the vaginal wall under prolapse and after laser treatment using cross-polarization optical coherence tomography: a pilot study. Diagnostics. 2023; 13(22): 3487. https://dx.doi.org/10.3390/diagnostics13223487
- Gambacciani M., Palacios S. Laser therapy for the restoration of vaginal function. Maturitas. 2017; 99: 10-5. https://dx.doi.org/10.1016/j.maturitas.2017.01.012
- Рамазанова М.О., Аполихина И.А., Шершакова Е.И. Современные проблемы и возможности в лечении генитоуринарного менопаузального синдрома на этапе развития медицинской науки и технологии. Акушерство и гинекология. 2024; 5: 24-31. [Ramazanova М.О., Apolikhina I.A., Shershakova E.I. Modern problems and opportunities in the treatment of genitourinary syndrome of menopause in the context of medical science and technology development. Obstetrics and Gynecology. 2024; (5): 24-31 (in Russian)]. https://dx.doi.org/10.18565/aig.2024.77
- Gubarkova E., Potapov A., Krupinova D., Shatilova K., Karabut M., Khlopkov A. et al. Compression optical coherence elastography for assessing elasticity of the vaginal wall under prolapse after neodymium laser treatment. Photonics. 2023; 10(1): 6. https://dx.doi.org/10.3390/photonics10010006
- Meston C.M., Freihart B.K., Handy A.B., Kilimnik C.D., Rosen R.C. Scoring and interpretation of the FSFI: what can be learned from 20 years of use? J. Sex. Med. 2020; 17(1): 17-25. https://dx.doi.org/10.1016/j.jsxm.2019.10.007
- Аполихина И.А., Казакова С.Н., Яроцкая Е.Л., Горбунова Е.А., Бычкова А.Е. Языковая, культурная адаптация и валидация опросника вульвовагинальных симптомов среди пациенток с вульвовагинальной атрофией. Акушерство и гинекология. 2023; 10: 177-82. [Apolikhina I.A., Kazakova S.N., Yarotskaya E.L., Gorbunova E.A., Bychkova A.E. Linguistic and cultural adaptation and validation of the questionnaire on vulvovaginal symptoms among the patients with vulvovaginal atrophy. Obstetrics and Gynecology. 2023; (10): 177-82 (in Russian)]. https://dx.doi.org/10.18565/aig.2023.228
- Шкарупа Д.Д., Кубин Н.Д., Пешков Н.О., Комяков Б.К., Писарев А.В., Зайцева А.О. Русскоязычные версии опросников для оценки качества жизни больных с пролапсом тазовых органов и стрессовым недержанием мочи. Экспериментальная и клиническая урология. 2016; 1: 94-7. [Shkarupa D.D., Kubin N.D., Peshkov N.O., Komyakov B.K., Pisarev A.V., Zaytseva A.O. Russian version of questionnaires for life quality assessment in patients with pelvic organ prolapse and stress urinary incontinenc. Experimental and Clinical Urology. 2016; 1: 94-7 (in Russian)].
- Rahn D.D., Good M.M., Roshanravan S.M., Shi H., Schaffer J.I., Singh R.J. et al. Effects of preoperative local estrogen in postmenopausal women with prolapse: a randomized trial. J. Clin. Endocrinol. Metab. 2014; 99(10): 3728-36. https://dx.doi.org/10.1210/jc.2014-1216
- Montoya T.I., Maldonado P.A., Acevedo J.F., Word R.A. Effect of vaginal or systemic estrogen on dynamics of collagen assembly in the rat vaginal wall. Biol. Reprod. 2015; 92(2): 43. https://dx.doi.org/10.1095/biolreprod.114.118638
- Villa P., Tagliaferri V., Amar I.D., Cipolla C., Ingravalle F., Scambia G. et al. Local ultra-low-dose estriol gel treatment of vulvo-vaginal atrophy: efficacy and safety of long-term treatment. Gynecol. Endocrinol. 2020; 36(6): 535-9. https://dx.doi.org/10.1080/09513590.2019.1702016
- Weidlinger S., Schmutz C., Janka H., Gruetter C., Stute P. Sustainability of vaginal estrogens for genitourinary syndrome of menopause – a systematic review. Climacteric. 2021; 24(6): 551-9. https://dx.doi.org/10.1080/13697137.2021.1891218
- Gaspar A., Addamo G., Brandi H. Vaginal fractional CO2 laser: A minimally invasive option for vaginal rejuvenation. Am. J. Cosmet. Surg. 2011; 28(3): 156-62. https://dx.doi.org/10.1177/074880681102800309
- Zerbinati N., Serati M., Origoni M., Candiani M., Iannitti T., Salvatore S. et al. Microscopic and ultrastructural modifications of postmenopausal atrophic vaginal mucosa after fractional carbon dioxide laser treatment. Lasers Med. Sci. 2015; 30(1): 429-36. https://dx.doi.org/10.1007/s10103-014-1677-2
- Salvatore S., Nappi R.E., Zerbinati N., Calligaro A., Ferrero S., Origoni M. et al. A 12-week treatment with fractional CO2 laser for vulvovaginal atrophy: a pilot study. Climacteric. 2014; 17(4): 363-9. https://dx.doi.org/10.3109/13697137.2014.899347
- Di Donato V., D'Oria O., Scudo M., Prata G., Fischetti M., Lecce F. et al. Safety evaluation of fractional CO2 laser treatment in post-menopausal women with vaginal atrophy: a prospective observational study. Maturitas. 2020; 135: 34-9. https://dx.doi.org/10.1016/j.maturitas.2020.02.009
- Аполихина И.А., Малышкина Д.А., Бычкова А.Е., Паузина О.А. Применение неодимового лазера в практике акушера-гинеколога. Акушерство и гинекология. 2021; 1: 194-9. [Apolikhina I.A., Malyshkina D.A., Bychkova A.E., Pauzina O.A. The use of a neodymium laser in the practice of an obstetrician-gynecologist. Obstetrics and Gynecology. 2021; (1): 194-9 (in Russian)]. https://dx.doi.org/10.18565/aig.2021.1.194-199
Received 10.06.2025
Accepted 25.11.2025
About the Authors
Marina O. Ramazanova, obstetrician-gynecologist, PhD student, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology,Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, mar1naramazanova@yandex.ru, https://orcid.org/0000-0003-2508-7109
Inna A. Apolikhina, Dr. Med. Sci., Professor, Head of the Department of Aesthetic Gynecology and Rehabilitation, V.I. Kulakov National Medical Research Center
for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4; Professor at the Department of Obstetrics, Gynecology, Perinatology, and Reproductology, Institute of Professional Education, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia,
119991, Russia, Moscow, Trubetskaya str., 8-2, i_apolikhina@oparina4.ru, https://orcid.org/0000-0002-4581-6295
Ksenia V. Shatilova, PhD, Head of the Department of Medical Laser Technologies, Melsytech LLC, 606000, Russia, Nizhny Novgorod Region, Dzerzhinsk,
Igumnovskoye Highway, 11D, kshatilova@mail.ru, https://orcid.org/0000-0002-7056-9871
Andrey D. Khlopkov, Senior Researcher at the Department of Medical Laser Technologies, Melsytech LLC, 606000, Russia, Nizhny Novgorod Region, Dzerzhinsk, Igumnovskoye highway, 11D, anbion@yandex.ru, https://orcid.org/0000-0003-0786-9975
Ludmila M. Sencha, Senior Researcher at the Department of Medical Laser Technologies, Melsytech LLC, 606000, Russia, Nizhny Novgorod Region, Dzerzhinsk, Igumnovskoye highway, 11D, luda-sencha@mail.ru, https://orcid.org/0000-0001-7800-5475



