Comparison of the profile of serum autoantibodies in women with different forms of endometriosis
Endometriosis is a chronic inflammatory system disease with multifactorial etiopathogenesis, including dysregulation of immune surveillance. It shares similarities with autoimmune diseases, however, the role of autoantibodies in endometriosis is not well understood.Menzhinskaya I.V., Melkumyan A.G., Pavlovich S.V., Chuprynin V.D., Krechetova L.V.
Objective: To study the profile of serum autoantibodies in women with endometrioid ovarian cysts and deep infiltrative endometriosis.
Materials and methods: Using ELISA, antibodies to tropomyosin 3 (TPM), tropomodulin 3 (TMOD), α-enolase (ENO), estradiol (E), progesterone (PG), human chorionic gonadotropin (hCG), antiphospholipid (aPL) and antinuclear (ANA) antibodies were determined in patients with endometriomas (group 1, n=53), deep infiltrative endometriosis (group 2, n=21) and in women without endometriosis (group 3, n=27).
Results: In patients with endometriosis, antibodies to hormones, endometrial antigens and ENO were detected more often compared to aPL and ANA, and more often than in women without endometriosis. The three groups differed in the detection rate of antibodies to E, hCG, ENO and TPM. In group 1, there was an increase in the level of IgM antibodies to PG, E, hCG and TPM and IgG antibodies to E, hCG, ENO and TMOD; whereas in group 2, only the level of IgM antibodies to TPM and hCG was increased. The levels of IgG antibodies to E and TMOD in group 1 were higher than in group 2.
Conclusion: Antibodies to hormones, endometrial antigens and α-enolase are more often detected in patients with endometriosis than in healthy women. A wider spectrum of antibodies is observed in endometriomas compared with deep endometriosis. Autoantibodies may be involved in the pathophysiology of endometriosis and have diagnostic value.
Authors’ contributions: Menzhinskaya I.V., Melkumyan A.G., Pavlovich S.V. – developing the concept and design of the study; Menzhinskaya I.V., Melkumyan A.G., Chuprynin V.D. – collecting and processing the material; Menzhinskaya I.V. – statistical data processing; Menzhinskaya I.V., Melkumyan A.G. – writing the text; Pavlovich S.V., Chuprynin V.D., Krechetova L.V. – editing the article.
Conflicts of interest: The authors declare no possible conflicts of interest.
Funding: The study was carried out within the framework of the State Program, Ministry of Health of Russia.
Ethical Approval: The study was approved by the Ethical Review Board of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.
Patient Consent for Publication: All patients signed an informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Menzhinskaya I.V., Melkumyan A.G., Pavlovich S.V., Chuprynin V.D., Krechetova L.V. Comparison of the profile of serum autoantibodies in women with different forms of endometriosis.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (8): 78-85 (in Russian)
https://dx.doi.org/10.18565/aig.2023.105
Keywords
Endometriosis is a chronic systemic disease characterized by the presence of functional endometrial–like epithelial and stromal tissue outside the uterus. In many patients, it is usually associated with an inflammatory process and often leads to chronic pelvic pain, dysmenorrhea, dyspareunia and infertility. It significantly affects the quality of life of women and has significant social and economic consequences [1, 2]. Endometriosis occurs in 5–10% of women of reproductive age; it has no pathognomonic signs and symptoms and poses a serious problem due to the difficulties of making an accurate diagnosis. As an asymptomatic course of the disease is noted in 45–50% of patients with endometriosis, the start of the treatment is delayed by an average of 8–10 years; this contributes to the progression of the disease, the development of severe forms and complications [1, 3].
There are different forms of endometriosis depending on the subtypes and location of the foci: superficial peritoneal endometriosis, ovarian endometriotic cyst (endometrioma), deep endometriosis, bowel endometriosis, bladder endometriosis, extra-abdominal endometriosis, iatrogenic endometriosis, adhesions [4, 5]. Endometrioma is the most common form of endometriosis in women of reproductive age; it affects up to 55% of women with the confirmed diagnosis of endometriosis [6].
To date, the gold standard for diagnosing endometriosis remains laparoscopic identification of endometrioid foci with histopathologic confirmation, although this invasive procedure has a number of limitations [7]. The diagnostic possibilities of noninvasive methods, namely ultrasound and magnetic resonance imaging, are limited in the early stages of endometriosis. Such biomarkers as CA 125, CA 19-9, interleukin 6, and endometrial antibodies which are known today for noninvasive diagnosis of endometriosis are not enough sensitive and accurate to detect the early stages of the disease [2, 8].
Endometriosis is mainly caused by inflammatory immune response and dysregulation of immune surveillance which contributes to the growth of ectopic foci of the endometrium [2, 9, 10]. Endometriosis is similar to autoimmune diseases associated with the presence of autoantibodies, high levels of cytokines, therapeutic response to immunomodulators, cell-mediated disorders and other concomitant autoimmune pathology [11]. Autoimmune diseases such as systemic lupus erythematosus, fibromyalgia, Sjogren’s syndrome and inflammatory bowel diseases are reported to be more common in patients with endometriosis than in the general population [12]. Endometriosis is associated with a number of autoimmune diseases [13]. Dysregulation of the immune system which is observed in autoimmune diseases leads to the changes in cell-mediated and humoral immunity and contributes to the development of endometriosis. In addition, concomitant autoimmunity causes a more severe course of endometriosis [14].
A number of studies showed that patients with endometriosis have a decrease in the activity of cells that prevent endometrial implantation, such as natural killer cells, CD4+/CD8+ T-lymphocytes or B-lymphocytes, and an increase in the number of immunosuppressive cells, such as regulatory T-lymphocytes, Th2 (T-helper) cells and MDSCs (myeloid-derived suppressor cells), which may contribute to the implantation of endometrial cells and the progression of endometriosis [10, 12]. According to recent studies, B cells producing autoantibodies, namely anti-endometrial, antiphospholipid (aPL), anti-nuclear (ANA) and anti-DNA antibodies typical for other autoimmune diseases, are involved in the pathogenesis of endometriosis [11, 15]. However, the role of autoantibodies and B cells in the development of the disease remains understudied. Endocrine disorders characteristic of endometriosis with increased production of steroid hormones can contribute to the development of autoimmune processes [16].
Autoantibodies to molecules that play an important role in the pathophysiology of endometriosis, in particular to specific endometrial antigens (tropomyosin (TPM), tropomodulin (TMOD)), glycolytic enzyme α-enolase (ENO), steroid hormones, are considered to be promising markers for noninvasive diagnosis of endometriosis [17, 18]. A deeper understanding of the immune aspects of endometriosis may be useful for finding new treatment strategies.
The aim of the research is to study the profile of serum autoantibodies in women with ovarian endometriotic cysts and deep infiltrating endometriosis.
Materials and methods
The study groups included women aged 20 to 40 years with stage II–IV ovarian endometriotic cysts (group 1, n=53) and stage III–IV deep infiltrating endometriosis without the involvement of the ovaries (group 2, n=21). The control group included women without endometriosis (group 3, n=27). The diagnosis of endometriosis was made according to the classification of the American Society for Reproductive Medicine on the basis of laparoscopic identification of the foci of endometriosis with histopathologic confirmation. Blood for the identification of autoimmune markers was taken from women in the proliferative phase of the menstrual cycle.
The study did not include patients with oncological and autoimmune diseases and women who had contraindications for surgical treatment. The exclusion criteria were acute pelvic inflammatory diseases, infectious diseases, previous hormonal and anti-inflammatory therapy for three last months prior to the study.
Immunological methods of the study included the identification of aPL of IgM and IgG classes to cardiolipin (CL) and β2-glycoprotein-I (β2-GP-I), IgG class ANA which were measured by means of enzyme immunoassay kits of ORGENTEC Diagnostika (Germany). Antibodies to hormones (human chorionic gonadotropin (hCG), progesterone (PG) and estradiol (E)) were determined using modifications of indirect enzyme-linked immunosorbent assay (ELISA) which were described previously [19, 20]. Antibodies to TPM, TMOD, and ENO were determined by means ELISA modified variants using of appropriate recombinant proteins (Abcam, Great Britain), immobilized on Maxisorp microtiter plates (Nunc, Denmark) at a concentration of 2–5 μg/ml [18, 21]. Serum samples were diluted 1:100. The optical density (OD) was measured at a wavelength of 450 nm using a MULTISKAN EX microplate photometer (Thermo Electron (Shanghai) Instrument Co., China). The result was considered positive if the average OD of the test sample exceeded the average OD of the control samples by more than two standard deviations (2δ).
Statistical analysis
The obtained data were analyzed using the software packages Microsoft Office Excel 2010, MedCalc v. 12. The normal distribution of values in the samples was assessed using the Shapiro–Wilk test and the Kolmogorov–Smirnov test. In case of deviation from the normal distribution, quantitative data were represented by median values (Me) with a range (min; max), quantitative data were analyzed using nonparametric statistical methods. In order to compare the data in two groups, the Mann–Whitney U-test was used; the Kruskal–Wallis test with the Bonferroni correction was used for multiple comparison in three groups. To represent qualitative data, absolute (n) and relative values (%) were given, the differences between them were evaluated using the χ2 (chi-square) test. The relationship between the variables was calculated using the Spearman correlation coefficient. In order to assess the relationship between the risk factor and the disease, the relative risk (RR) was determined with the 95% confidence interval (95% CI).
Results
The analysis of the clinical and anamnestic data of the patients in three groups showed that all three groups were comparable in age of the patients (Table 1). Between the three groups there were statistically significant differences in the frequency of detection of ovarian endometriotic cysts, bowel endometriosis, endometriosis of the pelvic peritoneum/ligaments, adenomyosis and adhesions in the pelvis; this pathology was detected significantly more often in groups 1 and 2 than in group 3. It should be noted that all patients in group 1 were diagnosed with ovarian endometriotic cysts, and all patients in group 2 were diagnosed with endometriosis of the pelvic peritoneum/ligaments. Besides, patients in group 2 were more likely to have bowel endometriosis and adenomyosis than patients in group 1 (p<0.001; p=0.02). The patients in groups 1 and 2 often had adhesions in the pelvis; however, bladder endometriosis was revealed less often. The study groups did not differ in the frequency of primary and secondary infertility, uterine fibroids, chronic endometritis, chronic salpingoophoritis, polyps and endometrial hyperplasia.
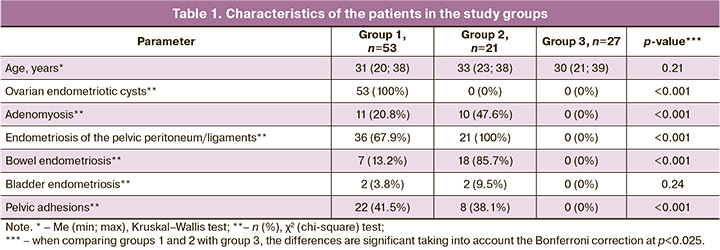
The results of determining the profile of autoantibodies showed that antibodies to hormones (E, PG, hCG), endometrial antigens (TMOD, TPM) and ENO were statistically significantly more often detected than aPL and ANA in patients with endometriosis (p<0.001 in group 1; p<0.05 in group 2). ANA were not found in all the study groups. It was shown that three groups differed in the frequency of detection of antibodies to E, hCG, ENO and TPM (p<0.025). These autoantibodies were revealed significantly more often in patients with endometriosis than in the group of women without endometriosis (Table 2). There were no significant differences between groups 1 and 2 in the frequency of detection of antibodies. However, the risk of detection of antibodies to PG, E, hCG, ENO, TPM, except for antibodies to TMOD, was 3.4–5.4 times higher in patients with ovarian endometriotic cysts than in women without endometriosis according to the values of the relative risk (RR) (p<0.05). The patients with deep infiltrating endometriosis without the involvement of the ovaries had a high risk of detection of only antibodies to E, ENO and TPM, which was 4.3–4.7 times higher than in the patients of the control group (p<0.05).
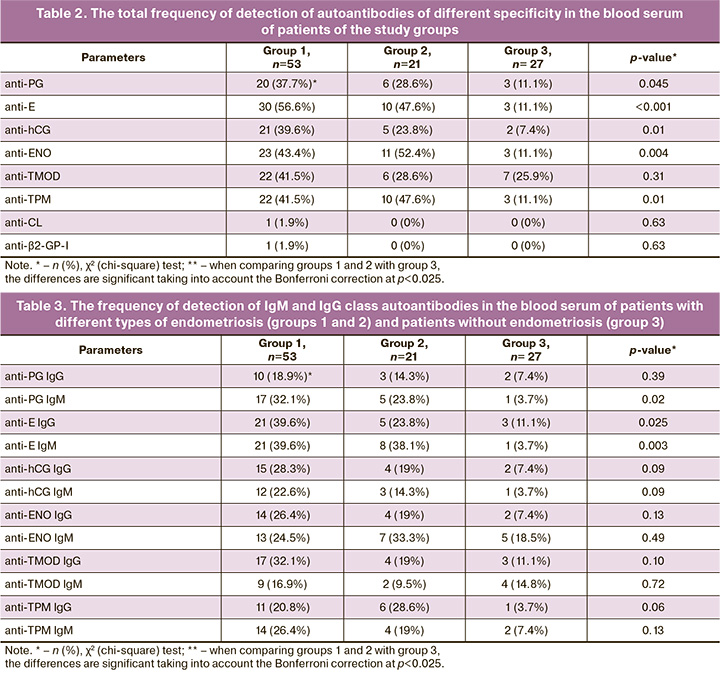
When the frequency of detection of IgG and IgM class antibodies was revealed in the study groups and the control group, differences were found in the frequency of detection of IgM and IgG antibodies to E and IgM antibodies to PG (p≤0.025) (Table 3). Pairwise comparison of patients from groups 1 and 2 revealed no significant differences in the frequency of detection of autoantibodies (p>0.05). However, the comparison of groups 1 and 2 with group 3 revealed that patients in group 1 showed a significantly higher frequency of detection of IgM and IgG class antibodies to E, IgM antibodies to PG (p<0.01), patients in group 2 showed a significantly higher frequency of detection of IgM antibodies to E and IgG antibodies to TPM than in the control group (p<0.025). According to the RR values, there was a high risk of detection of IgM and IgG antibodies to E in patients with ovarian endometriotic cysts (RR=10.7; 95% CI: 1.5–75.3; p=0.02; RR=3.6; 95% CI: 1.2–10.9; p=0.03) and IgM antibodies to PG (RR=8.7; 95% CI: 1.2–61.7; p=0.03). The patients with deep infiltrating endometriosis without the involvement of the ovaries had a high risk of detection of IgM antibodies to E (RR=10.3; 95% CI: 1.4–75.9; p=0.02) and IgG antibodies to TPM (RR=7.7; 95% CI: 1.0–59.26; p=0.049) in comparison with the patients without endometriosis. High RR values (>1.0) indicated the presence of a direct relationship between these autoantibodies and the above types of endometriosis.
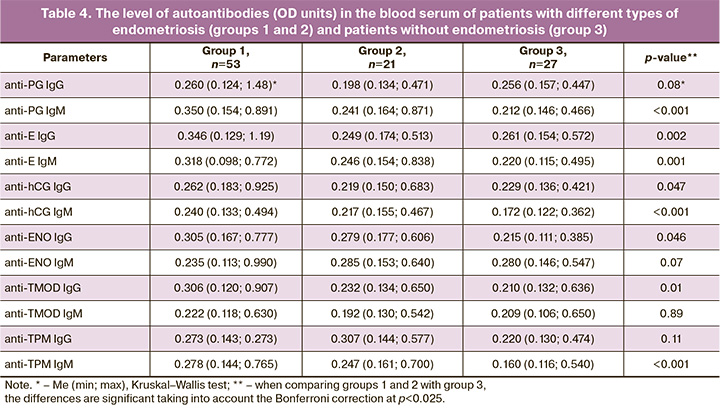
The comparison of serum levels of antibodies in patients of groups 1 and 2 with patients of group 3 revealed a significant difference in the levels of IgM antibodies to PG, E, hCG and TPM, as well as IgG antibodies to E and TMOD (p<0.025) (Table 4). The patients with ovarian endometriotic cysts had higher levels of IgM antibodies to PG, E, hCG and TPM, as well as IgG antibodies to E, hCG, ENO and TMOD than women without endometriosis (p<0.025). The patients with deep infiltrating endometriosis without the involvement of the ovaries showed an increase in the levels of IgM antibodies to TPM and hCG in comparison with the patients without endometriosis (p<0.001; 0.01). The comparison of groups of patients with different types of endometriosis showed higher levels of IgG antibodies to E and TMOD in group 1 than in group 2 (p<0.01; 0.02).
The patients with ovarian endometriotic cysts had a strong direct correlation between the levels of IgM and IgG antibodies to E and PG, as well as between the levels of IgM antibodies to TPM and TMOD (r correlation coefficient is 0.92, 0.75 and 0.85, respectively; p<0.001). In other cases, there was no correlation between the levels of IgG antibodies of different specificity; however, there was a direct correlation between the levels of IgM antibodies with values of r<0.7, and there was a more marked correlation between antibodies to hormones, as well as between anti-endometrial antibodies and antibodies to ENO.
The patients with deep infiltrating endometriosis had a direct correlation between IgG antibodies to TPM and TMOD (r=0.5; p=0.04), as well as between IgG antibodies to E and ENO (r=0.52; p=0.02) or TMOD (r=0.54; p=0.01). It should be noted that a direct correlation was also detected between IgM antibodies to hormones, TPM and TMOD (r>0.7; p<0.001).
Discussion
This study included patients with various types of endometriosis, namely ovarian endometriotic cysts and deep infiltrating endometriosis. All patients in group 1 were diagnosed with ovarian endometriotic cysts, which were most often detected in combination with endometriosis of the pelvic peritoneum/ligaments, and less often with bowel endometriosis, adenomyosis and bladder endometriosis. All patients in group 2 were diagnosed with deep infiltrating endometriosis; bowel endometriosis and adenomyosis occurred significantly more often in group 2 than in group 1. The patients of both groups had a high frequency of endometriosis-associated pelvic adhesions.
It should be noted that pathophysiology of endometriosis is largely unknown; however, there are numerous theories of pathogenesis of endometriosis. There is no consensus on whether different types of endometriosis have common pathogenesis, and whether one type of endometriosis can cause another. It is believed that the leading cause of deep endometriosis is embryological; therefore, the immune system plays a less important role in its development [10]. Whereas superficial peritoneal endometriosis and endometrioma are usually considered to result from the implantation of endometrial cells transferred to the abdominal cavity through the fallopian tubes due to retrograde menstruation. Impaired immune response and dysregulation of immune surveillance are critical factors leading to the development of endometriosis and possible consequences including infertility.
The results of this study demonstrated a high detection rate and a wide spectrum of autoantibodies in patients with endometriosis, especially in women with ovarian endometriotic cysts, including antibodies to endometrial antigens (TMOD, TPM), steroid and gonadotropin hormones (E, PG, hCG) and enzyme ENO. It should be noted that these antibodies were found in patients with endometriosis significantly more often than aPL and ANA, as well as they were more common in women with endometriosis than in women without it.
The obtained results are consistent with the data of a systematic review (2017). This review analyzed 22 studies on the role of B lymphocytes in endometriosis and revealed an increased presence and/or activation of B cells, as well as excessive production of antibodies and proinflammatory cytokines in patients with endometriosis [15]. The presence of anti-endometrial antibodies has previously been shown both in the blood serum and in the peritoneal fluid [22]. Autoantibodies are believed to contribute to the development of endometriosis by stimulating the immune system and supporting inflammation. However, their role in the development of the disease, as well as the relationship with clinical symptoms, localization and severity of the disease remains understudied.
The comparison of three groups showed significant differences in the frequency of detection of IgG and IgM antibodies to E, hCG, ENO and TPM, which were revealed more often in endometriosis. Although groups 1 and 2 did not differ significantly in the frequency of detection of antibodies, patients with ovarian endometriotic cysts had a high risk of detection of antibodies to PG, E, hCG, ENO, TPM, whereas patients without ovarian lesions had only antibodies to E, ENO, and TPM in comparison with women without endometriosis. IgM and IgG antibodies to E and IgM antibodies to PG were significantly more frequently detected in group 1, and IgM antibodies to E and IgG antibodies to TPM were more often detected in group 2. Moreover, there was a high risk of detection of antibodies to E and PG in group 1, and there was a marked direct relationship of antibodies to steroid hormones with ovarian endometriotic cysts; patients with deep infiltrating endometriosis had a high risk of detection of not only antibodies to E, but also antibodies to TPM.
Excessive production of estrogens in the ovaries, ectopic foci of the endometrium, peripheral adipose tissue, as well as increased production of PG by stromal cells in endometrioid heterotopias may contribute to the increased formation of antibodies to steroid hormones in patients with endometriosis [23]. Endometriosis is known to be an estrogen-dependent disease when the local biosynthesis of estradiol in the foci of endometriotic lesions in combination with severe inflammation in the abdominal cavity creates an abnormal immune-endocrine microenvironment that promotes the growth and survival of cells in ectopic foci [9].
In addition, there is an abnormal transmission of PG signals in the endometrium in endometriosis which plays an important role in the impairment of decidualization and the development of ectopic lesions [24]. The action of PG is known to be crucial for decreasing inflammation in the endometrium, and abnormal transmission of PG signals leads to a pro-inflammatory phenotype. The consequence of the impaired action of PG in endometriosis may be resistance to PG, which is a key endometrial factor in the pathogenesis of infertility associated with endometriosis; it also leads to the ineffectiveness of hormone therapy in some patients. The high positivity of patients for antibodies to PG which was revealed in this study (37.7% in group 1 and 28.6% in group 2) suggests that antibodies to PG may contribute to pathophysiology of endometriosis, to the development of endometrial resistance to PG and consequently to the ineffectiveness of hormone therapy in seropositive patients.
The obtained results demonstrate an increase in the serum level of a wide spectrum of autoantibodies of different specificity in patients with ovarian endometriotic cysts, namely IgM antibodies to PG, E, hCG and TPM, as well as IgG antibodies to E, hCG, ENO and TMOD, in comparison with women without endometriosis. Whereas the patients with deep endometriosis without the involvement of the ovaries had a significant increase in the level of IgM antibodies to TPM and hCG only. The patients in group 1 had significantly higher levels of IgG antibodies to E and TMOD than the patients in group 2.
Autoantibodies may be involved in pathophysiology of endometriosis. When autoantibodies bind to antigens on the cell surface or form immune complexes with soluble antigens, they can increase inflammation and tissue damage by means of recruiting neutrophils and other myeloid and lymphoid cells into lesions, activating complement along the classical pathway through the interaction of the C1q component with Fc domains of antibodies in immune complexes. They can also cause changes in the function and activation of immune cells, including effector cells that carry Fcγ-receptors on the surface, such as monocytes, neutrophils, macrophages, dendritic cells and natural killers, and increase the production of proinflammatory cytokines. Increased cytokine concentration in ectopic foci of endometriosis caused by inflammation, in turn, stimulates the production of autoantibodies by B-lymphocytes.
Particular attention should be paid to the detection of antibodies to endometrial antigens (TPM, TMOD) involved in important mechanisms of endometriosis pathogenesis related to cell mobility and migration, invasion and adhesion at ectopic sites, cytoskeletal dynamics, transition to stationary morphology, apoptosis and necrosis [25, 26]. The glycolytic enzyme ENO promotes the invasion of endometrial cells in ectopic foci by providing plasmin activation and degradation of the extracellular matrix [27].
The increased expression of the molecules in endometrioid cells is likely to contribute to the formation of antibodies to these molecules. This hypothesis is confirmed by the results of a study of the lysate proteins of endometrial tissue obtained from superficial and deep foci of endometriosis. The results demonstrated different expression of numerous proteins participating in implantation of cells outside the endometrium and involved in the progression of the disease [28]. In particular, there was an increased expression of TPM in ectopic endometrial tissue compared to eutopic tissue and its rise was especially noted in the secretory phase of the cycle. The study also showed an increased expression of TPM in foci of deep endometriosis, apparently due to a higher content of smooth muscle cells in the foci. This may explain the significant increase in the level of antibodies to TPM observed by us in patients with deep endometriosis.
The results of the study are consistent with the data obtained by other researchers on the possibility of using antibodies to ENO as a diagnostic marker of endometriosis [29] and a higher efficiency of determining antibodies to epitopes of TMOD and TPM for the diagnosis of endometriosis at early stages (I-II) in comparison with CA-125 and CA 19-9 [17].
Conclusion
Thus, serum autoantibodies to endometrial antigens, ENO, steroid and gonadotropin hormones were detected with higher frequency in patients with endometriosis than in women without endometriosis. However, there was no increase in the detection rate of aPL and ANA. The antibody profile was different in various types of endometriosis. A wider spectrum of antibodies was observed in patients with ovarian endometriotic cysts, including antibodies to hormones, TPM, TMOD and ENO, whereas antibodies to TPM prevailed in patients with deep endometriosis. Antibodies to steroid hormones were mainly associated with endometriomas. Antibodies to PG may contribute to the development of endometrial resistance to PG. Autoantibodies may be involved in the pathophysiology of endometriosis and have diagnostic value.
References
- Taylor H.S., Kotlyar A.M., Flores V.A. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet. 2021; 397(10276): 839-52. https://dx.doi.org/10.1016/S0140-6736(21)00389-5.
- Gajbhiye R.K. Endometriosis and inflammatory immune responses: Indian Experience. Am. J. Reprod. Immunol. 2023; 89: e13590.https://dx.doi.org/10.1111/aji.13590.
- O D.F., Flores I., Waelkens E., D'Hooghe T. Noninvasive diagnosis of endometriosis: Review of current peripheral blood and endometrial biomarkers. Best Pract. Res. Clin. Obstet. Gynaecol. 2018; 50: 72-83.https://dx.doi.org/10.1016/j.bpobgyn.2018.04.001.
- Tomassetti C, Johnson N.P., Petrozza J., Abrao M.S., Einarsson J.I., Horne A.W. et al.; International Working Group of AAGL, ESGE, ESHRE and WES. An international terminology for endometriosis, 2021. Hum. Reprod. Open. 2021; 2021(4): hoab029. https://dx.doi.org/10.1093/hropen/hoab029.
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Эндометриоз. 2020. [Ministry of Health of the Russian Federation. Clinical guidelines Endometriosis. 2020. (in Russian)].
- Гаспарян С.Н., Василенко И.А., Попова О.С., Лифенко Р.А. Эндометриома: новая парадигма диагностики и лечебной тактики. Проблемы репродукции. 2019; 25(6): 78-85. [Gasparyan S.A., Vasilenko I.A., Popova O.S., Lifenko R.A. Endometrioma: a new paradigm of diagnostics and therapeutic tactics. Russian Journal of Human Reproduction. 2019; 25(6): 78 85. (in Russian)]. https://dx.doi.org/10.17116/repro20192506178.
- Becker C.M., Attila Bokor A., Heikinheimo O., Horne A., Jansen F., Kiesel L. et al.; ESHRE Endometriosis Guideline Group. ESHRE guideline: endometriosis. Hum. Reprod. Open. 2022; 2022(2): hoac009. https://dx.doi.org/10.1093/hropen/hoac009.
- Nisenblat V., Bossuyt P.M.M., Shaikh R., Farquhar C., Jordan V., Scheffers C.S. et al. Blood biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst. Rev. 2016; 5: CD012179. https://dx.doi.org/10.1002/14651858.CD012179.
- Symons L.K., Miller J.E., Kay V.R., Marks R.M., Liblik K., Koti M., Tayade C. The immunopathophysiology of endometriosis. Trends Mol. Med. 2018; 24(9):748-62. https://dx.doi.org/10.1016/j.molmed.2018.07.004.
- Maksym R.B., Hoffmann-Młodzianowska M., Skibińska M., Rabijewski M., Mackiewicz A., Kieda C. Immunology and immunotherapy of endometriosis. J. Clin. Med. 2021; 10(24): 5879. https://dx.doi.org/10.3390/jcm10245879.
- Greenbaum H., Galper B.-E. L., Decter D.H., Eisenberg V.H. Endometriosis and autoimmunity: Can autoantibodies be used as a non-invasive early diagnostic tool? Autoimmun. Rev. 2021; 20(5): 102795. https://dx.doi.org/10.1016/j.autrev.2021.102795.
- Greenbaum H., Weil C., Chodick G., Shalev V., Eisenberg V.H. Evidence for an association between endometriosis, fibromyalgia, and autoimmune diseases. Am. J. Reprod. Immunol. 2019; 81(4): e13095. https://dx.doi.org/10.1111/aji.13095.
- Shigesi N., Kvaskoff M., Kirtley S., Feng Q., Fang H., Knight J.C. et al. The association between endometriosis and autoimmune diseases: a systematic review and meta-analysis. Hum. Reprod. Update. 2019; 25(4): 486-503.https://dx.doi.org/10.1093/humupd/dmz014.
- Vanni V.S., Villanacci R., Salmeri N., Papaleo E., Delprato D., Ottolina J. et al. Concomitant autoimmunity may be a predictor of more severe stages of endometriosis. Sci. Rep. 2021; 11(1): 15372. https://dx.doi.org/10.1038/s41598-021-94877-z.
- Riccio L.G.C., Baracata E.C., Chapron C., Batteux F., Abrão M.S. The role of the B lymphocytes in endometriosis: a systematic review. J. Reprod. Immunol. 2017; 123: 29-34. https://dx.doi.org/10.1016/j.jri.2017.09.001.
- Taneja V. Sex hormones determine immune response. Front. Immunol. 2018; 9: 1931. https://dx.doi.org/10.3389/fimmu.2018.01931.
- Gajbhiye R., Bendigeri T., Ghuge A., Bhusane K., Begum S., Warty N.et al. Panel of autoimmune markers for noninvasive diagnosis of minimal-mild endometriosis. Reprod. Sci. 2017; 24(3): 413-20.https://dx.doi.org/10.1177/1933719116657190.
- Менжинская И.В., Мелкумян А.Г., Павлович С.В., Чупрынин В.Д., Ванько Л.В., Сухих Г.Т. Аутоиммунные маркеры для неинвазивной диагностики эндометриоза у женщин. Биомедицинская химия. 2020; 66(2): 162-6. [Menzhinskaya I.V., Melkumyan A.G., Pavlovich S.V., Chuprynin V.D., Vanko L.V., Sukhikh G.T. Autoimmune markers for noninvasive diagnosis of endometriosis in women. Biomedical Chemistry. 2020; 66(2): 162-6.(in Russian)]. https://dx.doi.org/10.18097/PBMC20206602162.
- Менжинская И.В., Гладкова К.А., Сидельникова В.М., Сухих Г.Т. Антипрогестероновые антитела в клинике привычной потери беременности Иммунология. 2008; 29(1): 34-7. [Menzhinskaya I.V., Gladkova K.A., Sidelnikova V.M., Sukhikh G.T. Antiprogesterone antibodies in the clinic of reccurent pregnancy loss. Immunology. 2008; 29(1): 34-37(in Russian)].
- Менжинская И.В., Кашенцева М.М., Ванько Л.В., Сухих Г.Т. Иммунохимические свойства аутоантител к хорионическому гонадотропину у женщин с невынашиванием беременности. Иммунология. 2015; 36(1): 30-5. [Menzhinskaya I.V., Kashentseva M.M., Vanko L.V., Sukhikh G.T. Immunochemical properties of autoantibodies to chorionic gonadotropin in women with pregnancy loss. Immunology. 2015; 36(1): 30-5 (in Russian)].
- Gajbhiye R., Sonawani A., Khan S., Suryawanshi A., Kadam S., Warty N. et al. Identification and validation of novel serum markers for early diagnosis of endometriosis. Hum. Reprod. 2012; 27(2): 408-17. https://dx.doi.org/10.1093/humrep/der410.
- Yeol S.G., Won Y.S., Kim Y.I., Lee J.W., Choi Y.J., Park D.C. Decreased Bcl-6 and increased Blimp-1 in the peritoneal cavity of patients with endometriosis. Clin. Exp. Obstet. Gynecol. 2015; 42(2): 156-60.
- Bulun S.E., Yilmaz B.D., Sison C., Miyazaki K., Bernardi L., Liu S. et al. Endometriosis. Endocr. Rev. 2019; 40(4): 1048-79. https://dx.doi.org/10.1210/er.2018-00242.
- Patel B.G., Rudnicki M., Yu J., Shu Y., Taylor R.N. Progesterone resistance in endometriosis: origins, consequences and interventions. Acta Obstet. Gynecol. Scand. 2017; 96(6): 623-32. https://dx.doi.org/10.1111/aogs.13156.
- Manstein D.J., Meiring J.C.M., Hardeman E.C., Gunning P.W. Actin–tropomyosin distribution in non muscle cells. J. Muscle Res. Cell. Motil. 2020; 41(1):11-22. https://dx.doi.org/10.1007/s10974-019-09514-0.
- Parreno J., Fowler V.M. Multifunctional roles of tropomodulin-3 in regulating actin dynamics. Biophys. Rev. 2018; 10(6): 1605-15. https://dx.doi.org/10.1007/s12551-018-0481-9.
- Cappello P., Principe M., Bulfamante S., Novelli F. Alpha-Enolase (ENO1), a potential target in novel immunotherapies. Front. Biosci. (Landmark Ed). 2017; 22(5): 944-59. https://dx.doi.org/10.2741/4526.
- Irungu S., Mavrelos D., Worthington J., Blyuss O., Saridogan E., Timms J.F. Discovery of non‑invasive biomarkers for the diagnosis of endometriosis. Clin. Proteomics. 2019; 16: 14. https://dx.doi.org/10.1186/s12014-019-9235-3.
- Nabeta M., Abe Y., Kagawa L., Haraguchi R., Kito K., Ueda N. et al. Identification of anti-α-enolase autoantibody as a novel serum marker for endometriosis. Proteomics Clin. Appl. 2009; 3(10): 1201-10. https://dx.doi.org/10.1002/prca.200900055.
Received 21.04.2023
Accepted 14.07.2023
About the Authors
Irina V. Menzhinskaya, Dr. Med. Sci., Leading Researcher, Laboratory of Clinical Immunology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-11-83, i_menzinskaya@oparina4.ru, 4, Oparina str., Moscow, Russia, 117997.Arika G. Melkumyan, postgraduate student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-52-25, dr.melkumyan@gmail.com, 4, Oparina str., Moscow, Russia, 117997.
Stanislav V. Pavlovich, PhD, Scientific Secretary, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-52-25, s_pavlovich@oparina4.ru, 4, Oparina str., Moscow, Russia, 117997.
Vladimir D. Chuprynin, PhD, Head of the Surgical Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-35-75, v_chuprynin@oparina4.ru, 4, Oparina str., Moscow, Russia, 117997.
Lubov V. Krechetova, Dr. Med. Sci., Head of the Laboratory of Clinical Immunology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-11-83, l_krechetova@oparina4.ru, 4, Oparina str., Moscow, Russia, 117997.



