Current non-invasive methods for diagnosing cervical intraepithelial neoplasia and their effectiveness
Khachaturian A.R., Yarmolinskaya M.I.
Objective: To evaluate the diagnostic characteristics of fluorescence spectroscopy using LuViva technology for diagnosing cervical intraepithelial neoplasia.
Materials and methods: This study included 156 patients. The median patient age was 32 years (Q1–Q3: 27–39 years). All patients underwent fluorescence spectroscopy and cytological examination of the cervical and cervical canal scrapings using liquid cytology. Testing for high-risk human papillomavirus (hrHPV) using real-time PCR in cervical canal discharge was performed in 138 patients (88.5%), and extended colposcopy and targeted biopsy (as indicated) were performed in 52 women.
Results: The positive predictive value of fluorescence spectroscopy for detecting cytological changes of grade ≥ASCUS was 28.7% and the negative predictive value was 88.4%. The diagnostic characteristics of the methods used were compared with those of histological examination of cervical biopsy specimens. In the presence of hrHPV (43% of patients), the odds of detecting HSIL were almost 20 times higher (odds ratio [OR]=9.96, 95% CI 1.04–384.61). The sensitivity of the cytological test for LSIL and HSIL was 35.3% and 50%, respectively, whereas the specificity was 91.4% and 93.2%, respectively. The positive predictive values of cytological examination for LSIL and HSIL were 66.7% and 57.1%, respectively, while the negative predictive values were 74.4% and 91.1%, respectively. The positive predictive values of fluorescence spectroscopy for LSIL and HSIL were 42.5% and 15%, respectively, while the negative predictive values were 100% and 83.3%, respectively.
Conclusion: Fluorescence spectroscopy has higher sensitivity than liquid cytology. The non-invasive technique of fluorescence spectroscopy can act as a triage test for patients with conflicting results of cytology and HPV testing and determines the choice between immediate colposcopy and dynamic observation.
Authors’ contributions: Khachaturian A.R., Yarmolinskaya M.I. – conception and design of the study; Khachaturyan A.R. – material collection and processing, statistical analysis, drafting of the manuscript; Yarmolinskaya M.I. – editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The study was conducted within the framework of the Federal Research Institute "Strategy of maintaining health of women with gynecological and endocrine diseases at different ages: pathogenetic basis of drug rehabilitation and development of new directions of organ-preserving surgical interventions", state registration number of the topic in the EGISU NIOKTR No. 1021062812154-3-3.2.2.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the D.O. Ott Research Institute for OG&R.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Khachaturian A.R., Yarmolinskaya M.I. Current non-invasive methods for
diagnosing cervical intraepithelial neoplasia and their effectiveness.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (8): 106-113 (in Russian)
https://dx.doi.org/10.18565/aig.2024.98
Keywords
The significance of exploring ways to prevent cervical cancer is highlighted by the prevalence of this disease, especially among women in their reproductive years. The effectiveness of secondary prevention (screening), including cytological examination (PAP test) and detection of high-risk human papillomavirus (hrHPV) DNA, is influenced not only by the extent of population coverage but also by the sensitivity and specificity of the diagnostic tests used.
The cytological test, widely adopted as the standard for cervical cancer screening in many countries, shows low sensitivity (51–60%) for detecting pathology, but relatively high specificity for identifying normal results [1]. According to a recent meta-analysis, the sensitivity of cytological examination for cervical intraepithelial neoplasia grade 3 or higher (CIN3+) ranges from 52.8% to 75.4%, while the specificity ranges from 78.0% to 85.6%. The low sensitivity of this test is attributed to the significant role of the human factor in its implementation, which includes the quality of the sample obtained for the study, the extent of the transformation zone captured, and the subjectivity of the cytologist interpreting the results.
Currently, efforts are being made to develop new laboratory tests that can enhance the diagnostic value of cytological research. One such method is liquid cytology, which improves the quality and adequacy of samples. In the aforementioned meta-analysis, the diagnostic efficiency of atypical squamous epithelial cells of undetermined significance (ASC-US +) and low-grade squamous intraepithelial lesions (LSIL+) in liquid cytology was also assessed [1]. The sensitivity and specificity of ASC-US+ for detecting CIN2+ were 52–94% and 73–97%, respectively, and those for detecting CIN3+ were 52–98% and 73–97%, respectively. The sensitivity and specificity of LSIL+ for detecting CIN2+ were 42–87% and 90–99%, respectively, and those for detecting CIN3+ were 48–93% and 92–98%, respectively.
Another approach to increase the sensitivity of cytological screening is to combine it with various HPV testing options. HPV type 45 accounts for approximately 5% of cervical cancer cases, HPV 18 accounts for approximately 15%, and the most carcinogenic HPV type 16 accounts for approximately 60% [2]. A recent Canadian retrospective study compared HPV genotyping with cytological examination as a triage test in 1,396 HPV-positive women. The positive predictive value (PPV) for detecting CIN2+ in the first year of observation was calculated. For cytological findings of ASC-US or higher, the PPV was 20.9%, compared with 31.8% for cases with detected HPV type 16 [3].
Studying the expression levels of cell proliferation markers is another method to enhance the diagnostic characteristics of cytological tests. A large European multicenter study analyzed the diagnostic efficiency of assessing hypermethylation of the promoter regions of the tumor suppressor genes FAM19A4/mir124-2 in 2,384 HPV-positive women for detecting CIN2+ over two years of observation. The sensitivity for detecting CIN3 was 77.2%, and for CIN2, it was 46.8%. The overall specificity of this test was 78.3% [4]. Recently, the diagnostic value of an immunocytochemical test that detects the expression level of protein markers of cell proliferation through additional staining of cytological preparations has been actively studied. The p16ink4α protein inhibits cell differentiation by blocking the transition from the G1 phase to the S phase. The increased presence of this protein in cells infected with hrHPV is due to the need to slow cell cycle progression. The p16ink4α protein can be detected in atypical cells and squamous cell metaplasia of the columnar epithelium [5]. The Ki-67 antigen is a nuclear protein associated with cell proliferation and the transcription of ribosomal RNA, making it a marker of cell proliferation. In normal cells with intact physiological control of division, differentiation, and programmed apoptosis, the expression of p16ink4α and Ki-67 proteins is mutually exclusive; therefore, their simultaneous detection in one cell (known as "double labeling") signals a disturbance in the cell cycle [6].
Another reason for the relevance of additional diagnostic tests is the wide range of management options available for patients with abnormal cytological test results. For instance, when an ASC-US result is obtained, two management options are offered: a repeat cytological test within a year or additional testing for hrHPV carriage. If the hrHPV test is positive, colposcopy and biopsy are recommended [7, 8].
It is important to note that all the proposed diagnostic methods require specialized laboratories, specific equipment, and highly qualified physicians to perform colposcopy. Additionally, the waiting time for test results can increase patient anxiety, necessitate multiple visits to the doctor, and in cases of false positives, lead to unnecessary invasive procedures with associated complications, potentially impacting the overall screening outcome. Therefore, informative diagnostic methods, including those utilizing artificial intelligence technologies, would allow for better "sorting" (triage testing) of patients, thereby justifying the need for invasive diagnostic methods.
Among the most relevant diagnostic methods are real-time technologies such as optoelectronic techniques based on the fluorescence effect in various light spectra. For example, MediSpectra uses fluorescence in the ultraviolet spectrum, Lifespex in the visible and ultraviolet spectra, and TruScreen in the infrared and visible-light spectra [9]. Nuclear-cytoplasmic rearrangement in individual cells and changes in the spatial relationships of cells in squamous intraepithelial lesions (SIL) of varying severity lead to alterations in the physicochemical and optical characteristics of the tissue. Differences in the ability to absorb and reflect optical signals of different wavelengths enables differentiation between normal and atypically altered tissue areas. The assessment of tissue absorption and reflection of ultraviolet light signals, coupled with the analysis of spectroscopic parameters, increases the diagnostic value of cervical biopsy by 25% in traditional colposcopy, with biopsy based on a false-positive examination result occurring in only 4% of cases [10].
The most common optoelectronic SIL detection system is TruScreen technology, which analyzes the tissue's ability to absorb and reflect infrared and visible light spectra (combining wavelengths of 525 nm, 660 nm, and 936 nm). Using a hand probe equipped with biosensors to measure tissue optical properties, results from multiple points were recorded by contact (with an average of 21 scanned points, depending on the lesion area). During the examination, a "stop/continue" indicator on the hand probe signals the operator to continue moving the tip along the cervical surface. The choice of scanning zones is empirical and determined by the researcher. The scanning depth range is 200–300 µm, and the procedure required 1–2 min. After scanning sufficient points, the operator receives a signal indicating the completion of the procedure. The system automatically compares the data with an expert database of histological examination results of over 2,000 samples included in the software and issues a report on the presence or absence of SIL on paper, with results categorized as "Normal" (normal squamous epithelium, columnar epithelium, physiological metaplasia) or "Abnormal" (CIN 1–3, invasive cancer) [11, 12].
Analysis of the data showed that TruScreen's sensitivity in detecting SIL was 81.3% (83.3% for high-grade SIL (HSIL) and 78.6% for LSIL), with specificity for normal tissue at 50.0%. The positive predictive value for SIL detection was 64.6%, whereas the negative predictive value was 70.0% (for HSIL, 37.2% and 84.6%, respectively) [13]. These technologies exemplify the use of artificial intelligence for diagnostic purposes. The LuViva Advanced Cervical Scan diagnostic system is another example of fluorescence spectroscopy, an innovative medical device that uses white-light scanning to detect chemical and physical cellular changes. LuViva functions as a laboratory and diagnostic test in one device, providing immediate and objective results at the time of examination [14].
The LuViva Advanced Cervical Scan system is a spectroscopic technology that combines fluorescence spectroscopy of intracellular structures and reflectance spectroscopy. It can detect both chemical and physical changes in these structures, such as differences in luminescence between healthy and atypical cells, epithelial thickening, compaction, changes in the size of the nucleus, and angiogenic features [15]. The system uses low levels of ultraviolet radiation and visible light (300–800 nm), and has a scanning depth of 4–5 mm. Scanning times are 1 min and 20 s.
LuViva consists of a handheld unit and a base unit connected by a fiber-optic cable. The handheld unit is used with a disposable tube probe that acts as a patient interface. It provides a barrier between the patient and equipment, sets the optical distance, and excludes room lighting during the procedure. The tube probe includes a reference mark for device calibration before each use. It also saves the obtained data and compares them with the results of more than 2,000 cervical tissue samples available in the device database.
After scanning, the examination results are displayed as a risk level for high-grade cervical lesions (low, medium, or high) [16]. The scanning technique involves entering patient information and calibrating the device, inserting a gynecological speculum, preparing the cervix for examination, and inserting a tube probe until it touches the cervix. The axis of the probe should be centered on the focal axis before scanning begins.
Upon completion of the examination, the saved result is displayed as "before" and "after" images of the cervix. Comparing these images ensures objectivity and helps exclude errors due to patient movement. The scanning was performed automatically, without the need for a researcher to use a point probe, similar to the TruScreen method.
If the "before" and "after" images match on the monitor screen, the examination result is saved and compared with the database from the device memory. A conclusion in the form of low, medium, or high risk of detecting cervical intraepithelial neoplasia (CIN) appears on the monitor screen. The examination protocol can be obtained in paper form or saved electronically for archiving purposes.
In the case of a "medium risk" result, the patient has two options for further care. They can undergo immediate colposcopy, which is available and often performed in the Russian Federation, with the possibility of biopsy and/or cervical curettage if indicated. Alternatively, they can choose to observe with a repeat screening examination within 4–6 months.
The aim of our study was to evaluate the diagnostic performance of fluorescence spectroscopy using LuViva technology for diagnosing CIN.
Materials and methods
The study included 156 patients aged 20–65 years who visited the Ott Research Institute for OG&R for routine preventive examination in 2020. The median patient age was 32 years (Q1–Q3:27–39 years). Exclusion criteria for the study were pregnancy, a history of radiation therapy to the genitourinary system within the last year, cervical abnormalities (e.g., cervical duplication), any heavy vaginal discharge during the study, contact bleeding, and hypersensitivity to light (porphyria, lupus erythematosus, phototherapy, recent fluoroquinolone, or retinoid therapy).
All the patients provided informed consent to participate in the study.
Research methods
All patients (n=156) underwent fluorescence spectroscopy of the cervical surface using LuViva technology and cytological examination of scrapings from the cervical surface and from the cervical canal using liquid cytology. No side effects were detected during the spectroscopy. The Bethesda classification system was used to describe the results of the cytological examination, according to which the conclusions were divided into the following categories: NILM (Negative for Intraepithelial Lesion or Malignancy) – absence of intraepithelial malignant changes or signs of malignancy in the examined smear; ASC-US; ASC-H (Atypical squamous cells cannot exclude HSIL) – squamous epithelial cells with atypia of unclear significance, not excluding HSIL; LSIL; HSIL. Along with the cytological test and fluorescence spectroscopy, 138/156 patients (88.5%) underwent a test for hrHPV in discharge from the cervical canal using real-time polymerase chain reaction (real-time PCR) to determine 14 types of viruses. Patients with the results of cytological examination of ASC-US and higher and with a positive HPV test (according to clinical recommendations) underwent extended colposcopy and biopsy according to indications with subsequent histological examination (n=52).
Statistical analysis
The analysis and visualization were performed using IBM SPSS Statistics 26 software. Quantitative variables are presented as the median (Me) and lower and upper quartiles (Q1 and Q3). Categorical variables are presented as counts and percentages. Comparisons of percentages in the analysis of 2×2 contingency tables were performed using Fisher's exact test (if the expected frequency was less than 10) and Pearson's chi-squared test (if the expected frequency was greater than or equal to 10). The effect size (strength of the relationship between features) was estimated using Cramer's V. As a quantitative measure of the effect of relative indicators, we used the odds ratio (OR) with 95% confidence interval (95% CI). The critical level of statistical significance for the null hypothesis (absence of significant differences or factor influences) was set at 0.05.
The initial matrix for calculating the performance measures of the diagnostic test consisted of four values obtained from the 2 × 2 contingency table (Table 1).
The main diagnostic characteristics are the sensitivity, specificity, and predictive value of positive and negative test results. Sensitivity (Se) is the probability that the test result will be positive in the presence of the disease: Se=TPR/(TPR+FNR). Specificity (Sp) is the probability that the test result will be negative in the absence of the disease: Sp=TNR/(TNR+FPR). The positive predictive value (PPV) is the probability that the disease is present when the test is positive; PPV=TPR/(TPR+FPR). The negative predictive value (NPV) is the probability that the disease is not present when the test is negative, and NPV=TNR/(TNR+FNR). Binomial 95% CI were calculated for the diagnostic characteristics of the test. The dividing line between cytological norms and pathology for assessing the diagnostic characteristics of the test was the ASC-US conclusion, according to the Bethesda terminology system, and the detection of ASC-H was equated to HSIL. A result ≥ASC-US was considered a positive result.
Graphical data processing was performed using Microsoft Office Excel 2019 and GraphPad Prism 9.1.0.
Results
hrHPV testing using real-time PCR was performed in 138 patients and was positive in 59/138 (43%) women, allowing us to assess the prevalence of papillomavirus infection in the study group. Multiple HPV types were detected simultaneously in 10/59 (17%) patients; the predominant HPV type in our sample was HPV type 16 in 41% (24/59). The distribution of the HPV types is shown in Figure 1.
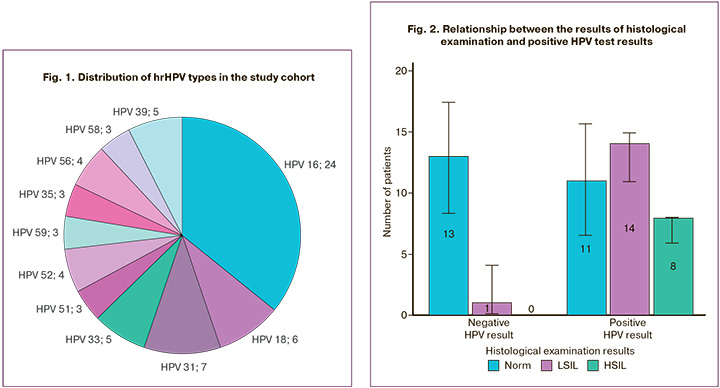
Analysis of the association between HPV positivity and HSIL based on histological examination of cervical biopsy specimens showed statistically significant differences (p=0.01). Thus, in the presence of HPV, the odds of detecting HSIL were almost 20 times higher (OR=19.96, 95% CI 1.04–384.61) (Fig. 2). A relatively strong relationship was observed between the compared features (V=0.478).
A wide 95% CI of OR was associated with insufficient statistical power, which requires further research.
As previously noted, cytological examination of scrapings from the surface of the cervix and from the cervical canal using liquid cytology and fluorescence spectroscopy using LuViva technology was performed in all patients.
Based on the results of LuViva fluorescence spectroscopy, a “low risk” of detecting intraepithelial lesions was detected in 69/156 (44%) patients (we considered this result negative). To standardize the fluorescence spectroscopy results, we combined patients with the “high” and “intermediate risk” conclusions into one group and considered this result positive; it was obtained in 87/156 women (56%).
Based on the cytological examination results, NILM was detected in the majority of patients (124/156), ASC-US was detected in 8/156 (5.13%), LSIL was diagnosed in 15/156 patients (9.6%), ASC-H in 6/156 patients, and HSIL in 3/156 patients (i.e., 9/156 (5.8%) patients had high-grade intraepithelial lesions).
Histological examination of the cervical samples was performed in 52/156 patients (33%) included in the study. According to the results, patients without intraepithelial lesions on cervical biopsy were predominant (n=26). LSIL was detected in 18 women, and HSIL in 8. Thus, among the patients included in the study, varying degrees of SIL were detected in 26 patients (50% of the total number of biopsies performed). We determined the diagnostic characteristics of the cytological test compared with the histological examination of cervical biopsies. ASC-US conclusions and higher were considered positive results of the cytological examination. According to our results, the sensitivity with respect to LSIL and HSIL was 35.3% and 50%, respectively, and the specificity was 91.4% and 93.2%, respectively. The positive predictive values of the cytology test for LSIL and HSIL were 66.7% and 57.1%, respectively, and the negative predictive values were 74.4% and 91.1%, respectively (Table 2).
Next, we compared the diagnostic performance of LuViva fluorescence spectroscopy with the histology of the cervical biopsy specimens. The sensitivity of fluorescence spectroscopy in detecting LSIL and HSIL was 100% and 75%, respectively, and the specificity was 34.3% and 22.7%, respectively. The positive predictive value (as already noted, we combined the “high” and “intermediate” risk scan conclusions into a positive result) for LSIL and HSIL was 42.5% and 15%, the negative predictive value of fluorescence spectroscopy (the conclusion is “low” risk) for LSIL and HSIL was 100% and 83.3%, respectively (Table 3).
Thus, fluorescence spectroscopy using the LuViva method in relation to histological examination in our study had sufficient sensitivity and high negative predictive value but showed low specificity.
We analyzed the diagnostic performance of LuViva fluorescence spectroscopy compared to cytology for different grades of SIL (ASC-US, LSIL, and HSIL) separately and then combined. This allowed us to conclude that the positive predictive value of fluorescence spectroscopy for detecting a cytology grade ≥ASC-US was 28.7%, and the negative predictive value was 88.4% (Table 4).
Therefore, LuViva fluorescence spectroscopy has a high negative predictive value and sensitivity for SIL of varying degrees, which increases its diagnostic value compared with cytological examination. LuViva allows for real-time results and the ability to perform more in-depth examinations, such as extended colposcopy and cervical biopsy, if necessary. Fluorescence spectroscopy also has significantly higher sensitivity and negative predictive value than histological and cytological methods, although its specificity is lower.
Currently, there is ongoing research to develop new noninvasive diagnostic methods that can improve the effectiveness of cervical screening. Many studies have been conducted to identify precancerous changes, increase the sensitivity of cytological examinations, and detect hrHPV. However, further research is required to determine the clinical significance and algorithms for using these methods.
The diagnostic performance measures of screening technologies, such as sensitivity, specificity, and positive and negative predictive values, are of great importance. Our data show that the LuViva diagnostic system has higher sensitivity than liquid cytology. Further studies with a larger number of patients are needed; however, based on our current results, we can conclude that non-invasive fluorescence spectroscopy can serve as a triage test for patients with conflicting results from cytological examination and HPV testing. This can help to determine whether immediate colposcopy or observation is the best course of action. Our study's results enabled us to secure a patent for our invention [17].
Conclusion
Florescence spectroscopy proves to be more sensitive than liquid cytology. The noninvasive technique of fluorescence spectroscopy can be used as a triage test for patients with conflicting cytology and HPV test results, helping to determine whether immediate colposcopy or dynamic observation is the most appropriate approach.
References
- Koliopoulos G., Nyaga V.N., Santesso N., Bryant A., Martin-Hirsch P.P., Mustafa R.A. et al. Cytology versus HPV testing for cervical cancer screening in the general population. Cochrane Database Syst. Rev. 2017; 8(8): CD008587. https://dx.doi.org/10.1002/14651858.CD008587.pub2.
- Venetianer R., Clarke M.A., van der Marel J., Tota J., Schiffman M., Dunn S.T. et al. Identification of HPV genotypes causing cervical precancer using tissue-based genotyping. Int. J. Cancer. 2020; 146(10): 2836-44. https://dx.doi.org/10.1002/ijc.32919.
- El-Zein M., Bouten S., Abdrabo L.S., Siblini A., Louvanto K., Franco E. et al. Genotyping and cytology triage of high-risk HPV DNA positive women for detection of cervical high-grade lesions. J. Low Genit. Tract. Dis. 2023; 27(1): 12-8. https://dx.doi.org/10.1097/LGT.0000000000000706.
- Bonde J., Floore A., Ejegod D., Vink F.J., Hesselink A., van de Ven P.M. et al. Methylation markers FAM19A4 and miR124-2 as triage strategy for primary human papillomavirus screen positive women: A large European multicenter study. Int. J. Cancer. 2021; 148(2): 396-405. https://dx.doi.org/10.1002/ijc.33320.
- Cosper P.F., Bradley S., Luo L., Kimple R.J. Biology of HPV mediated carcinogenesis and tumor progression. Semin. Radiat. Oncol. 2021; 31(4): 265-73. https://dx.doi.org/10.1016/j.semradonc.2021.02.006.
- Olivas A.D., Barroeta J.E., Lastra R.R. Overview of ancillary techniques in cervical cytology. Acta Cytol. 2023; 67(2): 119-28. https://dx.doi.org/10.1159/000528931.
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Цервикальная интраэпителиальная неоплазия, эрозия и эктропион шейки матки. 2020. [Ministry of Health of the Russian Federation. Clinical guidelines. Cervical intraepithelial neoplasia, erosion, and cervical ectropion. 2020. (in Russian)].
- Perkins R.B., Guido R.L., Saraiya M., Sawaya G.F., Wentzensen N., Schiffman M. et al. Summary of current guidelines for cervical cancer screening and management of abnormal test results: 2016-2020. J. Womens Health (Larchmt). 2021; 30(1): 5-13. https://dx.doi.org/10.1089/jwh.2020.8918.
- Сингер А. Новые оптико-электрические методики в скрининге предраковых заболеваний шейки матки. Материалы Международной научно-практической конференции «Профилактика рака шейки матки: взгляд в будущее». Москва, 31 марта-3 апреля 2008 г. М.; 2008: 130-1. [Singer A. New optical-electric techniques in cervical precancerous disease screening. Materials of the International Scientific and Practical Conference "Prevention of cervical cancer: a look into the future." Moscow, March 31-April 3, 2008. Moscow; 2008: 130-1. (in Russian)].
- Kendrick J.E., Huh W.K., Alvarez R.D. LUMA cervical imaging system. Expert Rev. Med. Devices. 2007; 4(2): 121-9. https://dx.doi.org/10.1586/17434440.4.2.121.
- Singer A., Coppleson M., Canfell K., Skladnev V., Mackellar G., Pisal N. et al. A real time optoelectronic device as an adjunct to the Pap smear for cervical screening: a multicenter evaluation. Int. J. Gynecol. Cancer. 2003; 13(6):804-11. https://dx.doi.org/10.1111/j.1525-1438.2003.13393.x.
- Pruski D., Kedzia W., Przybylski M., Józefiak A., Kedzia H., Spaczyński M. Ocena przydatności metody optoelektronicznej w wykrywaniu śródnabłonkowej neoplazji szyjki macicy [Assesment of real optoelectronic method in the detection of cervical intraepithelial neoplasia]. Ginekol. Pol. 2008; 79(5):342-6. (in Polish).
- Минкина Г.Н., Храмова О.К., Фириченко С.В. Клиническая эффективность оптикоэлектронной технологии TruScreen в диагностике цервикальной интраэпителиальной неоплазии. Вестник РГМУ. 2011; 4: 37-42. [Minkina G.N., Khramova O.K., Firichenko S.V. Clinical efficacy of TruScreen oprical-electronic technology in the diagnosis of cervical intraepithelial neoplasia. Bulletin of RSMU. 2011; (4): 37-42. (in Russian)].
- Wade R., Spackman E., Corbett M., Walker S., Light K., Naik R. et al. Adjunctive colposcopy technologies for examination of the uterine cervix--DySIS, LuViva Advanced Cervical Scan and Niris Imaging System: a systematic review and economic evaluation. Health Technol. Assess. 2013; 17(8): 1-240, v-vi. https://dx.doi.org/10.3310/hta17080.
- Awolude O.A., Akinwunmi B.O., Adewole I.F. Multimodal hyperspectroscopy screening in women at risk of cervical cancer: Results of a pilot study in a developing country. Trop. J. Obstet. Gynaecol. 2017; 34(2): 134-9. https://dx.doi.org/10.4103/TJOG.TJOG_31_17.
- Twiggs L.B., Chakhtoura N.A., Ferris D.G. Multimodal hyperspectroscopy as a triage test for cervical neoplasia: Pivotal clinical trial results. Gynecol. Oncol. 2013; 130(1): 147-51. https://dx.doi.org/10.1016/j.ygyno.2013.04.012.
- Патент на изобретение «Способ определения тактики ведения пациенток, которые составляют группу риска обнаружения плоскоклеточных интраэпителиальных поражений шейки матки». Хачатурян А.Р., Ярмолинская М.И. RU2751288C1, дата регистрации 12.07.2021. [Patent for the invention "Method for determining the management tactics of patients who are at risk of detecting squamous intraepithelial lesions of the cervix". Khachaturian A.R., Yarmolinskaya M.I. RU2751288C1, registration date 12.07.2021. (in Russian)].
Received 19.04.2024
Accepted 26.07.2024
About the Authors
Armine R. Khachaturian, PhD, Senior Researcher at the Department of Gynecology and Endocrinology, D.O. Ott Research institute of Obstetrics, Gynecology and Reproductology, 199034, Russia, St. Petersburg, Mendeleevskaya line, 3; Head of the Department of Outpatient Gynecology, Saint Petersburg State University Hospital; Associate Professor at the Department of Obstetrics, Gynecology and Reproductology, Saint-Petersburg State University, armine2709@mail.ru, eLibrary SPIN: 2691-3910, https://orcid.org/0000-0003-2141-6307Maria I. Yarmolinskaya, Dr. Med. Sci., Professor of the RAS, Head of the Department of Gynecology and Endocrinology, Head of the Center of Diagnostics and Treatment of Endometriosis, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, 199034, Russia, St. Petersburg, Mendeleevskaya line, 3; Professor at the Department of Obstetrics and Gynecology, I.I. Mechnikov North-Western State Medical University, Saint-Petersburg, Russia, m.yarmolinskaya@gmail.com,
eLibrary SPIN:3686-3605, https://orcid.org/0000-0002-6551-4147
Corresponding author: Armine R. Khachaturian, armine2709@mail.ru



