The role of sensitization to progesterone in improving the treatment of endometriosis-associated pelvic pain
Objective: To evaluate the effectiveness of complex treatment of patients with endometriosis and chronic pelvic pain (CPP) considering the preliminary diagnosis of their sensitization to progesterone.Bezhenar V.F., Molchanov O.L., Pastushenkov V.L., Konstandenkova A.S., Kuzmina N.S., Kruglov S.Yu., Gramatikova A.G.
Materials and methods: The study included 100 women of reproductive age with confirmed endometriosis and CPP. The patients of the main group were divided into four subgroups depending on the results of the diagnosis of sensitization to progesterone and on the administered hormone therapy with dienogest and dydrogesterone (group 1: sensitization to progesterone – surgical treatment+dienogest; group 2: sensitization to progesterone – surgical treatment + dydrogesterone; group 3: without sensitization to progesterone – surgical treatment+dienogest; group 4: without sensitization to progesterone – surgical treatment+dydrogesterone). Six months after surgery and therapy there was an assessment of the dynamics of pain intensity using a visual analogue scale (VAS).
Results: There was a statistically significant decrease in pain on the VAS scale in all four subgroups of patients who had experienced CPP, dysmenorrhea, and dyspareunia. A decrease in CPP and dyspareunia in patients with sensitization to progesterone was more significant in the subgroup that was treated with hormone therapy with dydrogesterone. A decrease in dysmenorrhea was not different in patients who were treated with dydrogesterone and dienogest.
Conclusion: The most effective option for postoperative treatment of endometriosis for patients with CPP and sensitization to progesterone is the therapy with dydrogesterone. The high selectivity of dydrogesterone to progesterone receptors provides clinical efficacy and a favorable safety profile of the medication.
Authors’ contributions: Bezhenar V.F., Molchanov O.L., Constandenkova A.S., Kuzmina N.S., Kruglov S.Yu., Gramatikova A.G. – developing the concept and design of the study, collecting, processing and analysis of the material, writing the text and editing; Pastushenkov V.L. – developing the concept of the study, editing.
Conflicts of interest: The authors declare that there are no conflicts of interest.
Funding: The study was conducted without sponsorship.
Ethical Approval: The study was approved by the Ethical Review Board of the Pavlov First Saint Petersburg State Medical University, Ministry of Health of Russia.
Patient Consent for Publication: The patients provided an informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Bezhenar V.F., Molchanov O.L., Pastushenkov V.L., Konstandenkova A.S.,
Kuzmina N.S., Kruglov S.Yu., Gramatikova A.G. The role of sensitization to progesterone
in improving the treatment of endometriosis-associated pelvic pain.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (7): 109-118 (in Russian)
https://dx.doi.org/10.18565/aig.2023.73
Keywords
Endometriosis is a chronic hormone- and immune-dependent genetic disease characterized by a benign growth of tissue similar to the endometrium in its morphological structure, but located outside the uterine cavity [1–3]. Endometriosis is the most severe and common disease of reproductive-aged women which affects their fertility, as well as reduces the quality of life, working capacity and general condition mainly due to chronic pelvic pain (CPP) syndrome [1–6].
Aberrant progesterone signaling in the endometrium is known to play a significant role in impaired decidualization and ectopic implantation of the endometrium. Ectopic endometrial cells in women with endometriosis are not able to decrease the regulation of genes involved in the regulation of the cell cycle, and their inability leads to uncontrolled proliferation.
Several causes of endometrial resistance to progesterone have been suggested including congenital “preconditioning” when the intrauterine environment contributes to the infant’s susceptibility to neonatal uterine bleeding and endometriosis [7]. The effect of progesterone is important in reducing inflammation in the endometrium, and deviant progesterone signaling can lead to a pro-inflammatory phenotype; therefore, chronic inflammation can induce the phenomenon of progesterone resistance [8]. Genetic causes of resistance to progesterone include polymorphism of genes of protein biomolecules responsible for progesterone synthesis, altered microRNA expression and epigenetic modifications of progesterone receptors in target tissues and organs [9, 10]. Resistance to progesterone is also aggravated by cyclic reflux of endometrial tissue via the fallopian tubes which results in the chronic inflammation of the peritoneum [11].
It is important to separate the two concepts: resistance to progesterone and sensitization to progesterone in terms of the dialectical concept “cause and effect”. Sensitization is the cause, and resistance is the effect.
Treatment of endometriosis and endometriosis-associated pain includes stages of surgical treatment and subsequent hormone therapy [2, 3, 12–14]. The drugs of choice for postoperative treatment of endometriosis are progestogens [2, 3, 12–15]. The preparations with the active substance dydrogesterone (10–30 mg/day), dienogest (2 mg/day) are more frequently used as progestogens [2, 3, 16].
The choice of a specific treatment regimen depends on the needs of the patient, the severity of symptoms and the response to treatment. In 2023, leading experts of the Russian Society of Obstetricians and Gynecologists developed and published “Algorithms for the management of patients with endometriosis” which help to individualize therapy depending on age, the presence/absence of reproductive plans, performed/not performed surgery and other factors [17]. Individual approach to the treatment of endometriosis makes it possible to achieve better treatment results [17].
However, if we consider therapy from the point of view of sensitization of patients to progesterone, it is worth noting the difference in the molecular structure of dydrogesterone and dienogest. Hormone therapy is ineffective in half of patients with endometriosis due to impaired action of progesterone [1, 2, 4]. The patients with endometriosis are considered to be resistant to progestogen therapy because their progesterone receptor apparatus is blocked by circulating immune complexes. The progesterone molecule is a heterocyclic organic complex based on cyclopentane perhydro phenanthrene. Type-specific antibodies do not form due to its small size. Nevertheless, the transport protein common to human progesterone and its exogenous analogues which come from outside with food and medicines take part in the formation of structures related to the category of haptens. Antibodies are produced to these structures and they are able to cross-block the progesterone-transport protein complex. The transport complex can form both directly in the blood plasma developing a non-functional toxic circulating immune complex and in the receptor apparatus of tissues blocking the regulatory hormonal transmission to the links of cellular metabolism. Progesterone deficiency is detected in the laboratory in the first case, and tissue deficiency of the receptor apparatus is detected immunohistochemically in the second case. Both cases have a universal approach to therapy, namely, exogenous administration of progesterone-type drugs (progestogens) [18].
According to a study by Cucinelli E. et al. (2017), women with endometriosis showed a statistically significantly higher prevalence of chronic endometritis compared to women without endometriosis (33/78 (42.3%) versus 12/78 (15.4%) on the basis of hysteroscopy analysis. This is due to the presence of a number of common etiopathogenetic factors; therefore, preparations that will affect both conditions should be used for the treatment [19].
The structural identity of progesterone preparations to a biologically active hormonal molecule synthesized by the body is probably a positive property of a pharmacological agent, but in the case of sensitization to progesterone, this position seems to be debatable. Moreover, it is possible to understand the facts of the paradoxical effect of prescribing such preparations which are manifested as local and systemic autoimmune reactions to varying degrees of severity.
Therefore, molecules with progesterone activity, but with stoichiometric modification, seem to be advantageous to be used. A greater affinity to the receptor apparatus is considered to be a potentiating effect of progesterone activity; the dydrogesterone molecule, in particular, has similar properties [18, 20–22]. It is probably due to stoichiometric features that the complex of the dydrogesterone molecule with transport protein does not have such a high degree of immunogenicity like synthesized structural analogues of progesterone.
The aim of the study was to evaluate the effectiveness of complex treatment of patients with endometriosis and CPP considering the preliminary diagnosis of their sensitization to progesterone.
Materials and methods
The study was conducted in the gynecology department in the Clinic of Obstetrics and Gynecology of the Pavlov First Saint Petersburg State Medical University in the period from September 2019 to April 2022.
The participants of the study were reproductive-aged patients (n=100) with endometriosis which was confirmed by laparoscopy and CPP (at least 3 points on a visual analog scale (VAS)). The patients were divided into two groups: women with sensitization to progesterone and those without it; then they were divided into four subgroups (Fig. 1):
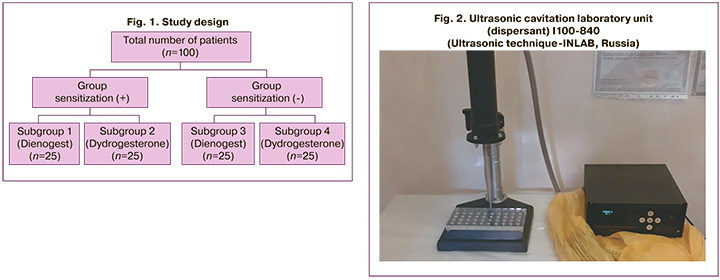
- subgroup 1 (n=25) included patients with sensitization to progesterone, CPP caused by endometriosis; women received surgical treatment and then hormone therapy with a drug with the active substance dienogest at a dose of 2 mg/day for 6 months continuously;
- subgroup 2 (n=25) included patients with sensitization to progesterone, CPP caused by endometriosis; women received surgical treatment and then hormone therapy with a drug with the active substance dydrogesterone at a dose of 20 mg/day in a prolonged cyclical regimen, from the 5th to the 25th day of the menstrual cycle for 6 months;
- subgroup 3 (n=25) included patients without sensitization to progesterone, CPP caused by endometriosis; women received surgical treatment and then hormone therapy with a drug with the active substance dienogest at a dose of 2 mg/day for 6 months continuously;
- subgroup 4 (n=25) included patients without sensitization to progesterone, CPP caused by endometriosis; women received surgical treatment and then hormone therapy with a drug with the active substance dydrogesterone at a dose of 20 mg/day in a prolonged cyclical regimen, from the 5th to the 25th day of the menstrual cycle for 6 months;
There were the following criteria for participation in the study: informed consent to participate in the study; diagnosis of endometriosis confirmed by clinical, laboratory and instrumental studies; CPP; age from 23 to 38 years; surgical treatment provided to all patients; dienogest administration after surgery to patients of the 1st and 3rd subgroups and dydrogesterone administration after surgery to patients of the 2nd and 4th subgroups; clinically insignificant concomitant diseases.
The exclusion criteria were as follows: personal refusal; mental, infectious, malignant pathologies; pregnancy; violation of the study protocol; age younger than 18 and older than 45 years; severe concomitant diseases.
All patients underwent laparoscopic surgical treatment. The patients underwent laparoscopic excision of infiltrative foci of endometriosis and ovarian cystectomy followed by histological verification of endometriosis.
Clinical examination. Taking a history; assessment of the CPP intensity, dyspareunia and dysmenorrhea using VAS; catamnestic survey of patients 6 months after surgical treatment and hormone therapy.
Instrumental studies. In order to determine the presence of sensitization to progesterone, we used the original author’s technique [23]: the patients’ blood plasma which was stabilized with buffered detergents and obtained regardless of the phase of the menstrual cycle was processed using an original ultrasonic laboratory cavitation unit (dispersant) (LLC Ultrasonic technique–INLAB, Russia) (Fig. 2) aimed at disintegration of circulating immune complexes (CIC) accompanied by the release of progesterone-transport protein complexes. Then the progesterone-transport protein complex was determined using a routine method with immunofluorescence detection. The level of progesterone in blood plasma was identified before and after ultrasound impact, and the presence of progesterone sensitization and the degree of its severity were assessed on the basis of an increase in progesterone levels.
The individual examples of changes in the progesterone content in blood serum before and after ultrasonic cavitation disintegration of immune complexes are presented in Table 1.
The study used the results which were obtained in the work on the initiative scientific project “Development of methods of ultrasonic disintegration of immune complexes in biological fluids to isolate free specific antigens and antibodies suitable for early and expert diagnosis” (code No. 59/06-31). The study was supported by Sevastopol State University.
There are the following stages in observation: the first stage of the study is the day of hospitalization; the second stage is the day of discharge from the hospital; the third stage is a 6-month period after the operation.
Statistical analysis
The statistical analysis of the obtained data was carried out on a personal computer using the R software environment, version 4.1.12. The variable “age” which has a normal distribution is represented as the mean and standard deviation M (SD). Normality was checked using the Shapiro–Wilk test. The data on the VAS scale are presented in the form of the median and interquartile intervals Me (Q1–Q3) for all subgroups regardless of the distribution; it is done for the convenience of comparing the values, since in some samples the distribution was not consistent with the Gaussian program. Binary data are presented in the form of the number and percentage of one of the n binary values (m%).
To identify the effect in the VAS dynamics, the Wilcoxon criterion was used. In order to compare the changes on the VAS scale, the Mann–Whitney U test was used taking into account the corrections for multiple comparisons using the Benjamini–Yekutieli method.
In all cases of hypothesis testing, a significance level of 0.05 was assumed to be statistically significant.
Results and discussion
A survey of the patients was conducted after their signing the informed consent and taking a history. Then all patients underwent laparoscopic excision of infiltrative foci of endometriosis and ovarian cystectomy followed by histological verification of endometriosis. It was histologically verified in 100% of the patients in the study.
Sensitization to progesterone was detected in 50% of patients, namely in subgroups 1 and 2; whereas there was no sensitization to progesterone in subgroups 3 and 4. The patients did not have any significant differences in the clinical characteristics of the types of endometriosis-associated pain assessed by VAS (Table 2), except for the difference in CPP between subgroups 1 and 2 (p<0.001) and between subgroups 2 and 3 (p=0.003).
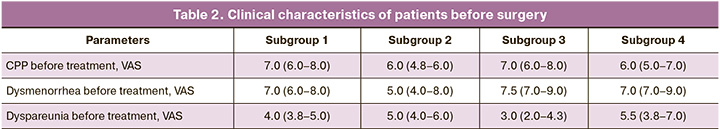
The survey of the patients which was conducted using VAS after the complex treatment showed differences among all the study subgroups according to the Kruskal–Wallis test; then a post-hoc analysis was carried out via pairwise comparisons using the Mann–Whitney U test taking into account the corrections for multiple comparisons using the Benjamini–Yekutieli method. The p-values for pairwise comparisons were adjusted according to this correction in the following analysis.
After the treatment, CPP was compared in the patients of the subgroups and significant differences were revealed among the subgroups, p<0.001 according to the Kruskal–Wallis test. After taking into account the correction, the pairwise comparison revealed differences between subgroups 1 and 2 (p<0.001), between subgroups 1 and 3 (p<0.001), between subgroups 1 and 4 (p<0.001) (Fig. 3). There were no significant differences between subgroups 2 and 3 (p=0.10), between subgroups 2 and 4 (p=1.00), and between subgroups 3 and 4 (p=0.06).
Pain in patients of subgroup 1 was reduced by 3.0 (2.0–4.0) points and it was less than in subgroup 2, namely by 4.0 (2.8–5.0) points (p=0.036), as well as in subgroup 3, by 6.0 (6.0–8.0) points (p<0.001), and in subgroup 4, by 4.5 (4.0–6.0) points (p<0.001). Pain reduction in subgroup 2 was significantly lower than in subgroup 3 (p<0.001). Pain reduction in subgroup 4 was lower than in subgroup 3 (p=0.007). There were no significant differences in pain reduction between subgroups 2 and 4 (p=0.20).
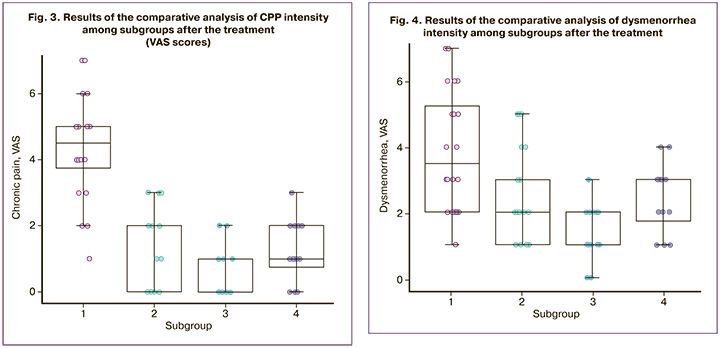
After the treatment, dysmenorrhea was compared in the patients of the subgroups and significant differences were revealed among the subgroups, p<0.001 according to the Kruskal–Wallis test. The pairwise comparison revealed significant differences between subgroup 1 with 3.5 (2.0–5.3) points and subgroup 2 with 2.0 (1.0–3.0) points (p=0.034), between subgroup 1 with 3.5 (2.0–5.3) points and subgroup 3 with 2.0 (1.0–2.0) points (p<0.001), as well as between subgroup 3 with 2.0 (1.0–2.0) points and subgroup 4 with 3.0 (1.8–3.0) points (p=0.023) (Fig. 4). There were no significant differences between subgroups 2 and 3 (p=0.46), as well as between subgroups 2 and 4 (p=1.00).
Pain in patients of subgroup 1 was reduced by 3.0 (2.0–4.0) points and it was less than in subgroup 3, namely by 6.0 (5.0-8.0) points (p=0.036), as well as in subgroup 4, by 4.5 (4.0–6.0) points (p=0.008). Pain in patients of subgroup 2 was reduced by 2.0 (2.0–4.3) points and it was less than in patients of subgroup 3, namely by 6.0 (5.0–8.0) points (p=0.002). There were no significant differences in pain reduction between subgroups 1 and 2 (p=1.00), subgroups 2 and 4 (p=0.06), and subgroups 3 and 4 (p=0.33).
After the treatment, dyspareunia was compared in the patients of the subgroups and significant differences were revealed among the subgroups, p=0.002 according to the Kruskal–Wallis test. However, the pairwise comparison showed differences only between subgroup 1 with 2.0 (1.0–3.0) points and subgroup 3 with 0.0 (0.0–1.0) points (p=0.003) (Fig. 5). There were no significant differences between subgroups 1 and 2 (p=0.39), between subgroups 1 and 4 (p=0.43), between subgroups 2 and 3 (p=0.38), between subgroups 2 and 4 (p=1.00), and between subgroups 3 and 4 (p=0.41).
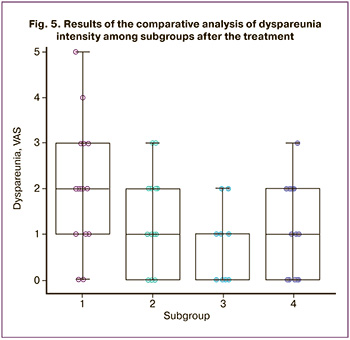
Pain in patients of subgroup 1 was reduced by 2.0 (1.8–3.0) points and it was lower than in subgroup 2, namely by 4.0 (2.8–5.0) points (p=0.038), as well as in subgroup 4, namely by 4.0 (2.0–6.3) points (p=0.030). Pain was reduced by 2.5 (1.8–4.0) points in subgroup 3, and it did not differ significantly from other subgroups, in particular, from subgroup 1 (p=1.00), subgroup 2 (p=0.46), and subgroup 4 (p=0.41). There were no differences between pain reduction in subgroups 2 and 4 (p=1.00), either.
The comparative analysis of the data obtained after the treatment showed significant differences in all parameters in all subgroups. The dynamics of changes in pain assessment parameters in the subgroups before and after the treatment was studied. The results of the study are presented in Tables 3, 4, 5, 6.
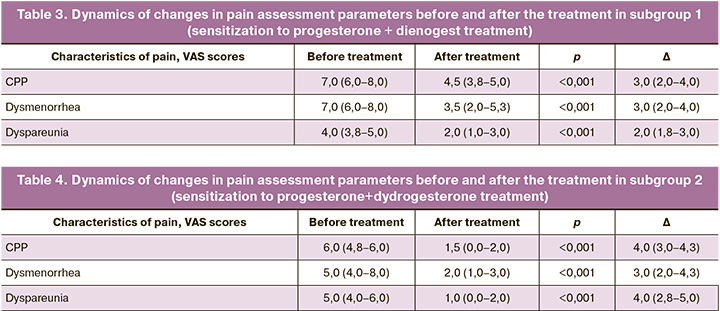
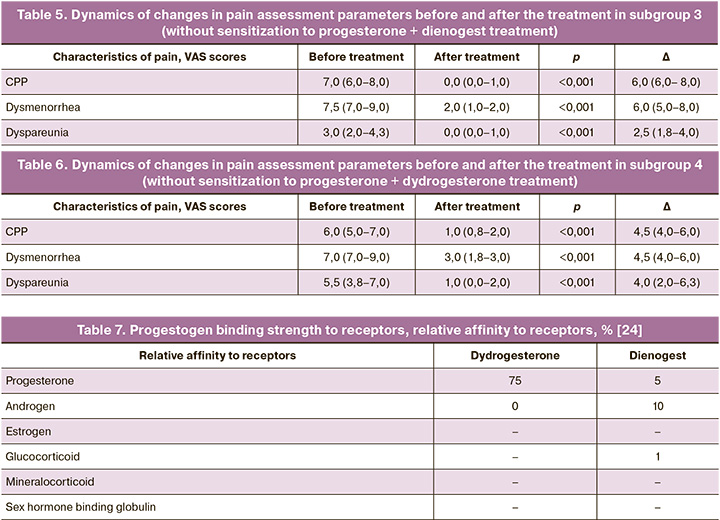
All the parameters decreased after the treatment. The results indicate an association between sensitization to progesterone and the effectiveness of complex therapy for endometriosis-associated pain.
Thus, the presence of sensitization to progesterone is a new and important criterion for the selection of hormone therapy after the surgical treatment of patients with endometriosis and CPP. The choice of a progestogen is determined by the degree of affinity of the drug molecule to progesterone receptors which determines its progestogenic activity, and by the degree of affinity with other receptors which determines additional, usually undesirable effects [24].
The results of the study demonstrate that the most effective treatment option for endometriosis in patients with sensitization to progesterone is dydrogesterone due to the lack of cross avidity of antibodies to the progesterone-transport protein complex in relation to the dydrogesterone-transport protein complex. The dydrogesterone molecule, unlike natural progesterone and its structural analogues, does not only have a typical radical structure, but it also has a characteristic stoichiometrically altered structure [18, 25]. It should be noted that high specificity and selectivity of dydrogesterone is due to the positioning of the C19 alpha-methyl group. A pharmacologically active metabolite with high progestogenic activity is formed as a result of metabolism [21].
Other studies have revealed that the unique features of the structure of the dydrogesterone molecule and its effect on the receptor apparatus of the cell determine a clinically significant value [12, 16, 18, 26–28]. The high selectivity of dydrogesterone to progesterone receptors provides a good safety profile of the drug [21]. Since there is no connection of dydrogesterone with androgen, glucocorticoid and mineralocorticoid receptors, many adverse reactions of this drug are missing [20] which limit the use of other progestogens (for example, dienogest) in women [29, 30]. Dienogest demonstrates affinity for progesterone receptors 15 times less, but the connection with other receptors increases the likelihood of undesirable side effects [20, 24] (Table 7).
Among all progestogens, dydrogesterone has the highest selectivity for progesterone receptors; it increases the effectiveness of therapy, especially in patients with reduced sensitivity of progesterone receptors at the time of treatment [24]. In comparison with progestogens used for the treatment of endometriosis, dydrogesterone does not have an antigonadotropic potential and does not cause the development of a hypoestrogenic condition in women; therefore, it can be prescribed to patients for a long time as a therapy for endometriosis.
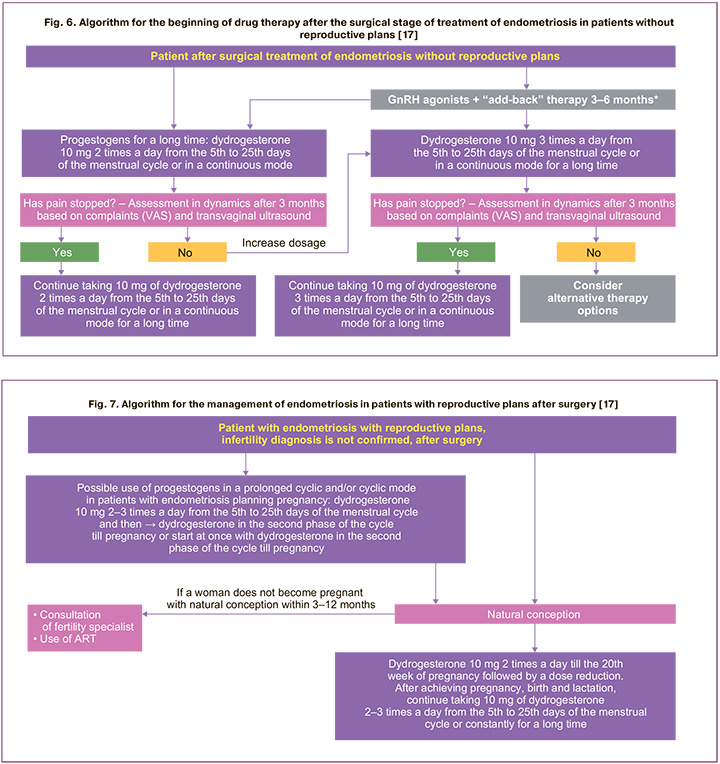
The results of our study are consistent with the position of leading experts in this area who issued algorithms for the management of patients with endometriosis [17]. The authors recommend to use the first line of therapy for the disease, that is progestogens and, in particular, dydrogesterone (Fig. 6, 7) after the surgical treatment (if necessary) [17].
Conclusion
Sensitization to progesterone is one of the most important criteria in the selection of hormone therapy in after surgical treatment for patients with endometriosis and CPP. The results of the study showed that progestogen therapy is an effective option for hormonal postoperative treatment in patients with endometriosis and CPP; a therapy with the drug with the active substance dydrogesterone should be preferred after the individual assessment of patients’ sensitization to progesterone.
The results of the study made it possible to develop and implement a personalized approach to the complex treatment of patients with endometriosis and CPP including the stage of determining sensitization to progesterone. This stage was identified on the basis of dynamic monitoring of the increase in progesterone content in blood serum after ultrasound cavitation. The new approach allows the clinicians to choose effective therapy and improve the quality of patients’ life in the postoperative period. Algorithms for the management of patients with endometriosis, and understanding of the mechanisms of progesterone resistance and medical tactics under these conditions are a universal tool that may help improve clinical approaches to the management of patients with endometriosis in everyday practice of each doctor.
References
- Министерство здравоохранения Российской Федерации. Эндометриоз. Клинические рекомендации. М.; 2020. [Ministry of Health of the Russian Federation. Endometriosis. Clinical guidelines. Moscow; 2020. (in Russian)].
- Дубровина С.О., Беженарь В.Ф., ред. Эндометриоз. Патогенез, диагностика, лечение. М.: ГЭОТАР-Медиа; 2020. 352с. [Dubrovina S.O., Bezhenar V.F., eds. Endometriosis. Pathogenesis, diagnosis, treatment. Moscow:GEOTAR-Media; 2020. 352 p. (in Russian)].
- ESHRE. ESHRE Guideline Endometriosis. 2 February, 2022.
- Адамян Л.В., Арсланян К.Н., Харченко Э.И., Логинова О.Н. Современные направления в медикаментозном лечении эндометриоза. Проблемы репродукции. 2019; 26(6): 58-66. [Adamyan L.V., Arslanyan K.N., Kharchenko E.I., Loginova O.N. Modern trends in the treatment of endometriosis. Russian Journal of Human Reproduction. 2019; 25(6): 58 66. (in Russian)].https://dx.doi.org/10.17116/repro20192506158.
- Ищенко А.И., Кудрина Е.А. Эндометриоз. Диагностика и лечение. М.: ГЭОТАР-МЕД; 2002. 104с. [Ishchenko A.I., Kudrina E.A. Endometriosis. Diagnosis and treatment. Moscow: GEOTAR-MED; 2002. 104p. (in Russian)].
- Краснопольский В.И., Буянова С.Н., Щукина Н.А., Попов А.А. Оперативная гинекология. 2-е изд. М.: МЕДпресс-информ; 2013. 320с. [Krasnopolsky V.I., Buyanova S.N., Shchukina N.A., Popov A.A. Operative gynecology. 2nd ed. Moscow: MEDpress-inform; 2013. 320p.(in Russian)].
- Sourial S., Tempest N., Hapangama D.K. Theories of the pathogenesis of endometriosis. Int. J. Reprod. Med. 2014; 2014: 179515.https://dx.doi.org/10.1155/2014/179515.
- Gargett C.E., Schwab K.E., Brosens J.J., Puttemans P., Benagiano G., Brosens I. Potential role of endometrial stem/progenitor cells in the pathogenesis of early-onset endometriosis. Mol. Hum. Reprod. 2014; 20(7): 591-8.https://dx.doi.org/10.1093/molehr/gau025.
- Al-Sabbagh M., Lam E.W., Brosens J.J. Mechanisms of endometrial progesterone resistance. Mol. Cell. Endocrinol. 2012; 358(2): 208-15. https://dx.doi.org/10.1016/j.mce.2011.10.035.
- Pabalan N., Salvador A., Jarjanazi H., Christofolini D.M., Barbosa C.P., Bianco B. Association of the progesterone receptor gene polymorphism (PROGINS) with endometriosis: a meta-analysis. Arch. Gynecol. Obstet. 2014; 290(5): 1015-22. https://dx.doi.org/10.1007/s00404-014-3308-3.
- Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004; 116(2): 281-97. https://dx.doi.org/10.1016/s0092-8674(04)00045-5.
- Беженарь В.Ф., Круглов С.Ю., Кузьмина Н.С., Крылова Ю.С., Сергиенко А.С., Абилбекова А.К., Жемчужина Т.Ю. Целесообразность длительной гормональной терапии эндометриоза после хирургического лечения. Акушерство и гинекология. 2021; 4: 134-42. [Bezhenar V.F., Kruglov S.Yu., Kuzmina N.S., Krylova Yu.S., Sergienko A.S., Abilbekova A.K., Zhemchuzhina T.Yu. Effectiveness of long-term hormone therapy for endometriosis after surgical treatment. Obstetrics and Gynecology. 2021; (4): 134-42. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.4.134-142.
- Улумбекова Г.Э., Худова И.Ю. Оценка демографического, социального и экономического эффекта применения гормональной терапии при эндометриозе и аномальных маточных кровотечениях. Оргздрав: Новости. Мнения. Обучение. Вестник ВШОУЗ. 2022; 8(1): 82-113. [Ulumbekova G.E., Khudova I.Yu. Demographic, social and economic effects of hormonal therapy in endometriosis and abnormal uterine bleeding. HEALTHCARE MANAGEMENT: News. Views. Education. Bulletin of VSHOUZ. 2022; 8(1): 82-113. (in Russian)]. https://dx.doi.org/10.33029/2411-8621-2022-8-1-82-113.
- Беженарь В.Ф. Гормональная терапия в лечении эндометриоза. Инновационная фармакотерапия. 2022; 2(6): 9-12. [Bezhenar V.F. Hormonal therapy in the treatment of endometriosis. Innovative Pharmacotherapy. 2022; 2(6): 9-12. (in Russian)].
- Баскаков В.П., Цвелев Ю.В., Кира Е.Ф. Эндометриоидная болезнь. СПб.: Издательство Н-Л; 2002. 452с. [Baskakov V.P., Tsvelev Yu.V., Kira E.F. Endometrioid disease. St. Petersburg: N-L Publishing House; 2002. 452p.(in Russian)].
- Сухих Г.Т., Адамян Л.В., Козаченко А.В., Дубровина С.О., Баранов И.И., Радзинский В.Е. и др. Дидрогестерон для лечения подтвержденного эндометриоза: ключевые результаты наблюдательного открытого многоцентрового исследования в условиях реальной клинической практики (исследование ОРХИДЕЯ). Акушерство и гинекология: новости, мнения, обучение. 2020; 8(4): 79-81. [Sukhikh G.T., Adamyan L.V., Kozachenko A.V., Dubrovina S.O., Baranov I.I., Radzinsky V.E. et al. Didrogesterone for the treatment of confirmed endometriosis: key results of an observational open multicenter study in real clinical practice (the ORCHID study). Obstetrics and Gynecology: News, Opinions, Training. 2020; 8(4): 79-81. (in Russian)]. https://dx.doi.org/10.24411/2303-9698-2020-14006.
- Сухих Г.Т., Серов В.Н., Адамян Л.В., Баранов И.И., Беженарь В.Ф., Габидуллина Р.И., Дубровина С.О., Козаченко А.В., Подзолкова Н.М., Сметник А.А., Тапильская Н.И., Уварова Е.В., Ших Е.В., Ярмолинская М.И. Алгоритмы ведения пациенток с эндометриозом: согласованная позиция экспертов Российского общества акушеров-гинекологов. Акушерство и гинекология. 2023; 5: 159-76. [Sukhikh G.T., Serov V.N., Adamyan L.V., Baranov I.I., Bezhenar V.F., Gabidullina R.I., Dubrovina S.O., Kozachenko A.V., Podzolkova N.M., Smetnik A.A., Tapilskaya N.I., Uvarova E.V., Shikh E.V., Yarmolinskaya M.I. Algorithms for the management of patients with endometriosis: an agreed position of experts from the Russian Society of Obstetricians and Gynecologists. Obstetrics and Gynecology. 2023; (5): 159-76. (in Russian)]. https://dx.doi.org/10.18565/aig.2023.132.
- Молчанов О.Л., Беженарь В.Ф., Аракелян Б.В., Коршунов М.Ю., Кира Е.Ф., Лебедева Я.А. К вопросу о механизме сенсибилизации к прогестерону. Вопросы гинекологии, акушерства и перинатологии. 2019; 18(4): 109-14. [Molchanov O.L., Bezhenar V.F., Arakelyan B.V., Korshunov M.Yu., Kira E.F., Lebedeva Ya.A. On the mechanism of progesterone sensitization. Gynecology, Obstetrics and Perinatology. 2019; 18(4): 109-14. (in Russian)]. https://dx.doi.org/10.20953/1726-1678-2019-4-109-114.
- Cicinelli E., Trojano G., Mastromauro M., Vimercati A., Marinaccio M., Mitola P.C., Resta L., de Ziegler D. Higher prevalence of chronic endometritis in women with endometriosis: a possible etiopathogenetic link. Fertil. Steril. 2017; 108(2): 289-95.e1. https://dx.doi.org/10.1016/j.fertnstert.2017.05.016.
- Schindler A.E., Campagnoli C., Druckmann R., Huber J., Pasqualini J.R., Schweppe K.W., Thijssen J.H. Classification and pharmacology of progestins. Maturitas. 2008; 61(1-2): 171-80. https://dx.doi.org/10.1016/j.maturitas.2008.11.013.
- Ших Е.В. Клинико-фармакологические аспекты применения дидрогестерона для сохранения беременности. Акушерство, гинекология, репродукция. 2010; 4(2): 6-9. [Shikh E.V. Clinical-pharmacological aspects of the use of dydrogesterone in pregnancy maintenance. Obstetrics, Gynecology and Reproduction. 2010; 4(2): 6-9. (in Russian)].
- Orazov M.R., Radzinskiy V.E., Nosenko E.N., Khamoshina M.B., Lebedeva M.G., Tokaeva E.S. et al. Combination therapeutic options in the treatment of the luteal phase deficiency. Gynecol. Endocrinol. 2017; 3(Suppl. 1): 1-4.https://dx.doi.org/10.1080/09513590.2017.1399695.
- Пастушенков В.Л., Беженарь В.Ф., Молчанов О.Л. Способ диагностики резистентности к прогестерону. Приоритетная справка на изобретение №2022115595 от 08.06.2022 входящий №032770. [Pastushenkov V.L., Bezhenar V.F., Molchanov O.L. Method of diagnosis of progesterone resistance. Priority certificate for invention No. 2022115595 dated 08.06.2022 incoming No. 032770. (in Russian)].
- Schindler A.E. Progestational effects of dydrogesterone in vitro, in vivo and on the human endometrium. Maturitas. 2009; 65(Suppl. 1): S3-11.https://dx.doi.org/10.1016/j.maturitas.2009.10.011.
- Торшин И.Ю., Громова О.А., Сухих Г.Т., Галицкая С.А., Юргель И.С. Молекулярные механизмы дидрогестерона. Полногеномное исследование транскрипционных эффектов рецепторов прогестерона, андрогенов и эстрогенов. Гинекология. 2009; 11(5): 9-15. [Torshin I.Yu., Gromova O.A., Sukhikh G.T., Galitskaya S.A., Yurgel I.S. Molecular mechanisms of didrogesterone. A genome-wide study of the transcriptional effects of progesterone receptors, androgens and estrogens. Gynecology. 2009; 11(5): 9-15. (in Russian)].
- Сидельникова В.М. Подготовка и ведение беременности у женщин с привычным невынашиванием: методические пособия и клинические протоколы. М.: МЕДпресс-информ; 2010. 224с. [Sidelnikova V.M. Preparation and management of pregnancy in women with habitual miscarriage: methodological manuals and clinical protocols. Moscow: MEDpress-inform; 2010. 224p.(in Russian)].
- Радзинский В.Е., Соловьёва А.В., Бриль Ю.А. Улучшение репродуктивных исходов: какими возможностями располагает современный врач? StatusPraesens. Гинекология, акушерство, бесплодный брак. 2018; 1: 45-50. [Radzinsky V.E., Solovyova A.V., Bril Yu.A. Improving reproductive outcomes: what opportunities does a modern doctor have? StatusPraesens. Gynecology, Obstetrics, Infertile Marriage. 2018; (1): 45-50. (in Russian)].
- Sukhikh G.T., Adamyan L.V., Dubrovina S.O., Baranov II, Bezhenar VF, Kozachenko AV, Radzinsky VE, Orazov MR, Yarmolinskaya M.I., Olofsson J.I. Prolonged cyclical and continuous regimens of dydrogesterone are effective for reducing chronic pelvic pain in women with endometriosis: results of the ORCHIDEA study. Fertil. Steril. 2021; 116(6): 1568-77.https://dx.doi.org/10.1016/j.fertnstert.2021.07.1194.
- Nirgianakis K., Vaineau C., Agliati L., McKinnon B., Gasparri M.L., Mueller M.D. Risk factors for non-response and discontinuation of Dienogest in endometriosis patients: A cohort study. Acta Obstet. Gynecol. Scand. 2021; 100(1): 30-40. https://dx.doi.org/10.1111/aogs.13969.
- Kim S.E., Lim H.H., Lee D.Y., Choi D. The long-term effect of dienogest on bone mineral density after surgical treatment of endometrioma. Reprod. Sci. 2021; 28(5): 1556-62. https://dx.doi.org/10.1007/s43032-020-00453-7.
Received 21.03.2023
Accepted 17.07.2023
About the Authors
Vitaly F. Bezhenar, Dr. Med. Sci., Professor, Head of the Department of Obstetrics, Gynecology and Neonatology/Reproductology, Head of the Clinic of Obstetrics and Gynecology, Pavlov First Saint Petersburg State Medical University, Ministry of Health of Russia, +7(812)338-78-66, bez-vitaly@yandex.ru,https://orcid.org/0000-0002-7807-4929, 197022, Russia, St. Petersburg, Leo Tolstoy str., 6-8.
Oleg L. Molchanov, Dr. Med. Sci., Professor at the Department of Obstetrics, Gynecology and Reproductology, Pavlov First Saint Petersburg State Medical University,
Ministry of Health of Russia, +7(812)338-78-66, moleg700@mail.ru, https://orcid.org/0000-0003-3882-1720, 197022, Russia, St. Petersburg, Leo Tolstoy str., 6-8.
Vlavimir L. Pastushenkov, Dr. Med. Sci., Professor, Chief Researcher, St. Petersburg Research Institute of Ear Throat and Speech, Ministry of Health of Russia,
+7(812)676-00-76, pastprof@mail.ru, https://orcid.org/0000-0002-4957-0181, 190013, Russia, St. Petersburg, Bronnitskaya str., 9.
Alina S. Konstandenkova, postgraduate student of the Department of Obstetrics, Gynecology and Reproductology, Pavlov First Saint Petersburg State Medical University, Ministry of Health of Russia, +7(812)338-78-66, dr.konstandenkova@gmail.com, https://orcid.org/0000-0002-6362-107X, 197022, Russia, St. Petersburg, Leo Tolstoy str., 6-8.
Natalya S. Kuzmina, PhD, Assistant at the Department of Obstetrics, Gynecology and Neonatology, Head of the Department of Oncogynecology No. 7 of the Clinic of Obstetrics and Gynecology, Pavlov First Saint Petersburg State Medical University, Ministry of Health of Russia, +7(812)338-78-66, https://orcid.org/0000-0002-1675-4144, 197022, Russia, St. Petersburg, Leo Tolstoy str., 6-8.
Svyatoslav Yu. Kruglov, PhD, Assistant at the Department of Obstetrics, Gynecology and Reproductology, Pavlov First Saint Petersburg State Medical University,
Ministry of Health of Russia, +7(812)338-78-66, skruglov89@mai.ru, https://orcid.org/0000-0002-7807-4929, 197022, Russia, St. Petersburg, Leo Tolstoy str., 6-8.
Ana G. Gramatikova, postgraduate student of the Department of Obstetrics, Gynecology and Neonatology, Pavlov First St. Petersburg State Medical University,
Ministry of Health of Russia, +7(812)338-78-66, frau.gramatikova@yandex.ru, https://orcid.org/0000-0001-7463-1831, 197022, Russia, St. Petersburg, Leo Tolstoy str., 6-8.



