Polymorphism of the CYP11A1, CYP17A1, and CYP19A1 genes in reproductive-aged women with polycystic ovary syndrome
Objective. To investigate the polymorphism of the CYP11A1, CYP17A1, and CYP19A1 genes in reproductive-aged women with polycystic ovary syndrome (PCOS) versus those without the latter.Beglova A.Yu., Elgina S.I., Gordeeva L.A.
Subjects and methods. The investigation enrolled 188 reproductive-aged women. Group 1 consisted of 94 women with PCOS; Group 2 included 94 women without PCOS. All the patients underwent molecular genetic analysis of SNP polymorphisms of the VNTR genes of the pentanucleotide ((tttta)n) polymorphism at position -528 of the CYP11A and CYP17A1 gene promoter region (-34T > C (MspA1), rs743572) and CYP19A1 (c.-39+15658 C>T, C40824T, rs2470152) using the test system made by the OOO SibDNA (Novosibirsk). The amplification reaction was carried out using a CFX96 real-time PCR detection system (Bio-Rad, USA), followed by statistical data processing.
Results. The frequency distribution of CYP11A1 (tttta)n genotypes, CYP17A1 rs743572 and CYP19A1 rs2470152 genes did not statistically significantly differ in PCOS and healthy women (p > 0.05). However, the CYP11A1 (tttta)n polymorphism tended to accumulate alleles with a larger number of (tttta)n repeats in PCOS women than in healthy ones. The VNTR genotypes with 6/6, 6/8, and 8/8 pentanucleotide repeats were typical.
Conclusion. The genetic factor did not seem to play a key role in the development of PCOS in the examined women. This study may be useful for subsequent meta-analyses that can reveal insights into the pathogenesis of the disease.
Keywords
According to available data, polycystic ovary syndrome (PCOS) is a common disease; it belongs to one of the most urgent problems in modern gynecology and is characterized by a wide individual variety of clinical manifestations [1]. PCOS is a heterogeneous group of disorders with wide clinical and biochemical variability, in which chronic anovulation is an outcome of the impaired feedback mechanism in the hypothalamic-pituitary system. This polyendocrine syndrome is accompanied by impaired function of the ovaries and other endocrine glands [2, 3]. In the population it can be found in every tenth woman of reproductive age, and according to some data – in every fifth woman. The frequency of PCOS ranges from 6.0% to 20.0% [4]. The mechanisms of PCOS development are studied extensively at the level of the hypothalamic-pituitary complex, ovaries, adrenal glands, adipose tissue.
PCOS is proved to be associated with infertility. However, the mechanisms through which PCOS affects the reproductive function remain relevant and controversial. PCOS is assumed to be associated with impairment of the gonadotropin and steroid secretion, folliculogenesis and ovulation defect that result in the disruption of the endometrium development, reduced secretion of estradiol in granulosa cells. Along with reproductive disorders, PCOS is associated with insulin resistance, impaired carbohydrate metabolism, mental status, and cardiovascular diseases. Despite the long history of the study, the details of the causes, pathogenesis and pathophysiology of the disease are not fully understood; the search for a single fundamental mechanism to explain true nature of the disease is not completed.
PCOS is a polygenic endocrine disorder caused by both hereditary and environmental factors. The contribution of genetic factors to the PCOS etiology is 79.0%, and that of the environment, lifestyle and individual case history is 21.0% [3]. Genetic theory of PCOS development is relevant and modern and it is actively studied in the development of the disease [5, 6].
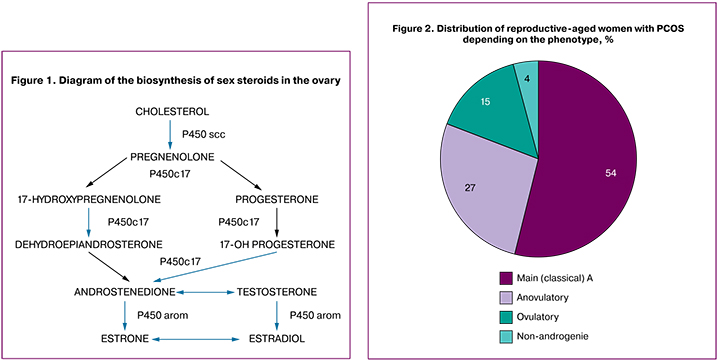
The secretion of all the steroidogenic precursors of the androgen biosynthesis occurs in the theca cells of the ovaries in women with PCOS. Cytochrome P450arom (CYP19A1) catalyzes aromatization of androgens into estrogens. The cytochrome P450 system plays a key role in ovarian function, folliculogenesis, follicle growth and development. The starting stage of steroidogenesis is the conversion of cholesterol into pregnenolone, which is catalyzed by the enzyme cleavage of the side chain of cholesterol or P450scc (Fig. 1). P450c17α catalyzes the synthesis of 17-hydroxypregnenolone and 17-OH progesterone from pregnenolone and progesterone, respectively, with the subsequent conversion of these steroids into dehydroepiandrosterone and androstenedione. P450c17α is the main link in the biosynthesis of androgens in the ovaries and adrenal glands [7].
A number of genetic polymorphisms associated with the enzymatic complex of cytochrome P-450 (CYP) are considered to play a pivotal role in the pathogenesis of PCOS.
The СYР11А1 gene (the gene of the enzyme of the side-chain cleavage of cholesterol encodes Р450ѕсс enzyme) is regarded as a gene candidate for PCOS. The increased enzyme activity of the CYP11A accounts for the increased production of androgens. Allelic variants of CYP11A1 gene may alter its expression and be associated with many diseases including PCOS [8]. The CYP17 gene encodes the P450c17α enzyme that has both 12 α - hydroxylase and 17, 20-lyase activity. The CYP19 gene encodes aromatase (P450arom), which converts C19-steroids (androgens) into C18-steroids (estrogens). It is assumed that genetic alterations in CYP19 may cause the decrease in aromatase activity in granulosa cells followed by an excess of androgens and blocking the development of follicles [9].
It can also be assumed that the decrease in aromatase activity is present in PCOS [10].
Genetic theory of PCOS is of critical importance in the pathogenesis of the disease and identification of genetic markers of pathology. Genetic polymorphism studies have the potential to provide insight into genetic aspects of PCOS etiology.
Materials and Methods
The study was conducted on the basis of the City Clinical Polyclinic №5, Kemerovo. Informed consent was obtained from all patients. The study was approved by the local ethics committee.
This was a retrospective analytical case-control study. All patients were divided into two groups: the main group enrolled 94 patients with PCOS and the comparison group involved 94 healthy women without PCOS. Women of reproductive age with the diagnosis of PCOS who signed an informed consent to participate in the study were eligible for inclusion in the main group. Exclusion criteria from the main group were age of women, namely under 18 and over 35 years; absence of consent to participate in the study; taking hormones and combined oral contraceptives. Criteria for inclusion in the comparison group were absence of PCOS, infertility, severe somatic diseases, or presence of somatic pathology in the stage of compensation in healthy women of reproductive age. Exclusion criteria from the comparison group were age of women, namely under 18 and over 35 years; infertility in women of reproductive age, severe somatic pathology in the stage of decompensation; refusal to participate in the study; taking hormonal therapy and combined oral contraceptives.
The diagnosis of PCOS was made on the basis of the clinical protocol criteria “PCOS in Women of Reproductive Age: Current Approaches to Diagnosis and Treatment” (Moscow, 2015) [17].
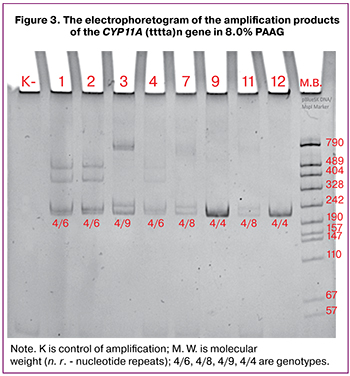
Distribution of reproductive-aged women with PCOS depending on the phenotype is given in Figure 2.
The most common phenotype in women of reproductive age with PCOS was the main (classical) type that occurred in 51 (54.2%) women. Other phenotypes were less common.
The analysis of the health status of reproductive-aged women was conducted on the basis of incidence of outpatient visits and preventive clinical examinations.
DNA isolation from peripheral blood lymphocytes was performed with the method of phenol-chloroform extraction followed by precipitation with ethanol (Sambrook J., Fritsch E.F., Maniatis T.). DNA samples were stored at the temperature -20° C.
 Genotyping. Test systems of “SibDNA” Company (Novosibirsk) were used for molecular genetic analysis of the SNP-polymorphisms of the VNTR of CYP11A gene (pentanucleotide ((tttta)n) polymorphism at position -528 of the promoter region of the CYP11A), CYP17A1 (rs743572) and CYP19A1 (rs2470152) genes.
Genotyping. Test systems of “SibDNA” Company (Novosibirsk) were used for molecular genetic analysis of the SNP-polymorphisms of the VNTR of CYP11A gene (pentanucleotide ((tttta)n) polymorphism at position -528 of the promoter region of the CYP11A), CYP17A1 (rs743572) and CYP19A1 (rs2470152) genes.
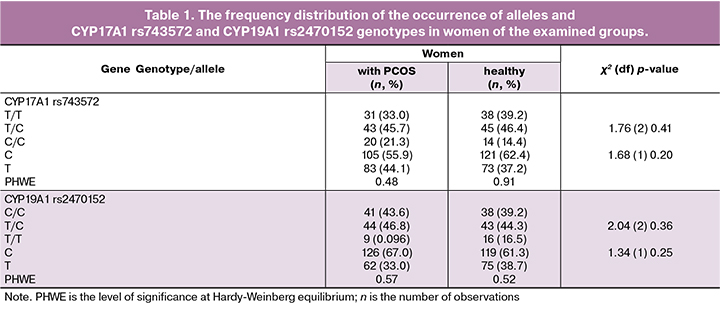
Pentanucleotide (tttta)n polymorphism of the CYP11A gene was determined by electrophoretic separation of amplification products in 8% polyacrilomide gel (PAAG) (Fig. 3). The VNTR alleles in the CYP11A1 gene were denoted as follows: allele 4 contained four tandem (tttta)n repeats; allele 6 — six tandem (tttta)n repeats; allele 8 — eight tandem (tttta)n repeats; allele 9 – nine tandem (tttta)n repeats; allele 10 — ten tandem (tttta)n repeats (Fig. 3).
The amplification reaction was carried out on a thermal cycler “Tertsik” (“DNA Technology”, Russia) under the following conditions: denaturation (95°C – 3 minutes), 32 cycles at 92°C - 10 seconds, 68°C – 10 seconds, 72°C – 10 seconds, final synthesis (72°C – 3 minutes). The total volume of the reaction mixture was 15 µl. Visualization of amplification products was carried out while applying a video system “GelDoc XR+ System” (Bio-Rad, USA) for documenting gels.
Polymorphism typing of the CYP17A1 (rs743572) gene was conducted with the method of asymmetric polymerase chain reaction in real-time PCR using a fluorescently-labeled oligonucleotide probe that was complementary to the DNA region under investigation. The total volume of the reaction mixture was 20 µl. The amplification reaction was performed under the following conditions: initial denaturation - 3 minutes at 96°C; then 40 cycles including denaturation at 96°C – 8 seconds, annealing primers and subsequent elongation at 60°C – 35 seconds (each step was accompanied by the registration of a fluorescent signal in the ranges corresponding to the fluorescence intervals of fluorophores).
The polymorphism typing of the CYP19A1 (rs2470152) gene was performed with TaqMan Real-time PCR. The amplification reaction was carried out under the following conditions: initial denaturation (96°C – 3 minutes); then 50 cycles including denaturation at 96°C – 8 seconds, annealing of primers at 58°C – 40 seconds and subsequent elongation at 72°C – 8 seconds. The total volume of the reaction mixture was 20 µl.
Amplification was performed using CFX96 (Bio-Rad, USA) thermal cycler.
Statistical processing of the findings was performed with StatSoft Statistica 6.1, IBM SPSS Statistics 20.0 application software package. The nature of the data distribution was estimated using the Shapiro-Wilk criterion. Quantitative data are presented by central trends and scattering: arithmetic mean (M) and standard deviation (SD) in M (SD) format.
The comparison of two independent groups with normal distribution was made with Student’s t-test. The null hypothesis was rejected at p≤0.05 in this case and in cases with other criteria.
The correspondence of genotype frequencies in gene polymorphic variants under the study to the Hardy-Weinberg equilibrium was checked by χ2 criterion. Pairwise comparison of frequencies of alleles and genotypes of the genes under the study was carried out using the χ2 criterion and the two-sided accurate Fisher test (at n <5).
Results
The main causes for outpatient visits of women with PCOS were infertility (primary - in 41 women (43.6%), secondary – in 11 women (11.7%); menstrual cycle disorders: oligo/amenorrhea - in 28 women (29.7%); menstrual cycle disorders – oligo/amenorrhea and primary infertility – in 14 women (14.8%).
The average age of women in the groups under the study had no statistically significant differences (28.2 (2.3) versus 28.6 (1.7)) (p = 0.92). At the next stage of the study, the effect of the genetic factor on the risk for PCOS development was studied.
The analysis of distribution of genotype frequencies of the CYP17A1 (rs743572) and CYP19A1 (rs2470152) genes in the groups of women with PCOS and healthy women for their compliance to Hardy-Weinberg equilibrium showed that the observed frequency of genotypes of the studied polymorphic variants of genes in both groups coincides with their expected frequencies (PHWE>0.05, Table 1).
The comparison of frequencies of alleles and genotypes of the CYP17A1 (rs743572) and CYP19A1 (rs2470152) genes in women from the examined groups did not reveal any statistical differences between them (p > 0.05).
According to literature sources, A2 (-34C) allele in the CYP17A1 gene has an enhanced transcription rate, so it is assumed that its carriers may experience an increase in the activity of the 17-alpha-hydroxylase enzyme and, accordingly, the synthesis of steroids is increased. The analysis of the literature shows that the relationship between the polymorphism of the CYP17A1 -34T>C (rs743572) gene and PCOS is not evident. Therefore, R. Kaur et al. (2018) pointed to the relationship of polymorphism - 34T>C of the CYP17A1 gene with PCOS; while others, on the contrary, consider that A2 (-34C) allele has a minor role in the development of PCOS, but may affect the hyperandrogenic phenotype [13-17].
Some genetic studies show the existence of association between polymorphism in the CYP19A1 (rs2470152) gene and the risk for PCOS [18]. N. Gharani proposed a hypothesis according to which the change in the structure of the gene encoding P450arom reduces the aromatase activity in granulosa cells. It is assumed that the CYP19A1gene is one of the key factors responsible for the PCOS etiopathogenesis, especially in adolescence. This may be caused by the activity of the aromatase enzyme. Gene polymorphism of the CYP19A1 (rs2470152) gene affects the activity of the enzyme aromatase, catalyzing the conversion of testosterone and androstenedione to estradiol and estrone. It is assumed that an excess of androgens in girls may contribute to menarche at an earlier age [19-21]. On the other hand, there are research articles that do not confirm the relationship of rs2470152 polymorphism in the CYP19A1 gene with the development of PCOS in women of certain ethnic groups [22-26]. On the basis of literature data and own research, it can be assumed that the polymorphic loci of CYP17A1 (rs743572) and CYP19A1 (rs2470152) genes are not major risk factors for PCOS in the women examined but they can affect the clinical picture of PCOS, thus, the study needs to be continued.
The polymorphism of the promoter region of the CYP11A1 gene is found to involve a different number of pentanucleotide repeats (tttta)n starting from position -528. The increased production of androgens proved to correlate with a large number of n-repeats (tttta)n in women and is associated with the risk for PCOS [9]. Therefore, we further studied the frequency distribution of genotype polymorphism of the CYP11A1 (tttta)n gene in two groups - in women with PCOS and in healthy women (Table 2).
Comparison of the genotype frequencies of the CYP11A1 (tttta)n gene in women of the groups involved in the study showed no statistically significant difference between them (p > 0.05). It should be noted that the most frequently genotype detected in women in both groups was 4/4 genotype (45.7% in PCOS and 51.5% in healthy women, respectively). At the same time, there was a tendency for women with PCOS to accumulate alleles with more (tttta) n-repeats than in healthy women. The VNTR genotypes with 6/6, 6/8 and 8/8 pentanucleotide repeats were typical. The number of the women examined may be not enough to allow us to identify any clear pattern of association of polymorphism of the CYP11A1 (tttta)n gene with PCOS, so it is necessary to continue the study.
Conclusion
The study revealed no association between the polymorphic loci of the CYP11A1 (tttta)n, CYP17A1 rs743572 and CYP19A1 rs2470152 genes and the risk of PCOS in the Russian population. The genetic factors in the examined women do not seem to play a key role in the development of PCOS. The study conducted demonstrates that these polymorphisms do not have any role, but this does not exclude the genetic factors and the possibility of the effect of other polymorphisms.
Thus, the study of genetic features of PCOS pathogenesis in women of an early reproductive age is a promising direction that will allow more accurate determination of the pathogenetic features. According to the results of the study, the genetic factors do not play a key role in the development of PCOS in the women under study. The study may be useful for subsequent meta-analyses that may reveal an understanding in the pathogenesis of the disease.
References
- Назаренко Т.А., Мишиева Н.Г. Бесплодие и возраст: пути решения проблемы. 2-е изд. М.: МЕДпресс-информ; 2014: 216–20. [Nazarenko T.A., Mishieva N.G. Besplodie i vozrast: puti resheniya problemy. 2nd ed. Moscow: MEDpress-inform; 2014: 216–20. (in Russian)]
- Lizneva D., Suturina L., Walker W., Brakta S., Gavrilova-Jordan L., Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil. Steril. 2016; 106(1): 6–15. https://dx.doi.org/10.1016/j.fertnstert.2016.05.003.
- Joham A.E., Teede H.J., Ranasinha S., Zoungas S., Boyle J. Prevalence of infertility and use of fertility treatment in women with polycystic ovary syndrome: data from a large community-based cohort study. J. Womens Health (Larchmt). 2015; 24(4): 299–307. https://dx.doi.org/10.1089/jwh.2014.5000.
- Joseph S., Barai R.S., Bhujbalrao R., Idicula-Thomas S. PCOSKB: A KnowledgeBase on genes, diseases, ontology terms and biochemical pathways associated with Polyсystic Ovary Syndrome. Nucleic Acids Res. 2015; 44(D1): D1032-5. https://dx.doi.org/10.1093/nar/gkv1146.
- Azziz R., Carmina E., Chen Z., Dunaif A., Laven J.S., Legro R.S. et al. Polycystic ovary syndrome. Nat. Rev. Dis. Primers. 2016; 2: 16057. https://dx.doi.org/10.1038/nrdp.2016.57.
- Найдукова А.А., Каприна Е.К., Донников А.Е., Чернуха Г.Е. Генетические аспекты формирования синдрома поликистозных яичников. Акушерство и гинекология. 2016; 3: 16–22. https://dx.doi.org/10.18565/aig.2016.3.16–22. [Naydukova A.A., Kaprina E.K., Donnikov A.E., Chernukha G.E. Development of polycystic ovary syndrome: Genetic aspects. Akusherstvo i ginekologiya/ Obstetrics and Gynecology. 2016; (3): 16–22. https://dx.doi.org/10.18565/aig.2016.3.16–22. (in Russian).]
- Ibanez L., Oberfield Sh.E., Witchel S.F., Auchus R.J., Chang R.J., Codner E. et al. An international consortium apdate: pathophysiology, diagnosis, and treatment of polycystic ovarian syndrome in adolescence. Horm. Res. Paediatr. 2017; 88(6): 371–95. https://dx.doi.org/10.1159/000479371.
- Day F.R., Hinds D.A., Tung J.Y., Stolk L., Styrkarsdottir U., Saxena R. et al. Causal mechanisms and balancing selection inferred from genetic associations with polycystic ovary syndrome. Nat. Commun. 2015; 6: 8464. https://dx.doi.org/10.1038/ncomms9464.
- Reddy K.R., Deepika M.L., Supriya K., Latha K.P., Rao S.S., Rani V.U., Jahan P. CYP11A1 microsatellite (tttta)n polymorphism in PCOS women from South India. J. Assist. Reprod. Genet. 2014; 31(7): 857–63. https://dx.doi.org/10.1007/s10815-014-0236-x.
- Kaur R., Kaur T., Kaur A. Genetic association study from North India to analyze association of CYP19A1 and CYP17A1 with polycystic ovary syndrome. J. Assist. Reprod. Genet. 2018; 35(6): 1123–9. https://dx.doi.org/10.1007/s10815-018-1162-0.
- Дубровина С.О. Синдром поликистозных яичников: современный обзор. Гинекология. 2016; 18(5): 14–7. [Dubrovina S.O. Polycystic ovarian syndrome: a modern overview. Ginekologiya. 2016; 18(5): 14–7. (in Russian).]
- Адамян Л.В., Андреева Е.Н., Гаспарян С.А., Геворкян М.А., Григорян О.Р., Гриняева Е.Н., Густовалова Е.А., Дедов И.И., Демидова Т.Ю., Карахалис М.Ю., Лизиева Л.Е., Спиридонова Н.Е., Сутурина Л.В., Тарасова М.А., Уварова Е.В., Филиппов О.С., Хамошина М.С., Чернуха Г.Е., Шереметьева Е.В., Ярмолинская М.В., Соболева Е.Л., Ярмолинская М.И. Синдром поликистозных яичников в репродуктивном возрасте (современные подходы к диагностике и лечению). Клинические рекомендации (протокол лечения). М.; 2015. Доступно по: https://kuzdrav.ru/special/guideline/cragmz.php?PAGEN_1=3 [Adamyan L.V., Andreeva E.N.,Gasparyan S.A., Gevorkyan M.A., Grigoryan O.R., Grinyaeva E.N., Gustovalova E.A., Dedov I.I., Demidova T.Yu., Karakhalis M.Yu., Lizieva L.E.,Spiridonova N.E., Suturina L.V., Tarasova M.A., Uvarova E.V., Filippov O.S.,Khamoshina M.S., Chernukha G.E., Sheremet’eva E.V., Yarmolinskaya M.V., Soboleva E.L., Yarmolinskaya M.I. Sindrom polikistoznykh yaichnikov v reproduktivnom vozraste (sovremennye podkhody k diagnostike i lecheniyu). Klinicheskie rekomendatsii (protokol lecheniya). Moscow; 2015. (in Russian). Available at: https://kuzdrav.ru/special/guideline/cragmz.php?PAGEN_1=3]
- Teede H.J., Misso M.L., Costello M.F., Dokras A., Laven J., Moran L. et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Clin. Endocrinol. 2018; 89(3): 251–68. https://dx.doi.org/10.1111/cen13795.
- Akgul S., Derman O., Alikaşifoglu M., Aktaş D. CYP1A1 polymorphism in adolescents with polycystic ovary syndrome. Int. J. Gynaecol. Obstet. 2011; 112(1): 8–10. https://dx.doi.org/10.1016/j.ijgo.2010.07.032.
- Chua A.K., Azziz R., Goodarzi M.O. Association study of CYP17 and HSD11B1 in polycystic ovary syndrome utilizing comprehensive gene coverage. Mol. Hum. Reprod. 2012; 18(6): 320–4. https://dx.doi.org/10.1093/molehr/gas002.
- Diamanti-Kandarakis E., Bartzis M.I., Zapanti E.D., Spina G.G., Filandra F.A., Tsianateli T.C. et al. Polymorphism T→ C (− 34 bp) of gene CYP17 promoter in Greek patients with polycystic ovary syndrome. Fertil. Steril. 1999; 71(3): 431–5. https://dx.doi.org/10.1016/s0015-0282(98)00512-3.
- Echiburú B., Pérez-Bravo F., Maliqueo M., Sánchez F., Crisosto N., Sir-Petermann T.Polymorphism T –> C (−34 base pairs) of gene CYP17 promoter in women with polycystic ovary syndrome is associated with increased body weight and insulin resistance: a preliminary study. Metabolism. 2008; 57(12): 1765–71. https://dx.doi.org/10.1016/j.metabol.2008.08.002.
- Park J., Lee E., Ramakrishna S., Cha D., Baek K. Association study for single nucleotide polymorphisms in the CYP17A1 gene and polycystic ovary syndrome. Int. J. Mol. Med. 2008; 22(2): 249–54.
- Unsal T., Konac E., Yesilkaya E., Yilmaz A., Bideci A., Ilke O.H. et al. Genetic polymorphisms of FSHR, CYP17, CYP1A1, CAPN10, INSR, SERPINE1 genes in adolescent girls with polycystic ovary syndrome. J. Assist. Reprod. Genet. 2009; 26(4): 205–16. https://dx.doi.org/10.1007/s10815-009-9308-8.
- Jin J.L, Sun J., Ge H.J., Cao Y.X., Wu X.K., Liang F.J. et al. Association between CYP19 gene SNP rs2414096 polymorphism and polycystic ovary syndrome in Chinese women. BMC Med. Genet. 2009; 10: 139. https://dx.doi.org/10.1186/1471-2350-10-139.
- Gharani N., Waterworth D.M., Batty S. Association of the steroid synthesis gene CYP11A with PCOS and hyperandrogenism. Hum. Mol. Genet. 1997; 6(3): 397–402. https://dx.doi.org/10.1093/hmg/6.3.397.
Received 31.05.2109
Accepted 21.06.2109
About the Authors
Angelika Yu. Beglova, Assistant of Department of Obstetrics and Gynecology, Kemerovo State Medical University, Phone: +7(903)9074757; е-mail: angelik-1986@mail.ru; https://orcid.org/0000-0001-5574-4275. 650056 Russia, Kemerovo, st. Voroshilova, 22a.Svetlana I. Elgina, MD, Professor, Professor of Department of Obstetrics and Gynecology, Kemerovo State Medical University. Phone: +7(3842)734856;
е-mail: elginas.i@mail.ru; https://orcid.org/0000-0002-6966-2681. 650056 Russia, Kemerovo, st. Voroshilova, 22a.
Lydmila A. Gordeeva, PhD, Leading Researcher, Laboratory of Immunogenetics, Institute of Human Ecology of the Siberian Branch of the Russian Academy of Sciences,
Phone: +7(913)3227899; е-mail: gorsib@rambler.ru. 650000 Russian Federation, Kemerovo, prospect Leningradskiy, 10.
For citation: Beglova A.Yu., Elgina S.I., Gordeeva L.A. Polymorphism of the CYP11A1, CYP17A1, and CYP19A1 genes in reproductive-aged women with polycystic ovary syndrome.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2019; 12: 148-53.(In Russian).
https://dx.doi.org/10.18565/aig.2019.12.148-153



