Features of the profile of proteins secreted by cells from the endometrioid foci and eutopic endometrium in women with external genital endometriosis in vitro culture
Objective. To comparatively assess the profile of proteins secreted in vitro culture by cells from endometrioid foci and eutopic endometrium of women with external genital endometriosis.Pavlovich S.V., Krechetova L.V., Vtorushina V.V., Vanko L.V., Melkumyan A.G., Yushina M.N., Savilova A.M., Makiyan Z.N., Yarotskaya E.L., Khilkevich E.G., Chuprynin V.D., Sukhikh G.T.
Material and methods. The cells isolated from the samples of endometrioid heterotopias and eutopic endometrium from 21 women with external genital endometriosis and the endometrial cells from 8 women without endometriosis were cultured in vitro. The surface phenotype of cells taken after Passage 1 was determined using a flow cytometer and a standard set of monoclonal antibodies. During Passage 2, a conditioned culture medium was used to estimate the concentration of various soluble analytes by a multiplexed analysis.
Results. The cells from the endometrioid foci and eutopic endometrium, which were cultured before Passage 2, adhered to plastic, showed fibroblast-like morphology, and phenotypically met the minimum criteria adopted for the identification of mesenchymal stromal cells. The culture supernatants displayed a different level of cell production of the test proteins from the foci of endometriosis and eutopic endometrium.
Conclusion. In vitro production of a wide range of proteins characterizing the functional state of stromal cells isolated from the ectopic foci of endometriosis indicates the increased cell activation in the endometrioid foci, which is an important pathogenetic factor in the development of endometriosis.
Keywords
External genital endometriosis is a common gynecological disease, in which endometrial cells (the inner layer of the uterine wall) grow outside the uterus. Participation of the immune system in the pathogenesis of endometriosis is deemed to be established. [1–4] Immune disorders in endometriosis include a decrease in cellular cytotoxicity reactions in relation to autologous endometrial cells and the development of autoimmune reactions [5–8]. Endometriosis is characterized by the development of local inflammation with systemic subclinical manifestation. [9–12]
For a detailed understanding of pathogenesis of external genital endometriosis, it seems important to study the role of angiogenic, neurogenic, lymphangiogenic factors, cytokines, chemokines, various growth factors in the formation and progression of lesions of the disease. The study of pathogenesis mechanisms and search for diagnostic markers is necessary for the development of early non-invasive diagnosis and pathogenetic therapy of the disease.
Peritoneal fluid – the most common material for study of pathogenesis of external genital endometriosis is used to study the phenotype and functional state of peritoneal macrophages, the content of cytokines, chemokines, and various growth factors [13–18]. However, obtaining peritoneal fluid is possible only for invasive interventions, and it limits the feasibility of the study.
Therefore, the study of pathogenesis of endometriosis is often carried out with the use of experimental models, in particular, in vitro. At present, an immortalized line of human endometrioid cells KC02-44D is already created, the cytoskeleton, gene expression and biochemical phenotype of which are similar to those in germinal endometrial stromal cells. This cell line is thought to be useful in the study of endometrial function and associated pathologies. [19] The procedures for creating and maintaining primary cell cultures of superficial epithelial cells of ovaries, tubular epithelium and human endometrium, the protocols for immortalization, clonal isolation and co-cultivation with stromal cells are described; it may be useful for studying the molecular and cellular functions of these types of epithelium in normal and pathological conditions. [20]
A comparative study of the phenotype and functional activity in cells cultivation in vitro from the eutopic endometrium and endometroid lesions of different localization seems to be a promising research line. [21, 22] The aim of this study was a comparative assessment of profile of secreted proteins by cells from endometroid lesions and eutopic endometrium in women with external genital endometriosis in in vitro culture.
Materials and methods
The samples of eutopic and ectopic endometium were collected in women during minimally invasive interventions – laparoscopy in combination with hysteroscopy in 21 patients with external genital endometriosis, who underwent in-patient treatment in the Departments of Surgical Gynecology and Surgery in the National Medical Research Center of Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov, Ministry of Health of the Russian Federation. The diagnosis “external genital endometriosis” was determined intraoperatively and histologically in all women. Paired eutopic endometrium and endometrioid heterotopia were sampled from 18 patients. Control samples of endometrium were collected from 8 patients, who underwent surgery of uterine fibroids (n = 3), incomplete and complete intrauterine septum (n = 4), and ovarian cysts (n = 1). The absence of endometriosis in these patients was confirmed during surgical intervention for the underlying disease.
The age of patients participating in the study was 25-40 years old.
Eutopic endometrium and endometriotic lesion samples were placed in sterile tubes and immediately transported to the laboratory. Tissue samples were washed in phosphate-buffered saline, mechanically crushed, and then incubated in a 0.07% type IA collagenase (Sigma-Aldrich, USA) at 37 °C for 30 min. The mixture was centrifuged at 2000 rpm for 5 min, the supernatant was removed and the precipitate was resuspended in DMEM/F-12 medium (PanEco, RF), containing 10% fetal calf serum (HyClone, UK), 50 U/ml penicillin, 50 μg/ml streptomycin, 2 mM L-glutamine (PanEco, RF).4
The cell suspension was transferred to culture vials of 25 cm2, the seeding density was 3-5×103 nucleated cells per 1 cm2.
The cell surface phenotype collected after the first passage was determined with the use of a standard set of monoclonal antibodies labeled with fluorescein isothiocyanate against antigens CD90, CD26, CD34, phycoerythrin against antigens CD105, CD146, CD14, CD200, HLA-DR, allophycocyanin against antigens CD45 (BD Bioscience, USA). The analysis was performed using a FACCСalibur flow cytometer (Becton Dickinson, USA).
The culture medium was changed every 3 days; cultures were passaged after the monolayer was reached. The collection of conditioned medium for analysis of soluble factors was carried out during the second passage when a monolayer was reached in the culture vial and centrifuged at 2000 rpm for 5 minutes. The supernatant was aliquoted in 1 ml, frozen at -20 ° C, stored at -80 °C until the study was conducted.
In the conditioned medium in the 2nd passage of ectopic and eutopic endometrial cultures, the content of various soluble analytes was determined: (interleukin (IL) -1β, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL -8, IL-9, IL-10, IL-12 (p70), IL-13, IL-15, IL-17A, interferon (IFN)-γ, tumor necrosis factor (TNF)α, monocytic chemotactic protein 1 (MCP-1), macrophage inflammation protein1α (MIP-1α), MIP-1β, IFN-γ, inducible protein-10 (IP-10), granulocyte colony stimulating factor (G-CSF), granulocyte macrophage colony stimulating factor (GM-CSF), platelet growth factor (PDGF)-bb, RANTES, eotaxin, vascular endothelial growth factor (VEGF), basic fibroblast growth factor basic form (bFGF) with application of multiplex method and the use of standard 27-plex test system Bio-Plex Pro Human Cytokine 27-plex Assay (Bio-Rad, USA) on the flow-based Bio-Plex 200 system (Bio-Rad, USA), and subsequenty the obtained results were processed by the Bio-Plex Manager 6.0 software application (Bio-Rad, USA). The concentration of analytes was calculated based on the production of one million cells in 1 ml of culture medium (pgG/mL/106kl).
Statistical processing of the results was carried out by standard procedure with the use of statistical package for Microsoft Office Excel 2007 and MedCalc12 program for Windows 7. Testing the normality of distribution was performed by Shapiro-Wilk test. Since the distribution data were different from normal, the Mann -Whitney U-test was used to assess the differences in the samples. The data in the tables were presented as the median (the 25th – 75th percentiles) of distribution. The differences were considered significant at p ˂ 0.05.
Results
The cells isolated from the tissues of the eutopic endometrium and ectopic endometrioid lesions, after the second passaging, were homogeneous in morphology and adhesion to plastic; their phenotype is presented in Table. 1.
The cultured cells isolated from the control and eutopic endometrium had phenotypic differences only in the number of CD90+ cells. The ratio of cells expressing the remaining studied markers was almost equal. The phenotypic characteristics of the composition of cultured cells that were isolated from endometrioid lesions significantly differed from phenotypic characteristics of cells from both normal and eutopic endometriosis. Cells from endometrioid lesions had maximal presence of CD90 and CD26 markers and minimal presence of CD105 and CD146 markers. In all cell cultures, the number of CD73+ cells was equal. In each culture there were less than 2% of cells positive for CD106, CD200, CD14, CD34, CD45, HLA-DR. Based on these data, we can conclude that all of the studied cells mainly belonged to the population of mesenchymal stromal cells.
In the protein profile examination of supernatants of the second passaging of cell cultures isolated from eutopic endometrium and endometrioid heterotopia, the concentration of cytokines (IL-1β, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12(p70), IL-13, IL-15, IL-17A, IFN-γ, TNFα), chemokines (IL-8, MCP-1, MIP-1α, MIP-1β, IP-10, RANTES, Eotaxin), and growth factor (IL-7, IL-9, CSF, GM-CSF, PDGF-bb, VEGF, FGF basic) were determined.
The results of the products evaluation of the analytes in the supernatants of the eutopic endometrium cultures of patients in the control group are presented in Table. 2.
It is noteworthy that the production by cells of IL-6 culture, chemokines IL-8 and MCP-1, and VEGF is very high.
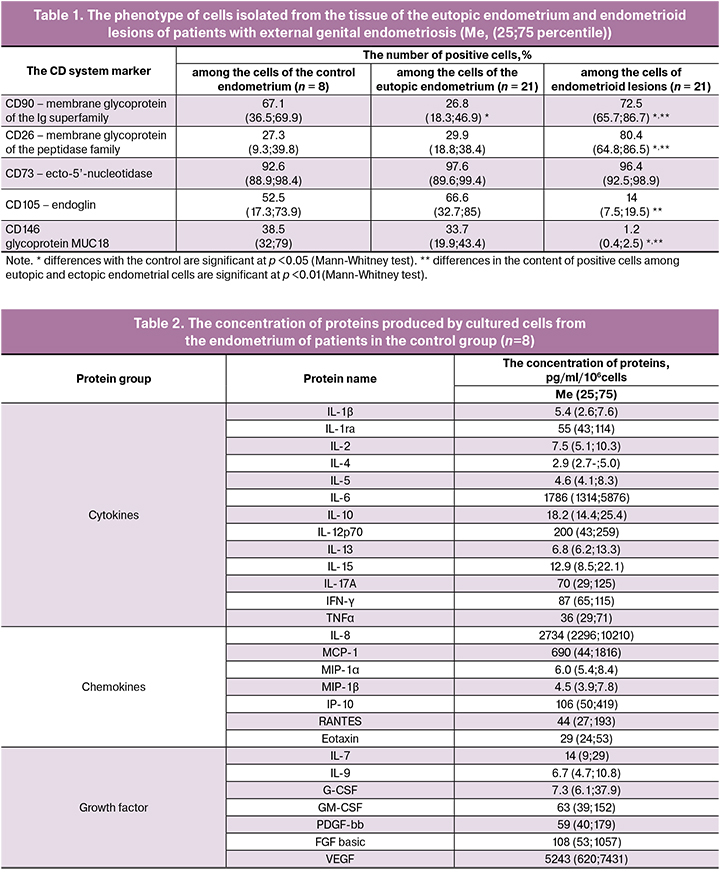
The results of the profile analysis of studied proteins in the cells supernatants of the eutopic endometrium of patients with external genital endometriosis compared with proteins in the cells supernatants of the endometrium of patients in the control group are presented in Fig.1.
According to the data presented in Fig. 1, the concentration of growth factors and chemokines in the supernatants of the eutopic endometrial cells did not differ from the control endometrial cells. During the examination of the level of cytokines, an increased production of TNF-α by eutopic endometrial cells (Me 102, 38-206 and Me 36, 29-71, respectively, р=0.042) and IL-4 (Me 6.9, 3.5-12.9 and Me 2.9, 2.7-5.0, respectively, p = 0.033) was detected.
The results of the profile analysis of soluble proteins in the supernatants of the cells from the endometrioid lesions of patients with external genital endometriosis, compared with soluble proteins in the supernatants of the control endometrial cells, are presented in Fig. 2.

According to the data presented in Fig.2, the concentration of all studied proteins in the supernatants of cells from the endometrioid lesions was significantly higher than the control values. The concentration of IL-6 was 213 times higher; eotaxin chemokine was 65 times higher; chemokine MCP-1 was 50 times higher; and growth factor G-CSF – 66 times higher.
A comparative analysis of the protein concentration in the cell culture supernatants from endometrioid heterotopias and eutopic endometrium of patients with external genital endometriosis based on the Wilcoxon signed-rank test for dependent samples is presented in Fig. 3.
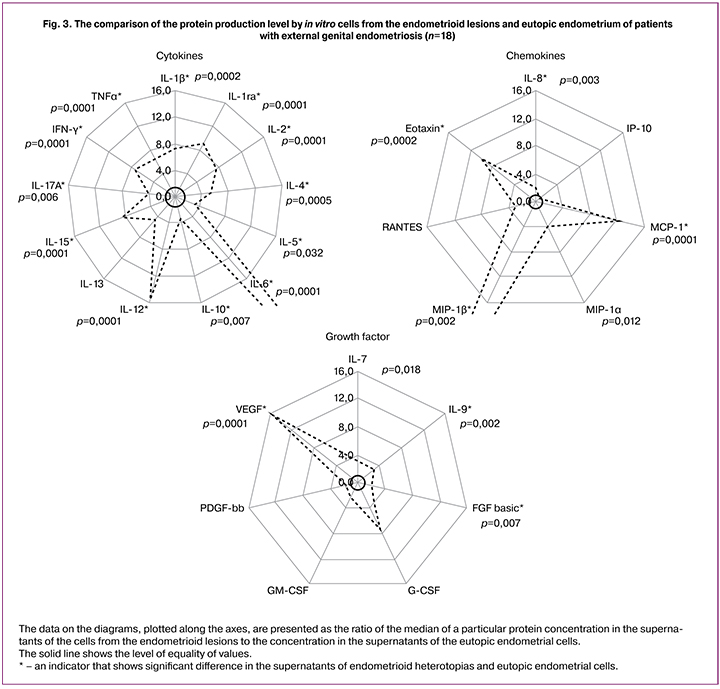
The data from Fig.1 let us to conclude that the concentration of the cytokines was significantly higher in the cell culture supernatants from endometrioid heterotopias (IL-6 – in 42.3 times). The chemokine concentration was also higher (MIP-1b – in 36 times), while there were no difference in the concentration between IP-10 and RANTES. The concentration of growth factors, especially VEGF, was also significantly higher, while there were no difference in concentration between G-CSF, GM-CSF, and PDGF-bb.
Discussion
In our study, we sampled and analyzed cell cultures from tissue of normal and eutopic endometrium, and endometrioid lesions. According to the presented data, most of the cells cultured before the second passaging were mesenchymal by their phenotype. The selected cells had the following characteristics: good adhesion to the culture plastic surface, fibroblast-like shape, and expression of CD90, CD26, CD73, and CD105 antigens with the absence of CD45, CD34, CD14. These are used to identify mesenchymal stromal cells (MSCs) [23, 24]. This led us to the conclusion that stromal cells prevailed in the cultures of the second passaging. Our results correspond with the data of Uder C. [25], who used a similar panel of cell surface markers (CD105 +, CD73 +, CD90 +, CD34-, CD14-) to identify MSCs.
The analysis of Chan et al. [20] on in vitro cytokine production by stromal cells isolated from similar tissue samples from patients with ovarian endometriosis showed that cells from both endometrioid lesions and eutopic endometrium are characterized by proliferative behavior, invasiveness, and adhesion that was differ from control endometrial cells.
Endometriosis is associated with impaired expression of many soluble factors: cell adhesion molecules, growth factors, cytokines, matrix metalloproteinases, and enzymes for estrogen synthesis and metabolism [26]. A systematic review, performed in 2014, showed that according to the findings of several researches chemokines and their receptors are suggested to be considered as potential biomarkers of endometriosis, especially such biomarkers as IL-8 or CXCL8 (in 51.6% of studies); MCP-1 or CCL-2 (in 38.7% of studies); RANTES or CCL5 (in 19.3% of studies) [27]. Since it is known that the IL-8 level increases in any inflammatory processes, it is more likely to be biomarker of the inflammatory process. That confirms the role of inflammation in the pathogenesis of endometriosis.
Over the past 5 years, a considerable amount of researches has been devoted to the analysis of cytokine content in the blood serum of patients with endometriosis with the use of different methods, including multiplex analysis. A high level of IL-1, IL-6 was found [28–30]. Some researchers consider that the marker of this pathology may be an increased level of anti-inflammatory cytokines IL-1Ra, IL-4, and IL-10 [29], while the others consider that the marker of this pathology may be an increased level of key proinflammatory cytokines IL 1β, IL-6, and TNF-α [30].
The content analysis of several cytokines (IL-1β, IL-6, IL-8, IL-12, IFN-γ, TNF, and VEGF-A) in the peritoneal fluid of patients with endometriosis revealed an association of endometriosis with the high levels of IL-6 and IL- 8 [31], high levels of IL-6, IL-18, eotaxin, and MCP-1, regardless of the menstrual cycle phase [32], high levels of IL-6 and transforming growth factor beta (TGF)-β, but without differences with control samples in the content of IL-10 and IL-17.
An in vitro experiment showed that IL-17 increases the secretion of IL-8 by endometrial stromal cells and thereby stimulates the appearance of the endometrioid lesions [33]. IL-17A is an important angiogenic and proinflammatory cytokine involved in the pathophysiology of chronic inflammatory diseases. The localization of IL-17A was immunohistochemically determined in the stroma of eutopic endometrium and endometrioid lesions samples; and the difference of IL-17A expression in these types of tissues was shown. Stimulation of in vitro endometrial stromal cells, Ishikawa and HUVEC cells with this cytokine led to a significant increase in angiogenic cytokines VEGF and IL-8, proinflammatory cytokines IL-6 and IL-1β, and chemokines G-CSF, CXCL12, CXCL1, and CX3CL1. Since surgical removal of ectopic lesions led to a drop in the systemic level of this cytokine, the authors conclude that endometriotic lesions produce IL-17A; and IL-17A plays an important role in creating an angiogenic and proinflammatory environment in the abdominal cavity and maintaining the ectopic lesions when they occur [34, 35].
In the study on cultivation of macrophages isolated from the peritoneal fluid of women with endometriosis and stimulated with in vitro lipopolysaccharide the same with control secretion level of IL-1β macrophages was found, with an increased level of secretion in the peritoneal fluid of patients. This led us to the conclusion that a violation in the level of the IL-1 family cytokines production is an important pathogenetic factor in the development of endometriosis. The conclusion is supported by an experiment showed that IL-1β, which stimulates the tryptophan catabolism, enhances the production of IL-6 and IL-8 by endometrial stromal cells through increased expression of tryptophan-2,3-dioxygenase [33]. The important role of IL-1β in the pathogenesis of endometriosis is also confirmed by a research that studied the effect of IL-1β on VEGF secretion by stromal cells from endometrioid lesions [34]. It was found that the addition of IL-1β to the stromal cell culture from endometrioid lesions and the eutopic endometrium increased the release of VEGF in both types of cultures, same as in the control sample, through increased expression of cyclooxygenase-2, an enzyme expressed by macrophages, fibroblasts and endothelial cells, and induced by cytokines or growth factors in inflammation.
A multiplex analysis of analytes in the culture supernatants of the second passaging revealed different levels of in vitro production of a wide range of the studied proteins by cells from the eutopic endometrium and endometrioid lesions compared with the control samples (Figs. 1-3). It was found that the protein production by cells from the eutopic endometrium differed from the control samples by a higher level of TNF-α and IL-4 (cytokines reflecting the processes of cellular activation), but did not differ in the production of chemokines and growth factors characterizing cell-cell interactions and proliferative activity, respectively. The functional state of in vitro cells isolated from the ectopic lesions of patients with external genital endometriosis differed significantly from the cells of both normal and eutopic endometrium in the production of all studied cytokines, chemokines, and growth factors (Fig. 2, 3). The most significant excess concentration had IL-6, eotaxin, chemokine MCP-1, and growth factors G-CSF and VEGF.
Conclusion
Results of our study on the in vitro production of a wide range of proteins characterizing the functional state of the stromal cells isolated from the eutopic endometrium and ectopic endometrioid lesions are consistent with the published data and indicate an increased activation state of cells from ectopic lesions that produce different types of cytokines, chemokines and growth factors into the culture medium. The correlation between the literature data and the results of our study indicate a significant contribution of soluble factors to the pathogenesis of endometriosis. These factors mediate various aspects of cell-cell interactions that contribute to the inflammatory process, angiogenesis and cell proliferation. However, the pathogenetic mechanisms complexity of external genital endometriosis development call for further researches, particularly on the role of cytokines and growth factors in the interaction of epithelial and stromal cells in endometriosis.
References
- Ahn S.H., Monsanto S.P., Miller C., Singh S.S., Thomas R., Tayade C. Pathophysiology and Immune Dysfunction in Endometriosis. Biomed. Res. Int. 2015; 2015: 795976.
- Miller J.E., Ahn S.H., Monsanto S.P., Khalaj K., Koti M., Tayade C. Implications of immune dysfunction on endometriosis associated infertility. Oncotarget. 2017; 8(4): 7138-47.
- Kobayashi H., Higashiura Y., Shigetomi H., Kajihara H. Pathogenesis of endometriosis: the role of initial infection and subsequent sterile inflammation (Review). Mol. Med. Rep. 2014; 9(1): 9-15.
- Králíčková M., Vetvicka V. Immunological aspects of endometriosis: a review. Ann. Transl. Med. 2015; 3(11): 153.
- Jeung I., Cheon K., Kim M.R. Decreased cytotoxicity of peripheral and peritoneal natural killer cell in endometriosis. Biomed. Res. Int. 2016; 2016:2916070.
- Caserta D., Mallozzi M., Pulcinelli F.M., Mossa B., Moscarini M. Endometriosis allergic or autoimmune disease: pathogenetic aspects--a case control study. Clin. Exp. Obstet. Gynecol. 2016; 43(3): 354-7.
- Thiruchelvam U., Wingfield M., O’Farrelly C. Natural killer cells: key players in endometriosis. Am. J. Reprod. Immunol. 2015; 74(4): 291-301.
- Eisenberg V.H., Zolti M., Soriano D. Is there an association between autoimmunity and endometriosis? Autoimmun. Rev. 2012; 11(11): 806-14.
- Agic A., Xu H., Finas D., Banz C., Diedrich K., Hornung D. Is endometriosis associated with systemic subclinical inflammation? Gynecol. Obstet. Invest. 2006; 62(3): 139-47.
- Sapkota Y., Low S.K., Attia J., Gordon S.D., Henders A.K., Holliday E.G. et al. Association between endometriosis and the interleukin 1A (IL1A) locus. Hum. Reprod. 2015; 30(1): 239-48.
- Sheveleva T., Bejenar V., Komlichenko E., Dedul A., Malushko A. Innovative approach in assessing the role of neurogenesis, angiogenesis, and lymphangiogenesis in the pathogenesis of external genital endometriosis. Gynecol. Endocrinol. 2016; 32(Suppl. 2): 75-9.
- Jiang L., Yan Y., Liu Z., Wang Y. Inflammation and endometriosis. Front. Biosci. (Landmark Ed). 2016; 21: 941-8.
- Barcz E., Milewski Ł., Dziunycz P., Kamiński P., Płoski R., Malejczyk J. Peritoneal cytokines and adhesion formation in endometriosis: an inverse association with vascular endothelial growth factor concentration. Fertil. Steril. 2012; 97(6): 1380-6.
- Соколов Д.И., Кондратьева П.Г., Ярмолинская М.И., Крамарева Н.Л., Селютин А.В., Рулев В.В., Ниаури Д.А., Сельков С.А. Содержание хемокинов и цитокинов в перитонеальной жидкости больных наружным генитальным эндометриозом различной степени тяжести. Медицинская иммунология. 2007; 9(1): 85-90.
- Sukhikh G.T., Sotnikova N.Y., Antsiferova Y.S., Posiseeva L.V., Veryasov V.N., Van’ko L.V. Cytokine production by immunocompetent cells of peritoneal fluid in women with external genital endometriosis. Bull. Exp. Biol. Med. 2004; 137(6): 568-71.
- Podgaec S., Rizzo L.V., Fernandes L.F., Baracat E.C., Abrao M.S. CD4(+) CD25(high) Foxp3(+) cells increased in the peritoneal fluid of patients with endometriosis. Am. J. Reprod Immunol. 2012; 68(4): 301-8.
- Barcz E., Milewski Ł., Dziunycz P., Kamiński P., Płoski R., Malejczyk J. Peritoneal cytokines and adhesion formation in endometriosis: an inverse association with vascular endothelial growth factor concentration. Fertil. Steril. 2012; 97(6): 1380-6.
- Yuhki M., Kajitani T., Mizuno T., Aoki Y., Maruyama T. Establishment of an immortalized human endometrial stromal cell line with functional responses to ovarian stimuli. Reprod. Biol. Endocrinol. 2011; 9: 104.
- Chan R.W., Mak A.S., Yeung W.S., Lee K.F., Cheung A.N., Ngan H.Y., Wong A.S. Human female reproductive tract epithelial cell culture. Methods Mol. Biol. 2013; 945: 347-63.
- Delbandi A.A., Mahmoudi M., Shervin A., Akbari E., Jeddi-Tehrani M., Sankian M. et al. Eutopic and ectopic stromal cells from patients with endometriosis exhibit differential invasive, adhesive, and proliferative behavior. Fertil. Steril. 2013; 100(3): 761-9.
- Huang F., Cao J., Liu Q., Zou Y., Li H., Yin T. MAPK/ERK signal pathway involved expression of COX-2 and VEGF by IL-1β induced in human endometriosis stromal cells in vitro. Int. J. Clin. Exp. Pathol. 2013; 6(10): 2129-36.
- Dominici M., Le B.K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D. et al. Minimal criteria for defining multipotent mesenchimal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006; 8: 315-7.
- Donnenberg A.D., Meyer E.M., Rubin J.P., Donnenberg V.S. The cell-surface proteome of cultured adipose stromal cells. Cytometry A. 2015; 87(7): 665-74.
- Uder C., Brückner S., Winkler S.., Tautenhahn H.M. Christ B. Mammalian MSC from selected species: Features and applications. Cytometry A. 2018; 93(1): 32-49.
- Bouquet De Jolinière J., Ayoubi J.M., Gianaroli L., Dubuisson J.B., Gogusev J., Feki A. Endometriosis: a new cellular and molecular genetic approach for understanding the pathogenesis and evolutivity. Front. Surg.2014; 1: 16.
- Borrelli G.M., Abrão M.S., Mechsner S. Can chemokines be used as biomarkers for endometriosis? A systematic review. Hum. Reprod. 2014; 29(2): 253-66.
- Sikora J., Mielczarek-Palacz A., Kondera-Anasz Z., Strzelczyk J. Peripheral blood proinflammatory response in women during menstrual cycle and endometriosis. Cytokine. 2015; 76(2): 117-22.
- Măluţan A.M., Drugan T., Ciortea R., Mocan-Hognogi R.F., Bucuri C., Rada M.P., Mihu D. Serum anti-inflammatory cytokines for the evaluation of inflammatory status in endometriosis. J. Res. Med. Sci. 2015; 20(7): 668-74.
- Malutan A.M., Drugan T., Costin N., Ciortea R., Bucuri C., Rada M.P., Mihu D. Pro-inflammatory cytokines for evaluation of inflammatory status in endometriosis. Cent. Eur. J. Immunol. 2015; 40(1): 96-102.
- Barcz E., Milewski Ł., Dziunycz P., Kamiński P., Płoski R., Malejczyk J. Peritoneal cytokines and adhesion formation in endometriosis: an inverse association with vascular endothelial growth factor concentration. Fertil. Steril. 2012; 97(6): 1380-6.
- Bersinger N.A., Dechaud H., McKinnon B., Mueller M.D. Analysis of cytokines in the peritoneal fluid of endometriosis patients as a function of the menstrual cycle stage using the Bio-Plex® platform. Arch. Physiol. Biochem. 2012; 118(4): 210-8.
- Hirata T., Osuga Y., Takamura M., Saito A., Hasegawa A., Koga K. et al. Interleukin-17А increases the secretion of interleukin-8 and the expression of cyclooxygenase 2 in endometriosis. Fertil. Steril. 2011; 96(1): 113-7.
- Urata Y., Koga K., Hirota Y., Akiyama I., Izumi G., Takamura M. et alIL-1β increases expression of tryptophan 2,3-dioxygenase and stimulates tryptophan catabolism in endometrioma stromal cells. Am. J. Reprod. Immunol. 2014; 72(5): 496-503.
- Ahn S.H., Edwards A.K., Singh S.S., Young S.L., Lessey B.A., Tayade C. IL-17A Contributes to the pathogenesis of endometriosis by triggering proinflammatory cytokines and angiogenic growth factors. J. Immunol. 2015; 195(6):2591-600.
Received 17.04.2019
Accepted 19.04.2019
About the Authors
Pavlovich, Stanislav V., M.D., Ph. D. – Academic secretary, National Medical Research Center of Obstetrics, Gynecology and Perinatology named after AcademicianV.I. Kulakov, Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +74954385225. E–mail: s_pavlovich@oparina4.ru
Krechetova L.V., Ph.D. in medical sciences, head of clinical immunology laboratory National Medical Research Center for Obstetrics, Gynecology and Perinatology
of Ministry of Healthcare of Russian Federation; 4, Oparin street, Moscow, Russian Federation, 117997, +7-(495)-438-11-83. e-mail: k_l_v_@mail.ru
Vtorushina Valentina V., Ph.D. in medical sciences, doctor of laboratory diagnostics in Laboratory of Clinical Immunology, “Research center for Obstetrics, Gynecology and Perinatology”, Ministry of Health of Russia. Address: 4, Acad. Oparin St., Moscow, Russian Federation 117997.Telephone: 8(495) 438 11 83. E-mail: vtorushina@inbox.ru
Vanko L.V., Doctor of Medicine, professor, leading research worker in Laboratory of clinical immunology, National Medical Research Center for Obstetrics, Gynecology and Perinatology of Ministry of Healthcare of Russian Federation; 4, Oparin street, Moscow, Russian Federation, 117997; +7 (495) 438-11-83; e-mail: lvanko@mail.ru.
Melkumyan, Arika G. postgraduate student, National Medical Research Center for Obstetrics, Gynecology and Perinatology of Ministry of Healthcare of Russian Federation; 4, Oparin street, Moscow, Russian Federation,: +79639633755, е-mail: dr.melkumyan@gmail.com
Yushina M.N., researcher, laboratory of molecular pathophysiology, National Medical Research Center for Obstetrics, Gynecology and Perinatology of Ministry
of Healthcare of Russian Federation; 4, Oparin street, Moscow, Russian Federation, phone +7 (977) 9977193.
Savilova, Anastasia M, Ph.D., Head of Information and Analytical Center for Biomedicine Research of the Institute of Translational Medicine of Pirogov Russian National Research Medical University, Ostrovitianov str. 1, Moscow, Russia, 117997, phone +7 (917)5610536, e-mail: 1savilova@gmail.com
Makiyan, Zograb N., Doctor of medicine, principal scientist, working in the department of operative gynecology, National Medical Research Center for Obstetrics, Gynecology and Perinatology of Ministry of Healthcare of Russian Federation; 4, Oparin street, Moscow, Russian Federation,
117997, phone +7 (495)438-11-83. E-mail: z_makiyan@oparina4.ru
Yarotskaya Ekaterina L., MD, Associate Professor, Head of the Department of International Cooperation, National Medical Research Center for Obstetrics,
Gynecology and Perinatology of Ministry of Healthcare of Russian Federation; 4, Oparin street, Moscow, Russian Federation
117997. Tel.: +7 (495) 438-1166; e-mail: e_yarotskaya@oparina4.ru
Khilkevich, Elena G., MD, obstetrician–gynecologist of the Surgery Department, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov, Ministry of Health of Russia.
117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +74954387783. E–mail: e_khilkevich@oparina4.ru. ORCID ID: 0000–0001–8826–8439
Chuprynin, Vladimir D., PhD, Head of the Surgery Department, National Medical Research Center for Obstetrics, Gynecology, and Perinatology named after Academician V.I. Kulakov, Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +7(495)4383575. E–mail: v_chuprynin@oparina4.ru
Sykhikh, Gennady T., MD, Professor, Academical of the Russian Academy of Sciences, Director of the National Medical Research Center for Obstetrics,
Gynecology and Perinatology named after Academician V.I.Kulakov of Ministry of Healthcare of Russian Federation.
117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +7(495)4381866. E-mail: g_sukhikh@oparina4.ru.
For citation: Pavlovich S.V., Krechetova L.V., Vtorushina V.V., Vanko L.V., Melkumyan A.G., Yushina M.N., Savilova A.M., Makiyan Z.N., Yarotskaya E. L., Khilkevich E.G., Chuprynin V.D., Sukhikh G.T. Features of the profile of proteins secreted by cells from the endometrioid foci and eutopic endometrium in women with external genital endometriosis in in vitro culture. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; 8:90-99 (In Russian).
https://dx.doi.org/10.18565/aig.2019.8.90-99



