Characteristics of methylation of HOXA10 and HOXA11 genes in patients with tubal and peritoneal factor infertility and previous unsuccessful IVF attempts
Objective. To assess methylation of HOXA10 and HOXA11 gene promoters in patients with tubal and peritoneal factor infertility and previous unsuccessful in vitro fertilization (IVF) attempts.Knyazeva Е.А., Kuznetsova М.V., Shubina Е.S., Goltsov А.YU., Dоnnikоv А.Е., Kalinina Е.А.
Materials and methods. Before starting the IVF program 47 patients were performed pippele endometrial biopsy with the assessment of methylation of HOXA10 and HOXA11 gene promoters. According to the outcomes of the IVF program following the pipelle biopsy, the patients were divided into two groups: group 1 included the women who achieved pregnancy, group 2 included the patients without pregnancy.
Results. During the study period, the analysis of methylation of 23 CpG islands located in the promoter regions of the HOXA10 gene was performed. In the study groups statistically significant difference in methylation was not revealed in any of CpG islands of HOXA10 and HOXA11.
Conclusion. The obtained results suggest that methylation of the HOXA10 and HOXA11 genes is rather a conservative parameter and it does not appear to influence the outcome of the IVF program in the women of the study group.
Keywords
According to the World Health Organization (WHO), female infertility causes from 8 to 29% of infertile cases and does not show a global tendency to decrease [1]. In addition to common infertility factors, namely male, endocrine, tubal and peritoneal factors, there is a significant increase in the combination of these factors [2]. According to American Society for Reproductive Medicine (ASRM), at least 25% of infertile couples have more than one cause of infertility; each of the spouses may be suffering from this problem and at the same time one spouse may have several factors [3]. The causes of infertility can be difficult to identify, since there are different combinations of infertility factors as well as unspecified causes in diagnosed infertility [4]. Moreover, in some cases after complete clinical laboratory evaluation the cause of infertility remains unidentified, and the couple is considered to be healthy. This factor is referred to unexplained or idiopathic infertility which is responsible for 10-30% of all infertility cases [5]. According to ASRM, the cause of infertility cannot be detected in 10% of infertile couples. In case of unexplained infertility there is no single algorithm for the diagnosis of the problem, as a result treatment strategy can vary from the expectant management (ovulation induction, intrauterine insemination) to complex procedures in applying assisted reproductive technologies (ART) [6, 7]. Thus, unexplained infertility remains a serious challenge to modern reproductive medicine.
It is also worth noting that some couples with identified causes of infertility and ART treatment cannot achieve conception even after repeated attempts of in vitro fertilization (IVF). The problem of repeated implantation failures which refer to cases of three and more unsuccessful IVF cycles with transfer of one or two morphologically normal embryos in every cycle is relevant today, since it remains unsolved despite the improvement of the diagnostic methods and ART [8, 9]. The causes of unsuccessful IVF attempts can be associated with either the failure of the embryo to implant into the endometrium (embryonal factor) or with the decreased endometrial receptivity (uterine factor) [1013]. Endometrial receptivity is defined in literature as the ability of the endometrium to accept the invading blastocyst. It is known that after preimplantation genetic screening in many cases the transfer of embryo without genetic abnormalities does not result in conception, this failure may be suggestive of uterine factor infertility due to the impaired endometrial receptivity [12].
Embryo implantation is a complex multistage process. The embryo can implant only into the receptive endometrium and it is possible only within a certain period of the menstrual cycle which is called “implantation window”, the 6th – 8th day after the peak of luteinizing hormone (LH) and ovulation. The success of embryo implantation largely depends on both the quality of the embryo and morphological and functional condition of the embryo. Morphological changes of the endometrium, along with the expression of certain factors (hormones, cytokines, adhesion molecules, growth factors and etc.) in the endometrium in this period, are of crucial importance to the formation of appropriate implantation window and subsequent successful implantation of blastocyst. The above-mentioned factors were called “markers of endometrial receptivity” which include different instrumental and laboratory findings, their changes can influence clinical outcomes of IVF that are assessed by the pregnancy rate [13, 14].
It was determined that receptors of estrogen and progesterone can play an important role in endometrial receptivity. A specific ultrastructural marker of implantation window, namely the protrusions on the surface of the endometrium (pinopodes) which arise in the middle of the luteal phase, was also identified. There is some evidence that the initial processes of blastocyst adhesion to the endometrium take place on the surface of pinopodes [15]. However, previous studies have described the cases of normal ultrastructure of the epithelial cell surface in patients with unsuccessful IVF attempts, so the development of pinopodes cannot be the only marker characterizing endometrial receptivity [13]. Among other implantation markers, there are cellular adhesion proteins, including integrins and selectins which contribute to the attachment of the embryo to the endometrium [16]. In spite of the fact that there is some evidence of successful implementation of identifying integrins as markers of implantation window, the necessity of their detection in clinical practice remains a subject of debate [15]. Many researchers consider it appropriate to determine the levels of leukemia inhibitory factor expression, vascular endothelial growth factor (VEGF), transforming growth factor β1 and other factors as possible criteria for predicting relevance of endometrial receptivity [13]. Thus, currently there is no single and optimal marker of endometrial receptivity and, therefore, the search for new informative markers of implantation window which might predict the achievement of pregnancy with ART still continues.
According to the data in literature, the HOXA10 and HOXA11 genes are among the key regulators of the processes responsible for endometrial receptivity [17, 18]. It has also been shown that hypermethylation of the gene promoters can be connected with the development of ectopic pregnancy [19]. Both of these genes are known to be expressed in the epithelial cells of endometrial glands and in the stroma in different areas of the uterus. The expression of these genes significantly increases during implantation window [20]. The product of the HOXA10 gene was found to play an essential role in the regulation of the embryonic development as well as in the control of the cyclic transformation of the endometrium during the menstrual cycle [21]. Y. Yang et al. demonstrated that HOXA10 gene expression was significantly decreased in women with repeated failures of implantation [22, 23]. Blocking the HOXA10 gene results in a sharp increase in the number of pinopodes [16]. Moreover, HOXA10 and HOXA11 gene expression is considerably increased in the endometrial cells during the endometrial transformation in the middle of the cycle [24]. The decline in the level of a gene product can be due to genetic causes (gene polymorphism or mutation) and epigenetic regulation. In particular, methylation of the promoter regions of the gene can produce both the decrease in gene expression and its complete inactivation (“epigenetic silence”). The review of works carried out by H. Du and H.S. Taylor demonstrated that methylation of HOXA10 and HOXA11 gene promoters causes their reduced expression and decreased endometrial receptivity [24]. Thus, there is sufficient evidence that the HOXA10 and HOXA11 genes are essential for the formation of implantation window and identification of endometrial receptivity.
The purpose of this study was to establish the methylation status of promoter regions of the HOXA10 and HOXA11 genes in the endometrial samples of the women with tubal and peritoneal factor infertility and previous unsuccessful IVF attempts.
Materials and Methods
The study group consisted of 47 patients who planned to achieve pregnancy with the IVF program and presented to the Department of Assisted Reproductive Technologies in the National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow, Russia. Inclusion criteria for women were as follows: age between 27 and 40 years, tubal and peritoneal factor infertility without severe pathospermia in partner, two or more previous unsuccessful IVF attempts, normal ovarian reserve, no history of ovarian surgeries, informed consent to participate in the study. Before starting the IVF program all the patients underwent clinical and laboratory examination in accordance with the order of the Russian Ministry of Health №107n dated 30.08.2012 “On approval of the use of assisted reproductive technologies, contraindications and limitations to their use”. During the examination every patient was also performed pippele endometrial biopsy during implantation window, on the 7th - 9th days after ovulation, diagnosed with the help of ultrasound scanning and Clear Blue pregnancy tests (“Unipath Ltd”, Great Britain). The obtained endometrial samples were washed three times with PBS (phosphate-buffered saline), then frozen and stored at -80℃ in the biobank of the National Medical Research Center of Obstetrics, Gynecology and Perinatology, Moscow, Russia.
DNA extraction. The obtained endometrial samples were cut with a scalpel and homogenized. DNA was extracted using DNeasy Blood & Tissue Kit (Qiagen, USA).
Bisulfate conversion. Genomic DNA was isolated from 1 mg of every sample. Bisulfate conversion of genomic DNA was performed using the EZ DNA Methylation-Gold Kit (ZymoResearch, USA). This method made it possible to convert non-methylated sites of cytosine to uracil, which was read as thymine in the subsequent polymerase chain reaction (PCR), as methylated cytosines remained unmodified.
PCR amplification. DNA amplification was carried out in a reaction volume of 25 µl, containing 1 µl of forward and reverse primers (10 µM) and 2 µl of bisulfate-treated DNA. Two primers described by Wu Y. et al. were used in the identification of a CpG-rich fragment within HOXA10 gene promoter in the 5’ region upstream of exon I (the F I region): forward - 5’-TGGGGTAGTTTTTATAGTTTTTG-3’; reverse – 5’-AACCCTTTCTAACTAACATTTCTT-3’ (biotinylated) [25].
Amplification conditions. Amplification was performed at 95 for 1 min followed by 40 cycles at 95 for 30 sec, at 55 for 30 sec and at 72 for 30 sec. The final extension at 72 for 10 min was then carried out. The 261 bp PCR products were checked using electrophoresis on a 2.5% agarose gel.
Sequencing. Amplified fragments were sequenced using semiconductor sequencing method on the system Ion S5 (Thermo Fisher Scientific). Library preparation for sequencing and sequencing were performed according to the manufacturer’s instructions.
For determining the methylation degrees, sequences were aligned to the reference sequence using software package bowtie2. The number of nucleotides C and T (i.e. methylated and unmethylated nucleotides) was subsequently counted for each CpG island.
To control the level of bisulfate conversion, the ratio of T nucleotides to C nucleotides was counted in each site outside the CpG islands where before the conversion there was nucleotide C. The conversion status for the whole sample was determined as the mean value for all the positions and was not less than 95%.
All patients underwent IVF according to the standard protocol involving gonadotropin-releasing hormone (GnRH) antagonists. Ovarian stimulation was performed with recombinant follicle stimulating hormone. The trigger of ovulation was injected if the leading follicle was greater than or equal to 17 mm. Human chorionic gonadotropin was used for ovulation triggering. One good quality blastocyst (according to the classification of D. Gardner and W.B. Schoolcraft) was transferred on the 5th day after performing the transvaginal puncture of the ovary. Endometrial pathology was excluded. If the level of human chorionic gonadotropin in the blood serum increased in 14 days after embryo transfer to the uterine cavity, biological pregnancy was registered; visualization of gestation sac in the uterine cavity in 21 days after embryo transfer was evidence of clinical pregnancy.
Statistical analysis of the data was performed using software package Statistica 12 and SPSS Statistica 23 (USA).
For qualitative features, data were presented as absolute numbers and risks (%). Nonparametric method (Fisher’s exact test) was used for the comparison of categorical variables between two groups as well as for the assessment of significant differences between them. For quantitative findings, data were presented as median (25-75th percentiles). Nonparametric methods (the Mann – Whitney U test) were also used when comparing quantitative data in two groups. At a significance level of p<0.05 the results were considered statistically significant.
The research was approved by the local ethics committee of the National Medical Research Centre of Obstetrics, Gynecology and Perinatology, Moscow, Russia.
Results and Discussion
Depending on the outcomes of the IVF program following the pipelle biopsy, the patients were divided into two groups: group 1 included the women who achieved pregnancy, group 2 included the patients without pregnancy. Normal distribution of age and anthropometric data of the patients from the study groups was proved by the Kolmogorov-Smirnov test (p<0.05); however, due to the small sample size in each group, nonparametric methods were applied as well. The Mann-Whitney U test did not identify statistically significant differences in age, height, body weight, and as a result, body mass index (BMI) between the study groups (Table 1).
Statistically significant differences were not revealed in the assessment of the menstrual function (age of menarche, menstrual cycle length, duration of the menstrual bleeding) and sexual function (age of onset of sexual activity) (Table 1).
As for extragenital diseases, no statistically significant differences were revealed between the groups. According to Table 1, the study groups did not show any differences in gynecological diseases and obstetric history either. It is worth noting that there were no ovarian tumors in the history of the patients in both groups and, as a result, ovarian resections were not performed. Polycystic ovary syndrome (PCOS) was not revealed in any of the patients included in the study. In performing the comparative evaluation of the hormonal status no statistically significant differences were detected.
The suggestion was made that the patients in the study groups with positive and negative outcomes of the IVF program differ in methylation of HOXA10 and HOXA11 gene promoters that may influence the expression of the genes and as a result, endometrial receptivity. The gene promoter includes several regions in DNA sequences in which cytosine and guanine residues of CpG islands are located nearby. The enzymes carrying out the DNA methylation (DNA methyltransferases) are known to be able to attach methyl group to cytosine only in CpG islands region. Methylation of CpG islands in the gene promoter is eventually associated with the decreased expression of this gene and suppression of its function.
DNA methylation can be identified with a chemical reaction, namely bisulfate conversion. In single-stranded DNA bisulfate affects the residues of cytosine that results in their conversion to uracil. However, in case of the presence of methyl group on cytosine residues such a conversion does not occur. Given the sequence of the nucleotides in a normal human genome and DNA sequence after performing bisulfate conversion, it is possible to determine which CpG islands were methylated and which were not. In order to detect the sequence after conversion, a number of methods can be used, such as methylation specific PCR, Sanger sequencing, next generation sequencing (NGS). The latter makes it possible to determine accurately and quickly the sequence of the present DNA sequences in the mixture.
Due to the cells lysing in DNA extraction from tissue sample after pippele biopsy, ultimate mixture may contain different DNA molecules, some of which may have a certain CpG island methylated, and some of them not. Sequencing allows for detecting the presence of all possible variants of sequences in the mixture; therefore, methylation analysis of each CpG island is presented as the percentage of DNA molecules in the mixture where the certain CpG island is methylated.
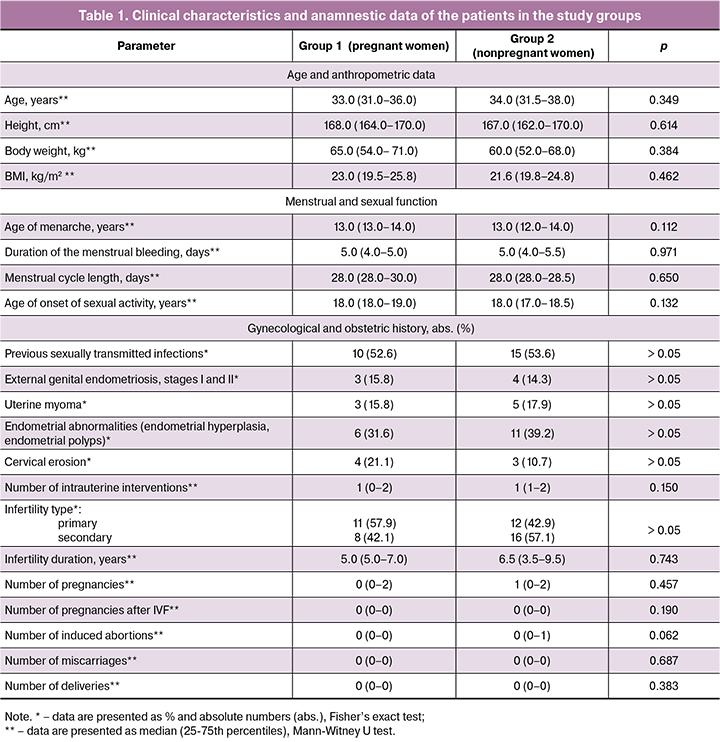
The comparison of methylation profiles of HOXA 10 and HOXA11 gene promoters is shown in Figures 1 and 2, respectively.
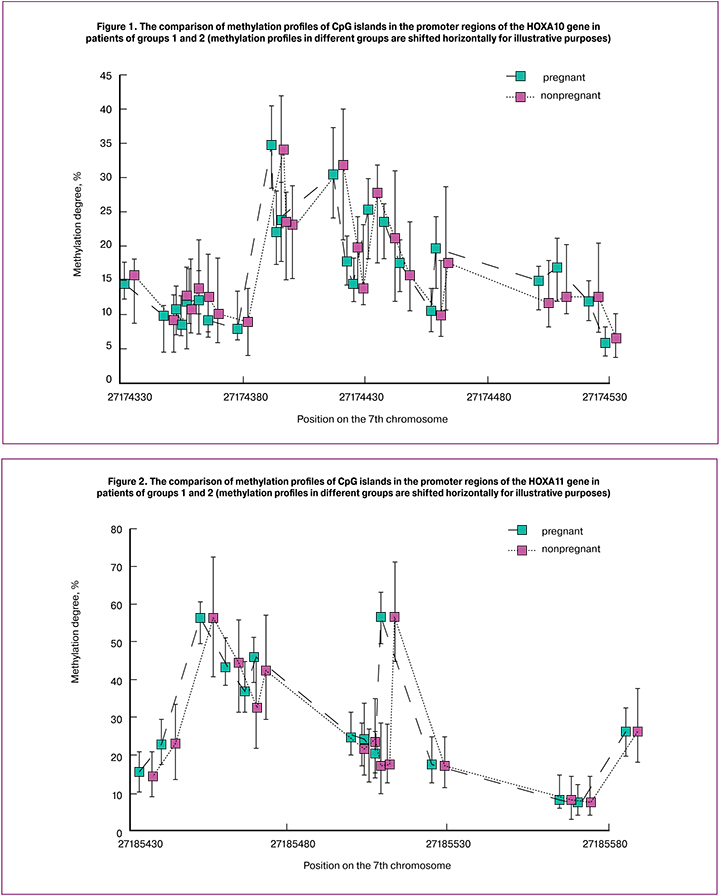
During the study period, methylation of 23 CpG islands in the promoter region of the HOXA10 gene and 15 CpG islands in the promoter region of the HOXA11 gene were analyzed. As a result, no significant difference in methylation of CpG islands in the genes was revealed. It suggests that methylation of the HOXA10 and HOXA11 genes is rather a conservative parameter for the women with the recurrent unsuccessful IVF attempts in the history and it does not influence prognosing the outcomes of IVF program.
There is a great number of studies where the connection of methylation of HOXA10 and HOXA11 gene promoters with different infertility factors is analyzed. However, the role of methylation of HOXA10 and HOXA11 gene promoters as a part of multifactorial models in women with tubal and peritoneal factor infertility has not been previously analyzed. The prospective observational study of Margioula-Siarkou et al. included 65 patients with different infertility etiologies: among them there were 13 patients with tubal and peritoneal factor infertility and 15 patients with unexplained infertility. The control group consisted of 25 healthy patients. During the study period it was determined that the HOXA11 expression in the endometrium in patients with unexplained infertility was statistically lower but patients with tubal and peritoneal factor infertility did not show the significant difference in the expression of these genes, whereas methylation of the genes was not analyzed during this study [27].
Another observational cohort study including 18 healthy controls, 12 women with recurrent implantation failures and 20 women with recurrent miscarriages demonstrated that the expression of the HOXA10 protein in the endometrium of healthy women is significantly higher than in patients from other groups that may suggest HOXA10 playing a role in unsuccessful outcomes of the IVF programs. However, methylation of the genes was not analyzed during this research either [28].
Conclusion
Thus, methylation status of the promotor regions of the HOXA10 and HOXA11 genes in patients with tubal and peritoneal factor infertility and previous unsuccessful IVF attempts has not been previously considered. Methylation of the promotor regions of the HOXA10 and HOXA11 genes does not appear to play a role in the prognosis of the IVF outcomes in the studied population, since methylation profile of the promoters of these genes is rather conservative.
References
- Доклад о репродуктивном здоровье. Всемирная организация здравоохранения, Европейское региональное бюро 2011 год. [Doklad o reproduktivnom zdorov’e. Vsemirnaya organizatsiya zdravookhraneniya, Evropeiskoe regional’noe byuro 2011. (in Russian).]
- Краснопольская К.В., Назаренко Т.А. Клинические аспекты лечения бесплодия в браке. М.: ГЭОТАР-Медиа; 2013. [Krasnopol’skaya K.V., Nazarenko T.A. Klinicheskie aspekty lecheniya besplodiya v brake. Moscow: GEOTAR-Media; 2013. (in Russian).]
- European Society of Human Reproduction and Embryology. ART fact sheet. [Электронный ресурс]. Available at: https://www.eshre.eu/-/media/sitecore-files/Guidelines/ART-fact-sheet_vFebr18_VG.pdf Accessed 02.10.2019.
- Корсак В. Сегодня существуют технологии, позволяющие вылечить бесплодие. Эффективная фармакотерапия. 2012; 18: 4-6. [Korsak V. Segodnya sushchestvuyut tekhnologii, pozvolyayushchie vylechit’ besplodie. Effektivnaya farmakoterapiya. 2012; (18): 4-6. (in Russian).]
- Leanza V., Coco L., Grasso F., Leanza G., Zarbo G., Palumbo M. Unexplained infertility and ovulatory induction with menopausal gonadotropins. Minerva Ginecol. 2014; 66(3): 303-7.
- McLachlan R.I. Approach to the patient with oligozoospermia. J. Clin. Endocrinol. Metab. 2013; 98(3): 873-80. https://dx.doi.org/10.1210/jc.2012-3650.
- van den Boogaard N.M., Bensdorp A.J., Oude Rengerink K., Barnhart K., Bhattacharya S., Custers I.M. et al. Prognostic profiles and the effectiveness of assisted conception: secondary analyses of individual patient data. Hum. Reprod. Update. 2014; 20(1): 141-51. https://dx.doi.org/10.1093/humupd/dmt035.
- Guo F., Zhou M.J., Zhang A.J. Advances in the treatment of reccurent implantation failure. Reprod. Dev. Med. 2017; 1(2): 123-6. https://dx.doi.org/10.4103/2096-2924.216860.
- Moura-Ramos M., Gameiro S., Canavarro M.C., Soares I. Assessing infertility stress: re-examining the factor structure of the fertility problem inventory. Hum. Reprod. 2012; 27(2): 496-505. https://dx.doi.org/10.1093/humrep/der388.
- Das M., Holzer H.E. Recurrent implantation failure: gamete and embryo factors. Fertil. Steril. 2012; 97(5): 1021-7. https://dx.doi.org/10.1016/j.fertnstert.2012.02.029.
- Корсак В.С., Смирнова А.А., Шурыгина О.В. Регистр центров ВРТ России. Отчет за 2013г. Проблемы репродукции. 2015; 21(6): 17-24. [Korsak V.S.,Smirnova A.A., Shurygina O.V. Russian ART register, 2013. Russian Journal of Human Reproduction/Problemy reproduktsii. 2015; 21(6): 17-24. (in Russian).]
- Herington J.L., Guo Y., Reese J., Paria B.C. Gene profiling the window of implantation: Microarray analyses from human and rodent models. J. Reprod. Health Med. 2016; 2(Suppl. 2): S19-25. https://dx.doi.org/10.1016/j.jrhm.2016.11.006.
- Краснопольская К.В., Назаренко Т.А., Ершова И.Ю. Современные подходы к оценке рецептивности эндометрия (обзор литературы). Проблемы репродукции. 2016; 22(5): 61-9. [Krasnopol’skaya K.V., Nazarenko T.A., Ershova I.Yu.Modern approaches to endometrial receptivity assessment (a review). Russian Journal of Human Reproduction/Problemy reproduktsii. 2016; 22(5): 61-9. (in Russian).] https://dx.doi.org/10.17116/repro201622561-69.
- Ginsburg E.S., Racowsky C., eds. In vitro fertilization: A comprehensive guide. New York: Springer; 2012.
- Боярский К.Ю., Гайдуков С.Н., Пальченко Н.А. Современный взгляд на проблему рецептивности и тонкого эндометрия в программах ВРТ (обзор литературы). Проблемы репродукции. 2013; 19(4): 51-60. [Boyarskii K.Yu.,Gaidukov S.N., Pal’chenko N.A. Modern look on endometrial receptivity and thin endometrium in art cycles (a review). Russian Journal of Human Reproduction/Problemy reproduktsii. 2013; 19(4): 51-60. (in Russian).]
- Коган Е.А., Калинина Е.А., Колотовкина А.В., Файзуллина Н.М., Адамян Л.В. Морфологический и молекулярный субстрат нарушения рецептивности эндометрия у бесплодных пациенток с наружно-генитальным эндометриозом. Акушерство и гинекология. 2014; 8: 47-52. [Kogan E.A., Kalinina E.A., Kolotovkina A.V., Faizullina N.M., Adamyan L.V. The morphological and molecular substrate of impaired endometrial receptivity in infertile patients with external genital endometriosis who enter an assisted reproductive technology program. Obstetrics and Gynecology/Akusherstvo i ginekologiya. 2014; (8): 47-52. (in Russian).]
- Князева Е.А., Алиева К.У., Калинина Е.А. Яичниковая беременность после программы экстракорпорального оплодотворения у пациентки со сниженной рецептивностью эндометрия. Акушерство и гинекология. 2018; 8: 180-5. [Knyazeva E.A., Alieva K.U., Kalinina E.A. Ovarian pregnancy after an IVF program in a patient with reduced endometrial receptivity. Obstetrics and Gynecology/Akusherstvo i ginekologiya. 2018; (8): 180-5. (in Russian).] https://dx.doi.org/10.18565/aig.2018.8.180-184.
- Князева Е.А., Калинина Е.А., Быстрицкий А.А., Алиева К.У., Байрамова Г.Р. Роль НОХ-генов при заболеваниях репродуктивной системы женщины, ассоциированных с бесплодием. Акушерство и гинекология. 2017; 11: 16-22. [Knyazeva E.A., Kalinina E.A., Bystritsky A.A., Alieva K.U., Bairamova G.R.Role of HOX genes associated with infertility in female reproductive system diseases. Obstetrics and Gynecology/Akusherstvo i ginekologiya. 2017; (11): 16-22. (in Russian).] https://dx.doi.org/10.18565/aig.2017.11.16-22.
- Ping L.I., Xia C.A.I. Effect of HOXA10 gene expression on embryonic implantation in patients with endometriosis. J. Int. Reprod. Health Fam. Plan. 2013; 32(6): 506-8.
- Taniguchi Y. Hox transcription factors: modulators of cell-cell and cell-extracellular matrix adhesion. Biomed. Res. Int. 2014; 2014: 591374. https://dx.doi.org/10.1155/2014/591374.
- Simon A., Laufer N. Repeated implantation failure: clinical approach. Fertil. Steril. 2012; 97(5): 1039-43. https://dx.doi.org/10.1016/j.fertnstert.2012.03.010.
- Yang Y., Chen X., Saravelos S.H., Liu Y., Huang J., Zhang J., Li T.C. HOXA-10 and E-cadherin expression in the endometrium of women with recurrent implantation failure and recurrent miscarriage. Fertil. Steril. 2017; 107(1): 136-43. e2. https://dx.doi.org/10.1016/j.fertnstert.2016.09.016.
- Bourdiec A., Ahmad S.F., Lachhab A., Akoum A. Regulation of inflammatory and angiogenesis mediators in a functional model of decidualized endometrial stromal cells. Reprod. Biomed. Online. 2016; 32(1): 85-95. https://dx.doi.org/10.1016/j.rbmo.2015.09.011.
- Du H., Taylor H.S. The role of Hox genes in female reproductive tract development, adult function, and fertility. Cold Spring Harb. Perspect. Med. 2016; 6(1): a023002. https://dx.doi.org/10.1101/cshperspect.a023002.
- Wu Y., Halverson G., Basir Z., Strawn E., Yan P., Guo S.W. Aberramt methylation at HOXA10 may be responsible for its aberrant expression in the endometrium of patients with endometriosis. Am. J. Obstet. Gynecol. 2005; 193(2): 371-80. https://dx.doi.org/10.1016/j.ajog.2005.01.034.
- Сухих Г.Т., Осипьянц А.И., Мальцева Л.И., Смолина Г.Р., Полозников А.А., Муйжнек Е.Л., Киселев В.И. Аномальное гиперметилирование генов НОХА10 и НОХА11 при бесплодии, ассоциированном с хроническим эндометритом. Акушерство и гинекология. 2015; 12: 69-74.[ Sukhikh G.T., Osipyants A.I., Maltseva L.I., Smolina G.R., Poloznikov A.A., Muizhnek E.L., Kiselev V.I. Abnormal hypermethylation of HOXА10 and HOXА11 genes in chronic endometritis-related infertility. Obstetrics and Gynecology/Akusherstvo i ginekologiya. 2015; (12): 69-74. (in Russian).]
- Margioula-Siarkou C., Petousis S., Milias S., Ravanos K., Kalogiannidis I., Mavromatidis G. et al. Endometrial expression of Leukemia Inhibitory Factor (LIF), LIF-receptor and HOXA11 but not HOXA-10 is significantly impaired in women with unexplained infertility during implantation window. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016; 206: e165-6. https://dx.doi.org/10.1016/j.ejogrb.2016.07.410.
Received 03.10.2019
Accepted 29.11.2019
About the Authors
Ekaterina A. Knyazeva, Post-graduate student of B.V. Leonov Department of Auxiliary Technologies in Infertility Treatment, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov. Tel.: +7 (495)438-25-01. E-mail: dr.knyazeva.ea@gmail.com. ORCID: 0000-0002-1472-2018.4 Oparina str., Moscow, 117997, Russian Federation.
Maria V. Kuznetsova, PhD, Researcher of the laboratory of molecular genetics methods, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov. Tel.: +7(495)438-22-92. E-mail: mkarja@mail.ru. ORCID: 0000-0003-3790-0427
Ekaterina S. Shubina, PhD, head of the Department of Laboratory genomic data, National Medical Research Center for Obstetrics, Gynecology and Perinatology named
after Academician V.I. Kulakov. Tel.: +7(926)721-87-17 Е-mail: e_shubina@oparina4.ru.
4 Oparina str., Moscow, 117997, Russian Federation.
Andrey Yu. Goltsov, Researcher of the laboratory of molecular genetics methods, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov. Tel.: +7(495)438-22-92. E-mail: andrey.goltsov@gmail.com. ORCID: 0000-0002-4004-4214.
4 Oparina str., Moscow, 117997, Russian Federation.
Andrey E. Donnikov, PhD, Head of the Department of Laboratory and Genetic Methods, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov. Tel.: +7(495)438-77-00. E-mail: a_donnikov@oparina4.ru. ORCID: 0000-0003-3504-2406.
4 Oparina str., Moscow, 117997, Russian Federation.
Elena A. Kalinina, MD, Associate Professor, Chief of B.V. Leonov Department of Auxiliary Technologies in Infertility Treatment named after professor B.V. Leonov
National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov. Tel.: +7(495)438-13-41.
E-mail: e_kalinina@oparina4.ru. ORCID: 0000-0002-8922-2878. 4 Oparina str., Moscow, 117997, Russian Federation.
For citation: Knyazeva Е.А., Kuznetsova М.V., Shubina Е.S., Goltsov А.Yu., Dоnnikоv А.Е., Kalinina Е.А. Characteristics of methylation of hoxa10 and hoxa11 genes in patients with tubal and peritoneal factor infertility and previous unsuccessful ivf attempts.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2020; 4: 140-147. (In Russian).
https://dx.doi.org/10.18565/aig.2020.4.140-147



