Diagnostic features, clinical picture and management of adnexal torsion in female adolescents
Background: The nonspecific clinical picture and the lack of early diagnosis of adnexal torsion (AT) lead to the persistence of high percentage of surgical removal of organs, including in children and adolescents.Khashchenko E.P., Uvarova E.V., Sheshko P.L., Kleymenova M.N., Kyurdzidi S.O., Salnikova I.A., Chuprynin V.D., Mamedova F.Sh., Asaturova A.V.
Objective: To evaluate the diagnostic features and clinical picture of adnexal torsion in female adolescents.
Materials and methods: A retrospective case-control study was carried out. The main group included 29 girls with confirmed adnexal torsion aged 11–17 years. The comparison group consisted of 27 somatically healthy girls of the same age. A comparative analysis of clinical, anamnestic, laboratory and instrumental data of patients and healthy girls before surgery and 3 months after surgery was carried out.
Results: In most cases, adnexal torsion was observed on the background of ovarian tumor (53.9%) and paraovarian cyst (46.2%). Most often, AT occurred on the right side (65.5%). According to multivariate analysis, significant diagnostic criteria for adnexal torsion in girls were acute abdominal pain (F=11.4; p=0.001), especially in combination with nausea and vomiting (F=5.8; p=0.20), that can be considered as an indication for emergency laparoscopy. The reduction in blood flow or absence of flow velocity according to color flow Doppler (F=15.6; p=0.000), the presence of additional ultrasound signs (spiral blood flow, increased ovarian volume and swelling of ovarian tissue, impaired visualization of the follicular in the parenchyma (F=8.42; 0.005)), may indicate adnexal torsion. Other parameters (hyperthermia (p=0.21), leukocythemia (p=0.07), CRP (p=0.44)) were not significant criteria for the diagnosis of adnexal torsion. In 89.7% of cases detorsion of uterine appendages was performed, and in 6.9% of cases, adnexectomy was performed due to complete necrosis; in 20.7% of patients, organ sparing surgery was supplemented with ovariopexy and shortening of infundibulopelvic ligaments.
Conclusion: In cases of elongated ovarian ligaments and recurrent torsion in the absence of other obvious causes of adnexal torsion in female adolescents, ovariopexy or plication of the ligaments was justified. No septic complications were observed after detorsion, including the cases when ovaries were stained black. Organ sparing surgery was performed in all cases, except for a long-term torsion in medical history and total tissue necrosis. 3 months after detorsion, despite the presence of black staining during surgery, the color Doppler ultrasound image indicated resumption of blood flow in the ovary.
Keywords
Adnexal torsion (AT) (ICD-10 code N83.5) ranks fifth in the occurrence of gynecologic surgical emergencies that require immediate surgical intervention. On average, AT occurs in 15% of girls and is 2.7% of all cases of acute abdominal pain in children, as well as 0.3–3.5% of cases of acute pathology of abdominal cavity per year [1–3]. Adnexal torsion is defined as twisting of the ovary, fallopian tube, or both twisting with the vascular pedicle, or more rarely, twisting of the ovary or tube alone [4].
Intact uterine appendages torsion is more common in children (15–50%), than in adults (8–18%) [4]. Among most common volumetric neoplasms, teratoma torsion is in 20–30% of children, torsion of cystadenoma in 20–30% and ovarian torsion in 20–25% [4]. Most common, AT is seen on the right side possibly due to the fact that the left ovary lies in close proximity to relatively immobile sigmoid colon, while the right ovary is located close to the hypermobile caecum and ileum [2]. Intact uterine appendages torsion that often occurs in children, most commonly is a consequence of congenital lengthening of the ovarian ligament, or weakness of the pelvic ligaments [5].
Specific indicators for AT do not exist in laboratory tests. Among the mandatory laboratory tests for the diagnosis of AT, the complete blood count (CBC) and human chorionic gonadotropin test (taking into account the patient's age and the sexual activity) are performed to exclude ectopic pregnancy. According to limited published data available in MEDLINE, PubMed, Scopus and Cochrane libraries regarding specific management of children with AT, only a small number of patients with AT had a high white blood cell (WBC) count (>12×109/L), at the same time different studies demonstrate various frequency of occurrence of this symptom (from 20% to 56%) [5–8]. Also, the patients with AT may have high levels of C-reactive protein (CRP) including at the stage of formation of appendage tissue necrosis [9]. However, most often a high level of CRP is associated with appendicitis, but not with AT [10].
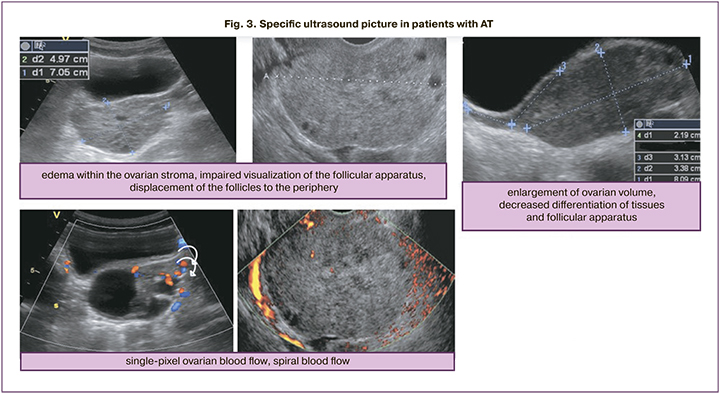
The Doppler ultrasound with color flow mapping (CFM) remains the most sensitive and specific method for diagnosing AT [11]. Ultrasound signs of AT include increased ovarian size, impaired blood flow, edema and heterogeneity of the ovarian parenchyma [4, 6, 12]. According to foreign researchers, the best quantitative determinants of AT are ovarian surface area (≥18.5 cm2), the ratio of the surface area of the damaged ovary to the surface area of the contralateral ovary (≥4.9), as well as ovarian volume (≥17 см3) [4, 6, 11–13]. The ovarian stroma may be heterogeneous due to edema, and the presence of hemorrhages, a decline in the of ovarian tissues differentiation is possible. [13]. Reduction or lack of blood flow seen by CFM was generally detected in most cases of AT along with enlarged ovarian size [4]. Ultrasound picture of the impaired blood flow in cases of AT may be different and depends on the degree of blood flow impairment, which in turn is determined by the period of the existence of adnexal torsion. One of the studies reported that in patients with confirmed AT, lack of arterial blood flow was detected only in 73% of cases [12]. Another study showed that a normal blood flow was in 60% of patients [14]. The blood flow may be present in cases of partial adnexal torsion, twisting of ovary or in cases when ultrasound is performed in the earliest stages of torsion [6]. Reduction or lack of arterial blood flow was most commonly detected with concomitant venous blood flow dysfunction (in 90–93% of cases) [12]. This can be explained by the fact that first of all, the torsion of the vascular pedicle affects the venous system of appendage, and then the arterial one [13].
Despite the fact that reduction or lack of blood flow indicates torsion, not all cases of AT are diagnosed in the presence of this sign [15, 16]. Some authors describe the typical diagnostic sign of AT as spiral blood flow in swirled appearance of the epididymal vessels, and this may be important to determine the viability of the ovary [2, 17, 18]. The twisted vascular pedicle is visualized by US as an echogenic roundish or beak-shaped mass with multiple concentric bands. Doppler ultrasound imaging shows circular or spiral arteries [17].
Moreover, only in cases of AT ultrasound detects multiple small cysts (of 8–12 mm up to 25 mm) around the periphery of the ovary due to displacement of follicles caused by venous congestion, as well as due to extravasation of fluid into follicles in cases of severe edema [11, 12, 19]. Findings of small cysts aroun the periphery of the ovary in combination with enlarged ovary on the background of abdominal pain is an important determinant of possible torsion [19].
AT is an emergency condition and indication for surgical intervention [2]. During many years, the standard approach to surgical treatment of AT was adnexectomy. The opposite conservative approach is unwinding of the twisted pedicle of the appendage and organ preservation [20–23]. The results of recent studies showed that laparoscopic detorsion could preserve the ovary even in cases when blood flow was impaired and the color of the ovarian tissue became lifeless, and it did not increase the risk of thromboembolism in the postoperative period [22–25]. A number of authors reported that after laparoscopic detorsion of appendages even when the tissue color was intraoperatively dark blue and black, restoration of ovarian function and resumption of blood flow occurred in 88–93% of cases 3 months after surgery [5, 24, 26–28]. The dark color of the ovary during surgical intervention possibly was a manifestation of venous and lymphatic stasis, but not arterial ischemia. This indicates the possibility of recovery of ovary function. In addition, it is critically important to shorten the time from torsion occurrence to surgical treatment. Due to this necrosis can be avoided and blood flow can be restored in the epididymis after laparoscopic detorsion [26, 29–31].
Thus, AT belongs to the group of pathologies characterized by severe abdominal pain and having no specific symptoms. Due to the fact that this pathology commonly manifests by the symptoms of "acute abdomen", a large number of patients are hospitalized to surgical departments, and the diagnostic stage does not include consulting with obstetrician-gynecologist. Delayed diagnosis and treatment can lead to harmful consequences, including irreversible damage to ovary and reduction of reproduction potential, as well as purulent-septic complications.
The aim of the study was to evaluate the diagnostic features and clinical picture of adnexal torsion in female adolescents.
Materials and methods
Considering the aim and objectives of the retrospective case-control study, medical histories of 29 patients with AT, who underwent treatment in the 2nd Gynecologic Department of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of the Ministry of Health of the Russian Federation in the period January 2017 – December 2021, were studied.
The comparison group consisted of 27 somatically healthy girls of the same age, who had no gynecologic and endocrine pathologies. The study was approved by the Ethics Committee of biomedical researches of the Center. The patients and their legal representatives have signed informed consent for participation in the study, the use of their personal data and publication of the results of the study.
The first stage of the study was analysis of anamnestic, laboratory and instrumental data of patients before laparoscopy versus the group of healthy girls (Fig. 1). Medical examination included the clinical methods (anamnestic data collection, assessment of complaints of pain, nausea, vomiting, and high temperature), laboratory methods (clinical analysis of blood, biochemical blood test, CRP test) and the Doppler ultrasound with CFM.
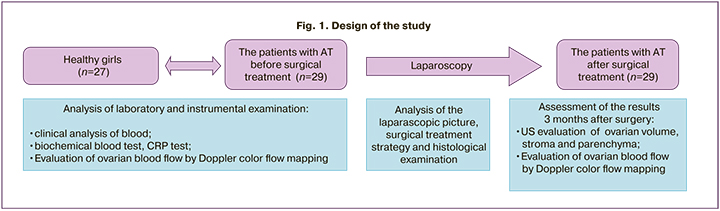
Then the laparascopic picture in each clinical case and the surgical treatment strategy were analyzed, and the results of surgery were assessed by subsequent pelvic ultrasound 3 months after surgery (including assessment of the volume of both ovaries and blood flow parameters).
The results of histological examination of the tissues resected during laparoscopy were studied, and the types of large ovarian mass involved in torsion were analyzed.
Statistical analysis
Statistical data processing was performed using software programs Microsoft Excel and Statistica 13.3 (Statsoft Inc.). Assessment of differences between the groups in non-parametric disctibution of quantitative variables for independent samples was performed using Mann–Whitney U-test, and Wilcoxon test was used for dependent samples. Comparison of variables with normal distribution provided that the homogeneity of dispersions in several independent groups was carried out using ANOVA. Assessment of categorical variables was carried out by calculation of frequencies and rates (%). Contingency tables were used to compare the differences and χ² test was used for calculations. Risk factors were assessed by multivariate analysis using logistic regression.
Results
Among 29 patients with diagnosed AT, 3 patients had recurrent AT. Moreover, recurrence of adnexal torsion was twice in one of these patients. In 19/29 (65.5%) cases, adnexal torsion was detected on the right side. Torsion of the large adnexal mass was found in 26/29 (89.7%), appendages remained unchanged in 3/29 (10.3%) female adolescents. Whole appendage torsion was in 15/29 (51,7%) girls, fallopian tube torsion and ovarian ligaments mass formation was in 7/29 (24.1%) girls, and only ovarian torsion was in 2/29 (69%) of girls, and only fallopian tube torsion was in 1/29 (3.4%) girl. Large adnexal masses were presented as paraovarian cyst in 12/29 (46.2%) girls, mature cystic teratoma in 8/29 (30.8%) girls, serous cystadenoma in 4/29 (15.4%), sex cord stromal ovarian tumor (benign) in 1/29 (3.85%) girl and as follicular cyst in 1/29 (3,85%) girl.
Analysis of the clinical picture showed the following regular patterns: 10/29 (34.5%) complained of severe pain, in some of them severe pain occurred on the background of intermittent pain. In total, 13/29 (44.8%) patients complained of intermittent pain occurrence. Among the patients with severe pain, there were girls who showed up in the Center within several hours or days from the time of seizure occurrence, as well as those, who showed up in the Center several months after the seizure. Pain was absent in 8/29 (27.6%) girls, and the reason for showing up was their wish to clarify the diagnosis and treatment tactics for the large adnexal mass (Fig. 2). Nausea and vomiting accompanied painful seizure in 6/29 (20.7%) patients, and hyperthermia was detected in 2/29 (6.9%).
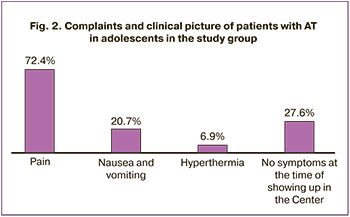
Analysis of categorical data was performed using ANOVA methods. It was found that significant risk factors were severe abdominal pain (F=11.4; p=0.001), nausea and vomiting (F=5.8; p=0.20). Hyperthermia was insignificant risk factor for AT (p=0.21). Further analysis showed that in girls complaining of intermittent pain, torsion was not tight. This probably explained the absence of the signs of persistent impairment of blood supply, and intermittent pain was associated with periodic twisting of the appendage.
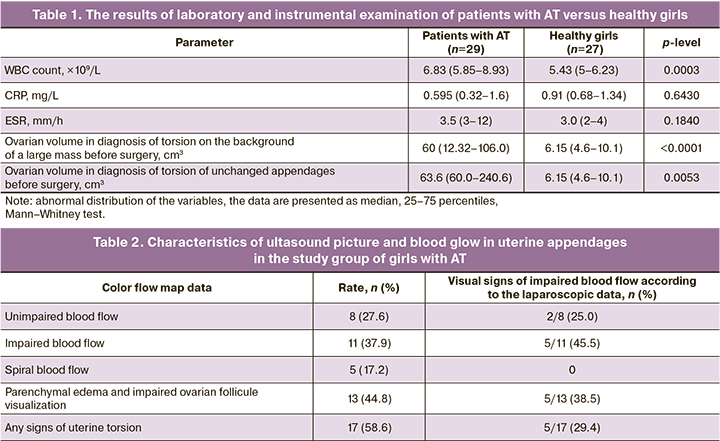
White blood cell (WBC) count, the levels of C-reactive protein and erythrocyte sedimentation rates (ESR) were analyzed in the study group. High reference levels of CRP were found in 4/29 (13.8%) cases in the group of patients with AT. In addition, in 2 of these cases US evaluation showed the sighs of impaired blood flow in the appendage, and laparoscopic and histological examination detected necrotic changes in 1 case. Higher levels of ESR (21.4%) were detected in 3 patients in the group with adnexal torsion, and necrotic changes in the uterine appendages were observed in 2 patients. The comparative analysis of the range of WBC count showed the difference in the values of this parameter in girls with AT and in healthy girls (6.83 (5.85–8.93) versus 5.43 (5.01–6.23), p=0.0003, Mann–Whitney test) (Table 1).
Assessment of risk factors using logistic regression showed that the values of WCB count, C-reactive protein and ESR are not significant predictors of AT in adolescent patients (χ2=3.1443, p=0.7620).
According to US assessment, ovarian volume was higher in patients with torsion of unchanged appendages and torsion on the background of a large mass versus healthy girls (63.6 (60.0–240.6) and 60 (12.32–106.0) versus 6.15 (4.6–10.1)). Doppler color flow mapping did not detect the signs of AT in 17/29 (58.6%) cases. В 8/29 (37,9%) impairment of ovarian blood flow (low or zero-flow) was detected. Spiral blood flow was visualized in 5/29 (17.2%) cases. In 8/29 (27.6%) cases, CFM detected unimpaired blood flow. At the same time, in 2 cases of laparoscopy impaired blood flow in the appendage was visualized, and histologically confirmed necrotic changes were detected in 1 case (Table 2).
In cases of laparoscopy, the signs of impaired blood supply and/or necrosis were most commonly visualized in the presence of impaired blood flow detected by Doppler ultrasound with CFM. In cases of spiral blood flow, torsion presented an average of 2 turns and was loose. Perhaps, this was due to the absence of visual signs of impaired blood supply to the appendage in cases of laparoscopy.
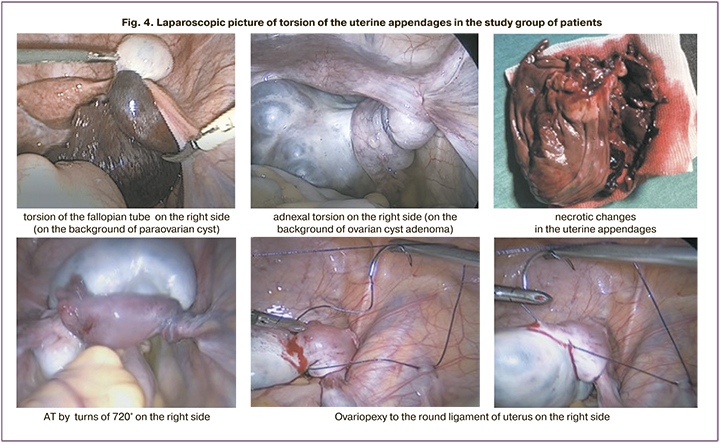
All patients underwent laparoscopic surgery. During laparoscopy, torsion of total adnexa was diagnosed with visual signs of impaired blood flow presented by in 8/29 (27.6%) cases. In addition, necrotic changes were seen in 3/29 (10.3%) cases, and blue color of the appendage tissues presented the signs of impaired blood supply in 5/29 (17.2%) cases. In cases of necrotic changes in the appendages, the tissues were dirty yellow in color, and often formed a conglomerate of surrounding tissues, and necrosis was confirmed by pathomorphological examination of the surgical specimens. In 2 patients, necrosis of the appendage was observed, due to this andexectomy was performed. One patient was diagnosed with fallopian tube necrosis, and she underwent tubectomy. 3 patients had total torsion of adnexa that was twisted for several turns (>720°), however, blood flow was preserved. The signs of impaired blood flow with tubal and/or ovarian edema with dark blue and black color of the appendage and formation of small cysts on ovarian surface were observed in 5 cases.
In all cases (26/29 (89.6%) of absent necrotic changes, laparoscopic detorsion was performed. In all cases (8/26 (27.6%) of impaired blood supply to uterine appendages after detorsion, restoration of the appendage color was seen under dynamic observation during 10 minutes. In other cases (21/29 (72.4%)), the appendages were visualized without the signs of ischemia or necrosis. Ovariopexy was performed in 6/29 (20.7%) patients: ovariopexy of the contralateral ovary after adnexectomy was performed in 1 girl; bilateral ovariopexy and plication of the ligaments due to pathological length change was performed in 2 girls; in 1 girl after tubectomy; in 1 girl with multifollicular ovaries and in 1 girl with recurrence of AT.
Further we analyzed the period from occurrence of the first and last painful seizures before laparoscopy. Among 10/29 (34.5%) patients with complaints of severe pain, the shortest period of time before surgery was several hours in 3 patients. Moreover, one of the patients had seizure for the first time, and impairment of blood flow in the appendage was seen during laparoscopy. In the second patient, the first seizure occurred several days before surgery, and impairment of blood supply to the viable appendage was also visualized during laparoscopy. In the third patient, the first painful seizure occurred 6 month before surgical intervention, but no signs of ischemia were observed during surgery due to loose adnexal torsion. Of two patients with diagnosed necrosis, one girl had severe pain two weeks before surgery, and another girl 2 months before surgery. In other 5 patients with severe pain, the time period before showing up for healthcare in hospital was from 10 days to 2 months. In one of them, the signs of ischemia of the viable appendage were detected, and the time period from painful seizure to surgery was 10 days. In the absence of clinical picture of severe pain, but in the presence of intermittent lower abdominal pain with muscle stretching in 13/29 (44.8%) patients, the period of pain presence before surgery was from 3 weeks to 7 months. No signs of impaired blood flow on the background of loose and total torsion were found in any of these patients. In 8/29 (27.6%) cases of asymptomatic AT, the signs of impaired blood flow with subsequent resumption were observed in 2 patients, and necrotic changes in the appendage were found in 1 patient.
Comparative assessment of the volume of twisted ovary before surgery versus ultrasound evaluation performed 3 months after surgery showed significant reduction in ovarian size 60.0 (14.3–97.0) cm3versus 16.7 (7.9–22.5) cm3, р-value =0.0015, the Wilcoxon test). Even in cases when blood flow to the ovary was impaired and blood turned a black color during surgery, reduction in the size of the ovary and restoration of stromal differentiation and resumption of blood flow to the ovarian tissue were observed 3 months after detorsion.
Discussion
The study confirmed the relevance of the issue of timely diagnosis of AT in adolescents. The obtained results correlated with published data on the incidence rate of adnexal torsion including the unchanged appendages and on the background of large ovarian mass in children and adolescents. At the same time, AT was most commonly observed on the right side, and paraovarian cyst or mature cystic teratoma was involved in torsion most often [23, 31]. The major clinical symptom, which prompted most girls to see the doctor, was lower abdominal pain. The significance of this symptom was described in many publications, reporting the study of the clinical picture of AT in adults [32–34], as well as in limited published studies of AT in adolescents [3, 35]. This study confirmed that acute pain was detected only in some patients [36]. In the study group, the clinical picture of acute pain versus poorly distinguished manifestation was present in fewer cases. Due to this, it was difficult to establish the diagnosis in girls.
Adnexal torsion is a diagnosis requiring immediate attention of obstetricians, gynecologists and surgeons, because due to the non-specific clinical picture of patients with “acute abdomen” are often admitted to surgical departments, and the diagnosis of AT can be missed. Moreover, it is known that due to impaired blood supply, the intensity of clinical symptoms and complaints of pain tend to decrease until the complete absence of complaints in patients with necrosis of the uterine appendages in cases of long-term AT. Analysis of anamnestic data showed that 8/29 (27.5%) patients had seizure of acute pain in anamnesis, and they were examined to rule out acute appendicitis or acute colitis, and adnexal torsion was not suspected. These patients were admitted to the gynecological department in the hospital, when recurrent attack occurred. Due to this, analysis of early symptoms of AT for timely diagnosis and emergency surgical care is of particular relevance. According to the results of the study, the significant parameters in diagnosis of AT were defined: acute abdominal pain. nausea and vomiting on the background of painful seizures, as well as ultrasound assessment and Doppler color flow mapping (reduction or lack of blood flow, spiral blood flow, increased ovarian volume and ovarian tissue edema, impaired visualization of ovarian follicular apparatus due to parenchymal edema), and they correlated with published data [3, 6, 36, 37].
The features of the clinical picture in the study group were associated with the degree of torsion of the uterine appendages. The patients without severe symptoms or with absent symptoms, had loose and total torsion or long-term necrosis of uterine appendages. Adnexal torsion was detected in some patients because of elective surgical intervention due to the presence of large ovarian mass. This confirms commonly observed situation, when the diagnosis of AT is delayed or missed, and surgery is also delayed.
Undoubtedly, instrumental research methods are of great importance in the diagnosis of AT. At the same time, during performance of pelvic ultrasound, it is necessary to assess the presence of the signs, such as increased ovarian volume and unclear stromal differentiation, impaired or spiral blood flow.
Laboratory methods are auxiliary Although, comparison between the parameters of white blood cell count in patients with AT versus healthy girls showed significantly high values, multifactoral analysis did not confirm the significance of the number of white blood cells, the level of C-reactive protein and ESR in diagnosis of AT in female adolescent patients.
AT is an indication for emergency surgery, since ischemia and tissue necrosis develop as a result of AT. Delayed diagnosis reduces the chances for maintaining a viable ovary, and this is especially relevant in childhood. According to the results obtained in the study group, in the vast majority of cases (27/29 (93.1%)) of AT, the ovary remained viable, including the cases of impairment of ovarian blood supply system. According to some others, it is known that about 88–93% of impairments of impaired blood supply to ovaries are reversible, and organ-sparing approach in management of patients with AT is justified. [5, 24, 26–28]. Assessment of the clinical picture and laparoscopic data showed that ischemia and necrosis generally develop in case of tight torsion, and most commonly acute symptoms are present. In cases of gradual twisting of the appendage the symptoms may be absent, but this does not rule out possible development of necrotic changes. Adnexal ischemia and necrosis could be suspected by Doppler ultrasound and CFM data. But the signs of ischemia were present not in all cases of impaired blood flow according to the results of laparoscopic Doppler sonography, and in the presence of visual signs of impaired blood supply, the ovary was retained. All this indicated that surgical tactics should be based on the organ-sparing approach, even in cases of impaired blood supply seen by Doppler sonography or visually. In the clinical research that involved 58 women, who underwent laparoscopic detorsion, restoration of ovarian function was in 94% of cases, despite the presence of dark blue and black color of the ovary [27]. In our study, in all women with dark blue and black color of the ovary during laparoscopy, physiological ovarian color restored within 10–15 minutes after performance of detorsion, and resumption of blood flow to the ovary, restoration of the size and stromal differentiation was confirmed by Doppler ultrasound with CFM 3 months after the surgery. Moreover, after detorsion of the appendage, even when the color of tissues was dark blue and black, no cases of thromboembolic or septic complications were registered in the postoperative period. This fact speaks in favor of maximal use of organ-sparing approach for surgical intervention in children.
Thus, management strategy for patients with AT in adolescents is based on organ-sparing approach and performance of detorsion of twisted ovary even in cases of serious blood flow impairment. In cases of congenital elongation of ovarian ligaments, recurrent torsion and in the absence of other obvious causes of adnexal torsion in female adolescents, ovariopexy to the round ligament of the uterus, as well as plication and shortening of ligaments was performed.
Conclusion
1. In most cases, AT in adolescent patients was observed on the background of mass formation in the ovary (53.9%) and paraovarian cyst (46.2%). Torsion of unchanged appendages was observed in 10,3% of cases, and AT was most often observed on the right side (65.5%).
2. In 80% of cases, adolescents with AT complained of seizures of acute abdominal pain (F=11,4; p=0.001), in one third of cases in combination of nausea and vomiting (F=5.8; p=0.20), which are significant signs in the diagnosis of AT and and indication for emergency surgery.
3. According to ultrasound assessment, Doppler color flow mapping displayed reduction or lack of blood flow to ovarian tissues (F=15.6; p=0.000), as well as spiral blood flow, increased ovarian volume and edema of ovarian tissue, impaired visualization of ovarian follicular apparatus due to parenchymal edema (F=8.42; p=0.005). Other parameters of clinical and laboratory tests did not present difference versus the group of healthy girls (hyperthermia (p=0.21), leukocythemia (p=0.07), the level of C-reactive protein (p=0.44)).
According to laparoscopic picture, tight torsion with the signs of impaired blood flow to the uterine appendages was observed in 27.6% of cases; loose torsion with preservation of physiological color of tissues was in 72.4% of cases; detorsion of uterine appendages was performed in 89.7% of cases, and adnexectomy was performed due to total necrosis in 6.9% of cases; organ sparing surgery was supplemented with ovariopexy and shortening of infundibulopelvic ligaments 20.7% of patients.
4. In the postoperative period, resumption of blood flow to the ovary according to Doppler ultrasound scan and reduction in ovarian size was observed 3 months after surgery (р=0.001). This confirms advisability of organ-sparing tactics for management of adolescent patients with AT.
References
- Батырова З.К., Чундокова М.А., Уварова Е.В., Кумыкова З.Х., Хащенко Е.П., Чупрынин В.Д., Луньков С.С., Киселева И.А., Латыпова Н.Х., Буралкина Н.А. Перекрут придатков матки. Органосохраняющая тактика. Акушерство и гинекология. 2017; 9: 148-52. [Batyrova Z.K., Chundokova M.A., Uvarova E.V., Kumykova Z.Kh., Khashchenko E.P., Chuprynin V.D., Lunkov S.S., Kiseleva I.A., Latypova N.Kh., Buralkina N.A. Adnexal torsion. Organ-sparing tactics. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2017; (9): 148-52. (in Russian)]. https://dx.doi.org/10.18565/aig.2017.9.148-52.
- Sasaki K.J., Miller C.E. Adnexal torsion: review of the literature. J. Minim. Invasive Gynecol. 2014; 21(2): 196-202. https://dx.doi.org/10.1016/j.jmig.2013.09.010.
- Adeyemi-Fowode O., Lin E.G., Syed F., Sangi-Haghpeykar H., Zhu H., Dietrich J.E. Adnexal torsion in children and adolescents: a retrospective review of 245 cases at a single institution. J. Pediatr. Adolesc. Gynecol. 2019; 32(1): 64-9. https://dx.doi.org/10.1016/j.jpag.2018.07.003.
- Kives S., Gascon S., Dubuc É., Van Eyk N. No. 341-diagnosis and management of adnexal torsion in children, adolescents, and adults. J. Obstet. Gynaecol. Canada. 2017; 39(2): 82-90. https://dx.doi.org/10.1016/j.jogc.2016.10.001.
- Pansky M., Smorgick N., Herman A., Schneider D., Halperin R. Torsion of normal adnexa in postmenarchal women and risk of recurrence. Obstet. Gynecol. 2007; 109(2, Pt 1): 355-9. https://dx.doi.org/10.1097/01.AOG.0000250969.15438.17.
- Shadinger L.L., Andreotti R.F., Kurian R.L. Preoperative sonographic and clinical characteristics as predictors of ovarian torsion. J. Ultrasound Med. 2008; 27(1): 7-13. https://dx.doi.org/10.7863/jum.2008.27.1.7.
- Kart C., Aran T., Guven S., Karahan S.C., Yulug E. Acute increase in plasma D-dimer level in ovarian torsion: An experimental study. Hum. Reprod. 2011; 26(3): 564-8. https://dx.doi.org/10.1093/humrep/deq378.
- Chang S.D., Yen C.F., Lo L.M., Lee C.L., Liang C.C. Surgical intervention for maternal ovarian torsion in pregnancy. Taiwan. J. Obstet. Gynecol. 2011; 50(4): 458-62. https://dx.doi.org/10.1016/j.tjog.2011.10.010.
- Tobiume T., Shiota M., Umemoto M., Kotani Y., Hoshiai H. Predictive factors for ovarian necrosis in torsion of ovarian tumor. Tohoku J. Exp. Med. 2011; 225(3): 211-4. https://dx.doi.org/10.1620/tjem.225.211.
- Tsafrir Z., Hasson J., Levin I., Solomon E., Lessing J.B., Azem F. Adnexal torsion: Cystectomy and ovarian fixation are equally important in preventing recurrence. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012; 162(2): 203-5. https://dx.doi.org/10.1016/j.ejogrb.2012.02.027.
- Graif M., Itzchak Y. Sonographic evaluation of ovarian torsion in childhood and adolescence. AJR Am. J. Roentgenol. 1988; 150(3): 647-9. https://dx.doi.org/10.2214/ajr.150.3.647.
- Albayram F., Hamper U.M. Ovarian and adnexal torsion: Spectrum of sonographic findings with pathologic correlation. J. Ultrasound Med. 2001; 20(10): 1083-9. https://dx.doi.org/10.7863/jum.2001.20.10.1083.
- Chang H.C., Bhatt S., Dogra V.S. Pearls and pitfalls in diagnosis of ovarian torsion. Radiographics. 2008; 28(5): 1355-68. https://dx.doi.org/10.1148/rg.285075130.
- Peña J.E., Ufberg D., Cooney N., Denis A.L. Usefulness of Doppler sonography in the diagnosis of ovarian torsion. Fertil. Steril. 2000; 73(5): 1047-50. https://dx.doi.org/10.1016/S0015-0282(00)00487-8.
- Linam L.E., Darolia R., Naffaa L.N., Breech L.L., O’Hara S.M., Hillard P.J. et al. US findings of adnexal torsion in children and adolescents: Size really does matter. Pediatr. Radiol. 2007; 37(10): 1013-9. https://dx.doi.org/10.1007/s00247-007-0599-6.
- Ben-Ami M., Perlitz Y., Haddad S. The effectiveness of spectral and color Doppler in predicting ovarian torsion: A prospective study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2002; 104(1): 64-6. https://dx.doi.org/10.1016/S0301-2115(02)00056-8.
- Lee E.J., Kwon H.C., Joo H.J., Suh J.H., Fleischer A.C. Diagnosis of ovarian torsion with color Doppler sonography: Depiction of twisted vascular pedicle. J. Ultrasound Med. 1998; 17(2): 83-9. https://dx.doi.org/10.7863/jum.1998.17.2.83.
- Vijayaraghavan S.B. Sonographic whirlpool sign in ovarian torsion. J. Ultrasound Med. 2004; 23(12): 1643-9. https://dx.doi.org/10.7863/jum.2004.23.12.1643.
- Graif M., Shalev J., Strauss S., Engelberg S., Mashiach S., Itzchak Y. Torsion of the ovary: sonographic features. Am. J. Roentgenol. 1984; 143(6): 1331-4. https://dx.doi.org/10.2214/ajr.143.6.1331.
- Breech L.L., Hillard P.J.A. Adnexal torsion in pediatric and adolescent girls. Curr. Opin. Obstet. Gynecol. 2005; 17(5): 483-9. https://dx.doi.org/10.1097/01.gco.0000179666.39548.78.
- Oelsner G., Shashar D. Adnexal torsion. Clin. Obstet. Gynecol. 2006; 49(3): 459-63. https://dx.doi.org/10.1097/00003081-200609000-00006.
- Rha S.E., Byun J.Y., Jung S.E., Jung J.I., Choi B.G., Kim B.S. et al. CT and MR imaging features of adnexal torsion. Radiographics. 2002; 22(2): 283-94. https://dx.doi.org/10.1148/radiographics.22.2.g02mr02283.
- Aziz D., Davis V., Allen L., Langer J.C. Ovarian torsion in children: is oophorectomy necessary? J. Pediatr. Surg. 2004; 39(5): 750-3. https://dx.doi.org/10.1016/j.jpedsurg.2004.01.034.
- Oelsner G., Cohen S.B., Soriano D., Admon D., Mashiach S., Carp H. Minimal surgery for the twisted ischaemic adnexa can preserve ovarian function. Hum. Reprod. 2003; 18(12): 2599-602. https://dx.doi.org/10.1093/humrep/deg498.
- Lo L.M., Chang S.D., Horng S.G., Yang T.Y., Lee C.L., Liang C.C. Laparoscopy versus laparotomy for surgical intervention of ovarian torsion. J. Obstet. Gynaecol. Res. 2008; 34(6): 1020-5. https://dx.doi.org/10.1111/j.1447-0756.2008.00806.x.
- Çelik A., Ergün O., Aldemir H., Özcan C., Özok G., Erdener A. et al. Long-term results of conservative management of adnexal torsion in children. J. Pediatr. Surg. 2005; 40(4): 704-8. https://dx.doi.org/10.1016/j.jpedsurg.2005.01.008.
- Shalev E., Bustan M., Yarom I., Peleg D. Recovery of ovarian function after laparoscopic detorsion. Hum. Reprod. 1995; 10(11): 2965-6. https://dx.doi.org/10.1093/oxfordjournals.humrep.a135830.
- Cohen S.B., Oelsner G., Seidman D.S., Admon D., Mashiach S., Goldenberg M. Laparoscopic detorsion allows sparing of the twisted ischemic adnexa. J. Am. Assoc. Gynecol. Laparosc. 1999; 6(2): 139-43. https://dx.doi.org/10.1016/S1074-3804(99)80091-7.
- Ben-Arie A., Lurie S., Graf G., Insler V. Adnexal torsion in adolescents: prompt diagnosis and treatment may save the adnexa. Eur. J. Obstet. Gynecol. 1995; 63(2): 169-73. https://dx.doi.org/10.1016/0301-2115(95)02228-7.
- Rossi B.V., Ference E.H., Zurakowski D., Scholz S., Feins N.R., Chow J.S. et al. The clinical presentation and surgical management of adnexal torsion in the pediatric and adolescent population. J. Pediatr. Adolesc. Gynecol . 2012; 25(2): 109-13. https://dx.doi.org/10.1016/j.jpag.2011.10.006.
- Rousseau V., Massicot R., Darwish A.A., Sauvat F., Emond S., Thibaud E. et al. Emergency management and conservative surgery of ovarian torsion in children: A report of 40 cases. J. Pediatr. Adolesc. Gynecol. 2008; 21(4): 201-6. https://dx.doi.org/10.1016/j.jpag.2007.11.003.
- White M., Stella J. Ovarian torsion: 10-year perspective. Emerg. Med. Australas. 2005; 17(3): 231-7. https://dx.doi.org/10.1111/j.1742-6723.2005.00728.x.
- Mazouni C., Bretelle F., Ménard J.P., Blanc B., Gamerre M. Diagnosis of adnexal torsion and predictive factors of adnexal necrosis. |Gynecol. Obstet. Fertil. 2005; 33(3): 102-6. https://dx.doi.org/10.1016/j.gyobfe.2005.02.014.
- Bider D., Mashiach S., Dulitzky M., Kokia E., Lipitz S., Ben-Rafael Z. Clinical, surgical and pathologic findings of adnexal torsion in pregnant and nonpregnant women. Surg. Gynecol. Obstet. 1991; 173(5): 363-6.
- Dasgupta R., Renaud E., Goldin A.B., Baird R., Cameron D.B., Arnold M.A. et al. Ovarian torsion in pediatric and adolescent patients: A systematic review. J. Pediatr. Surg. 2018; 53(7): 1387-91. https://dx.doi.org/10.1016/j.jpedsurg.2017.10.053.
- Schwartz B.I., Huppert J.S., Chen C., Huang B., Reed J.L. Creation of a Composite score to predict adnexal torsion in children and adolescents. J. Pediatr. Adolesc. Gynecol. 2018; 31(2): 132-7. https://dx.doi.org/10.1016/j.jpag.2017.08.007.
- Appelbaum H., Abraham C., Choi-Rosen J., Ackerman M. Key Clinical predictors in the early diagnosis of adnexal torsion in children. J. Pediatr. Adolesc. Gynecol. 2013; 26(3): 167-70. https://dx.doi.org/10.1016/j.jpag.2012.12.005.
Received 18.03.2022
Accepted 20.04.2022
About the Authors
Elena P. Khashchenko, PhD, Researcher at the 2nd Gynecological (Children and Adolescents) Department, Academician V.I. Kulakov NMRС for OG&P,Ministry of Healthcare of the Russian Federation, khashchenko_elena@mail.ru, https://orcid.org/0000-0002-3195-307X, 117997, Russia, Moscow, Academician Oparin str., 4.
Elena V. Uvarova, Dr. Med. Sci., Professor, Corresponding Members of the RAS, Head of the 2nd Gynecological (Children and Adolescents) Department,
Academician V.I. Kulakov NMRС for OG&P, Ministry of Healthcare of the Russian Federation, 117997, Russia, Moscow, Academician Oparin str., 4;
Professor of the Department of Obstetrics, Gynecology, Perinatology and Reproductology of the Institute of Professional Education, I.M. Sechenov First MSMU,
Ministry of Healthcare of the Russian Federation (Sechenov University),
119991, Russia, Moscow, Trubetskaya str., 8-2, elena-uvarova@yandex.ru, https://orcid.org/0000-0002-3105-5640
Polina L. Sheshko, obstetrician-gynecologist, oncologist at the Department of Innovative Oncology and Gynecology, Academician V.I. Kulakov NMRС for OG&P,
Ministry of Healthcare of the Russian Federation, p_sheshko@oparina4.ru, 117997, Russia, Moscow, Academician Oparin str., 4.
Marina N. Kleуmenova, student of the Faculty of Fundamental Medicine, M.V. Lomonosov Moscow State University, mkleymenowa@yandex.ru,
119192, Russia, Moscow, Lomonosovsky Ave., 27-1.
Stanislav O. Kyurdzidi, PhD student, Academician V.I. Kulakov NMRС for OG&P, Ministry of Healthcare of the Russian Federation, I.M. Sechenov First MSMU,
Ministry of Healthcare of the Russian Federation (Sechenov University), dr.kyurdzidis@gmail.com, https://orcid.org/0000-0002-6316-1325,
119991, Russia, Moscow, Trubetskaya str., 8-2.
Irina A. Salnikova, obstetrician-gynecologist, doctor of ultrasound diagnostics, Researcher at the 2nd Gynecological (Children and Adolescents) Department,
Academician V.I. Kulakov NMRС for OG&P, Ministry of Healthcare of the Russian Federation, 117997, Russia, Moscow, Academician Oparin str., 4.
Vladimir D. Chuprynin, PhD, Head of General Surgery Department, Academician V.I. Kulakov NMRС for OG&P, Ministry of Healthcare of the Russian Federation,
v_chuprynin@oparina4.ru, 117997, Russia, Moscow, Academician Oparin str., 4.
Fatima Sh. Mamedova, PhD, Doctor at the Department of Ultrasound Diagnostics in Neonatology and Pediatrics, Academician V.I. Kulakov NMRС for OG&P,
Ministry of Healthcare of the Russian Federation, mamedova_f@mail.ru, 117997, Russia, Moscow, Academician Oparin str., 4.
Aleksandra V. Asaturova, PhD, Head of the 1th Pathology Department, Academician V.I. Kulakov NMRС for OG&P, Ministry of Healthcare of the Russian Federation,
a_asaturova@oparina4.ru, https://orcid.org/0000-0001-8739-5209, 117997, Russia, Moscow, Academician Oparin str., 4.
Corresponding author: Elena P. Khashchenko, khashchenko_elena@mail.ru
Author’s contributions: Khashchenko E.P., Sheshko P.L., Kleimenova M.N., Kyurdzidi S.O. – the concept and design of the study, analysis of the published sources, article writing and editing; Khashchenko E.P., Sheshko P.L., Salnikova I.A., Uvarova E.V., Chuprynin V.D. – patient management and treatment, analysis and interpretation of patient data; Mamedova F.Sh. – performance of ultrasound examination of the pelvic organs and interpretation of the results; Asaturova A.V. – carrying out pathomorphological study and analysis of the results, Uvarova E.V., Chuprynin V.D. – final editing of the text of the article.
Conflicts of interest: The authors declare that they have no conflicts of interest regarding this publication.
Funding: The study was carried out with the financial support of the State assignment No. 18-A21 of Ministry of Health "The role of energy metabolism and immune defense disorders in the development of various forms of endometriosis, the development of personalized therapy and the forecast of its effectiveness in the early reproductive period (from menarche to 18 years)".
Ethical Approval: The study was approved by the Ethics Committee of biomedical researches of Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation.
Patient Consent for Publication: All patients provided informed consent for the publication of their data and associated images.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Khashchenko E.P., Uvarova E.V., Sheshko P.L., Kleymenova M.N., Kyurdzidi S.O., Salnikova I.A., Chuprynin V.D., Mamedova F.Sh., Asaturova A.V. Diagnostic features,
clinical picture and management of adnexal torsion in female adolescents.
Akusherstvo i Gynecologia/ Obstetrics and Gynecology. 2022; 5: 91-100 (in Russian)
https://dx.doi.org/10.18565/aig.2022.5.91-100



