Pain syndrome features in different forms of external genital endometriosis: a cross-sectional study
Pronina V.A., Chernukha G.E., Filatova E.G., Solopova A.E.
Objective: To assess pain syndrome in patients with different forms of external genital endometriosis.
Materials and methods: The one-stage study was conducted at the V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology & Perinatology of the Ministry of Health of the Russian Federation from 2021 to 2023, involving 200 patients (age 32.03 (7.15) years) with confirmed endometriosis through expert ultrasonography and magnetic resonance imaging of the pelvic organs. Patients were categorized into three groups based on the form of endometriosis: with peritoneal endometriosis (SUP), endometriomas (OMA), and deep endometriosis (DE); in case of combined pathology inclusion in one or another group was carried out according to the most severe form. The targeted history collection of women was conducted through questioning. The intensity of the pain syndrome was evaluated using the visual analog scale (VAS), followed by calculation of the pelvic pain index (PPI). The level of central sensitization (CS) was assessed using the CSI scale, and quality of life and sexual function were evaluated using the SF-12 questionnaire and 5-point Likert scale, respectively. Neuropathic components were assessed based on the results of the PainDetect questionnaire.
Results: Data analysis revealed that patients with OMA were predominantly not characterized by pain syndrome. The severity of dysmenorrhea, chronic pelvic pain, and PPI, according to VAS indicators, was minimal in isolated endometriomas, with a tendency for higher PPI when endometriomas were combined with SUP, DE, and all three forms of endometriosis, respectively. PPI determination revealed that a threshold score of ≥3.8 points can indicate the presence of DE. Additionally, a significant level of sensitization (≥40 points) was observed in nearly one in two women with DE, one in three women with SUP, and one in six women with OMA. The presence of a neuropathic component was generally less common in patients with endometriosis (5.0%).
Conclusion: The study results demonstrated that not only the presence of pain but also the degree of its severity play a significant role in the diagnosis of endometriosis. PPI can serve as a tool to identify women at risk of endometriosis at the outpatient examination stage. A PPI score ≥3.8 points may indicate DE. The study results led to the conclusion that CS significantly contributes to pain genesis in patients with endometriosis-associated pelvic pain, while the neuropathic component plays a lesser role.
Authors’ contributions: Chernukha G.E., Pronina V.A. – the conception and design of the study; Pronina V.A. – data collection and analysis, drafting and editing of the manuscript; Chernukha G.E., Filatova E.G., Solopova A.E. – editing of the manuscript and final approval of the version to be submitted.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Acknowledgements: We express our gratitude to N.D. Simich-Lafitsky (PhD) for statistical processing and mathematical description of the data obtained in the course of the study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors’ Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Pronina V.A., Chernukha G.E., Filatova E.G., Solopova A.E. Pain syndrome features in different forms of external genital endometriosis: a cross-sectional study.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (3): 80-88 (in Russian)
https://dx.doi.org/10.18565/aig.2023.280
Keywords
Pelvic pain is one of the primary clinical manifestations of endometriosis. Literature indicates that almost 95% of women with endometriosis report experiencing at least one form of pain syndrome [1]. The range of pain symptoms associated with endometriosis varies widely, from the most common, such as dysmenorrhea, to less common, such as chronic pelvic pain (CPP), dyspareunia, dyschezia, dysuria, myalgia, migraines, and others. However, the severity of pain does not always align with the extent of endometriosis observed during laparoscopy. In some cases, surgical excision of endometriotic lesions does not alleviate the severity of pelvic pain [2, 3]. The nature of endometriosis-associated pelvic pain is multifaceted; its genesis involves not only the activation of angiogenesis and neurogenesis but also central sensitization (CS), which plays a role in the chronicity of the pain syndrome and, in some instances, its persistence after radical surgical treatment [4, 5]. The quality of life of patients with endometriosis and CPP can be further affected by concurrent conditions associated with CS, such as interstitial cystitis, irritable bowel syndrome (IBS), myalgia, and vulvodynia [6].
Despite conflicting data in the literature regarding the relationship between the prevalence of endometriosis and pain intensity [3, 7–9], there appears to be a trend in the association between the severity of clinical symptoms and their number. For instance, approximately every second patient with endometriosis reports a combination of dysmenorrhea, dyspareunia, and CPP [10, 11]. Additionally, isolated studies have shown that ovarian endometriomas (OMA) do not typically present with pain syndrome, and the occurrence of pelvic pain is more often linked to other forms, such as peritoneal (superficial) endometriosis (SUP) and deep endometriosis (DE) [12, 13].
In clinical practice, the intensity of pelvic pain is assessed using an 11-point visual analog scale (VAS). For example, the likelihood of endometriosis increases with dysmenorrhea scores ≥ 6 points, CPP ≥ 2 points, and dyspareunia ≥ 3 points [14, 15]. It has been determined that not only the severity of symptoms but also their co-occurrence, considering the aforementioned threshold values, has clinical significance [14]. The quest for clinical predictors of endometriosis for timely diagnosis is ongoing. Ballard K.D. et al., pioneers in this field, revealed in 2008 a correlation between the risk of developing endometriosis and an increase in the number of associated symptoms and comorbid conditions [16]. A team from France subsequently proposed a 21-item online questionnaire based on their research and the ENDOPAIN-4D questionnaire, which evaluates the likelihood of endometriosis based on various pain characteristics and other clinical signs [17]. Other studies have also proposed predictive models for endometriosis risk based on patient’s’ medical and family histories, the severity of pelvic pain, and the presence of associated conditions [18, 19]. Nonetheless, these prognostic models are not without flaws, and the search for significant predictors of the development of endometriosis continues.
Therefore, this study aimed to evaluate pain syndrome in patients with various forms of endometriosis and determine the role of CS and the neuropathic component in the genesis of endometriotic pain.
Materials and methods
The one-stage study was conducted at V.I. Kulakov NMRC for OG&P of the Ministry of Health of Russia from 2021 to 2023. The study included 200 women aged 18–49 years (mean age 32.03 (7.15) years; mean body mass index 21.35 (3.35) kg/m2). All patients with suspected genital endometriosis underwent expert ultrasound and pelvic magnetic resonance imaging (MRI). In 54% of patients’ endometriosis was verified laparoscopically and histologically: in 63/200 (31.5%) patients during a previous surgical intervention and in 45/200 (22.5%) patients during the subsequent first elective operation.
Exclusion criteria were absence signs of endometriosis on MRI, current or history of oncological diseases of the female reproductive system, serious somatic diseases, pregnancy and lactation, and use of suppressive hormonal therapy for at least three months prior to enrollment in the study. The algorithm for forming a sample of patients is presented in Figure 1. The final number of women included in the study was 200, which is a representative sample as a whole and for each of the subgroups separately.
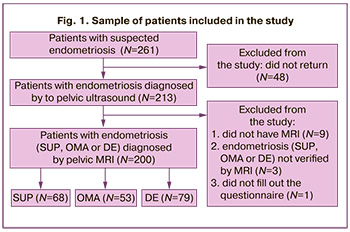
Pelvic MRI was performed using GE Signa Excite 1.5T and GE Signa Architect 3.0T machines, and contrast enhancement was used in cases where differential diagnosis of endometriosis was necessary as well as an auxiliary tool for visualizing extragenital forms of endometriosis. During MRI, signal intensity was assessed on T1-weighted (T1-WI) and T2-weighted images (T2-WI) performed in the sagittal, axial, and coronal planes, as well as on diffusion-weighted images, using a slice thickness of 0.3 cm and a field of view of 32–42 cm. Visual diagnosis of OMA, DE, and endometriosis was performed according to the recommendations of the European Society of Urogenital Radiology [20]. The presumptive diagnosis of SUP was established in the presence of MR signs of endometrioid heterotopias in the pelvic area with pickle-shaped heterogeneous contours less than 0.5 cm in size, with hypointense signal on T2-WI, localized along the serous covering of the uterus, on the ligamentous apparatus, including the uterosacral ligaments, peritoneum, and parametrial tissues [21–25]. Depending on the presumed form of endometriosis identified by MRI, patients were divided into three groups: with SUP, OMA, and DE; in case of combined pathology, inclusion in one or another group was made according to the most severe form of endometriosis.
All women underwent a medical history for the presence of non-gynecologic conditions associated with pain and completed a questionnaire. CPP was defined as the presence of acyclic lower abdominal pain not associated with menstruation, ovulation, or sexual intercourse for at least six months. The assessment of dyspareunia and calculation of the pelvic pain index (PPI) were performed only in sexually active women. Infertility was defined as the absence of pregnancy with regular unprotected intercourse for 12 months, and was assessed only in women planning to become pregnant.
Assessment of the severity of dysmenorrhea, dyspareunia, CPP, dyschezia and dysuria was carried out using an 11-point VAS, PPI was calculated, where D1 – dysmenorrhea, D2 – dyspareunia, D3 – dyschezia, D4 – dysuria, 5 – number of indicators:
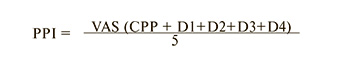
The Central Sensitization Inventory questionnaire (CSI) was used to assess the degree of CS, the PainDetect questionnaire was used to assess the presence of a neuropathic component of pain, the SF-12 questionnaire was used to assess quality of life, and a 5-point Likert scale was used to assess sexual function [26–28]. The cut-off points for the presence of CSI pis ≥40 points, for the neuropathic component ≥19 points. The scale scores were interpreted by a neurologist. All data were anonymized before calculation. This study was approved by the ethics committee.
Statistical analysis
Quantitative variables are expressed as mean (M) with standard deviation (SD), and median (Me) with interquartile range (Q1; Q3). Categorical variables were reported as frequencies and percentages. Quantitative variables showing a normal distribution were expressed as M (SD); otherwise, Me (Q1; Q3) was reported. Comparisons of two independent samples (quantitative characteristics) were performed using the Mann–Whitney test. Pairwise comparisons between the groups based on categorical characteristics were performed using Fisher's exact test. Bonferroni correction was used for both criteria. To estimate the threshold value of the PPI score, ROC analysis was used to determine the area under the curve (AUC) with a confidence interval. Data analysis was performed using IBM SPSS Statistics (v.26). The critical significance level for testing the statistical hypotheses was set at 0.05.
Results
In the endometriosis structure visualized by pelvic MRI, SUP was detected in 68/200 (34%), OMA in 53/200 (26.5%), and DE in 79/200 (39.5%) patients. DE was associated with bowel endometriosisin every fourth case (22/79, 27.8%) and extragenital endometriosis at other sites (bladder, scar, appendix, lung) in 7.6% of women. Isolated OMA was observed in only 4.5% of cases; in 22%, it was combined with SUP, and in 2.5%, with DE. All three forms of endometriosis were observed in every 5th patient (Fig. 2). The patients were further subdivided according to the most severe form of endometriosis into SUP (n=68), OMA (n=53), and DE (n=79).
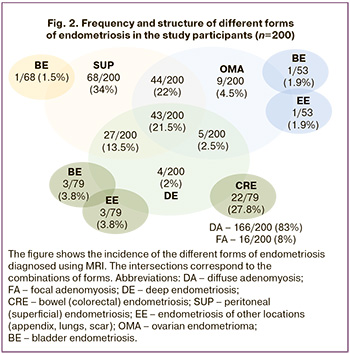
Patients in all three groups were comparable in their clinical characteristics, with the exception of older age and a higher frequency of previous surgical treatment, including multiple operations, in women with DE than with SUP (Table 1). Every 4th woman with SUP, every 3rd woman with OMA and every 2nd woman with DE had previous operations.
Every second patient received hormone therapy prior to enrollment, and there was no significant difference between the groups. Every 5th woman had a family history of endometriosis, and every 2nd woman had infertility.
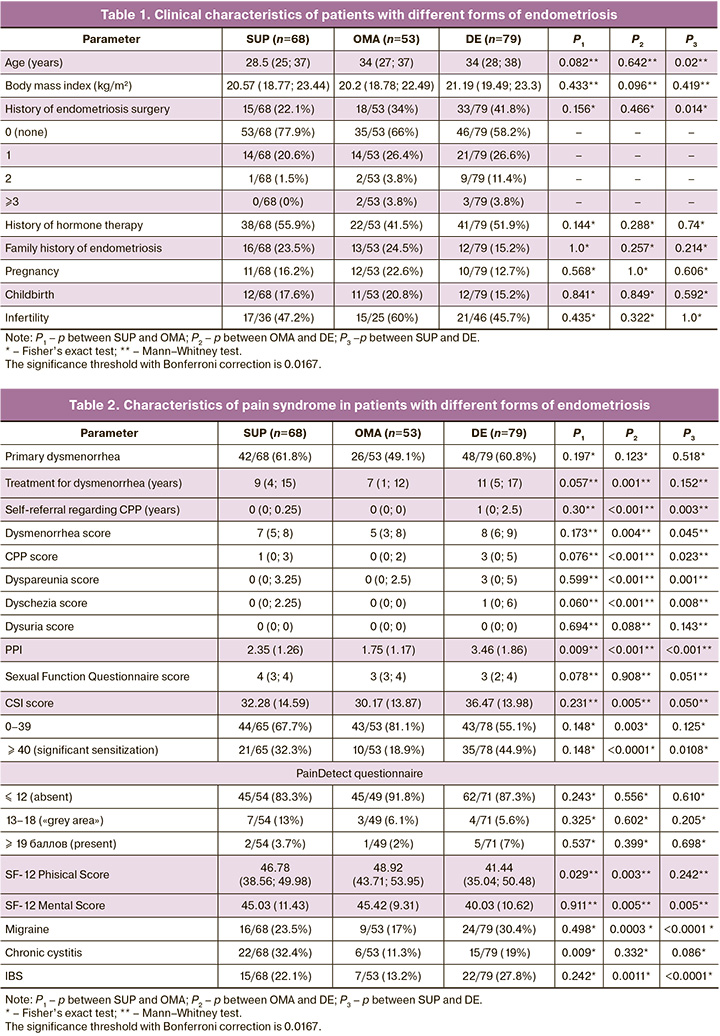
The data presented in Table 2 illustrates a statistically significant difference between the groups for all pain indicators. Patients with OMA had less severe pelvic pain, in contrast to women with SUP and DE, which is clearly demonstrated by the PPI: 3.46 (1.86) points for DE, 2.35 (1.26) points for SUP, and only 1.75 (1.17) points – for OMA (p<0.001). In addition, this group of women was predominantly characterized by the presence of dysmenorrhea of moderate intensity, whereas in SUP and DE, it was of high intensity.
Patients with DE had significantly higher mean CSI scores. The percentage of significant sensitization (≥ 40 points) was found in almost every second woman with DE, every third woman with SUP, and every fifth woman with OMA. In a pairwise comparison of the groups, patients with DE were significantly more likely to have sensitization than patients with OMA (p<0.0001) and SUP (p=0.0108). A similar trend was observed for non-gynecological conditions associated with CS. Pairwise comparisons showed that the presence of concomitant migraine and IBS was more frequent in patients with DE than in those with SUP (p<0.0001 in both comparisons) or OMA (p=0.0003 and p=0.0011 for migraine and IBS, respectively) (Table 2).
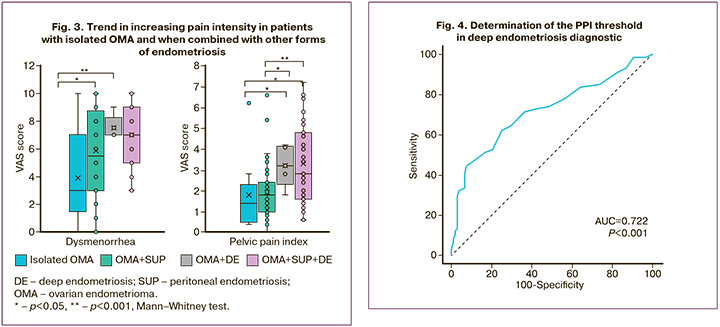
A more detailed analysis of the data obtained revealed a trend towards the lowest severity of pain in terms of dysmenorrhea and PPI in isolated OMA and their combination with SUP, with an increase in the intensity of pelvic pain in the combination of cysts with DE and in the presence of all three forms of endometriosis (Figure 3).
The results of the PainDetect questionnaire showed that only 5% of patients with endometriosis had a high probability of neuropathic component, 8% of patients were in the so-called "gray area", characterized as an indeterminate result, meaning the possible presence of a neuropathic component.
In a survey of patients with relevant complaints, it was found that in the general cohort of women with endometriosis, the mean time from the onset of dysmenorrhea to confirmation of the diagnosis was 10 (3.5; 15) years, and from the onset of CPP, it was only 1 (0; 3) years. Patients with OMA had a statistically significantly shorter interval to diagnosis of 7 (1; 12) years, which is probably due to the fact that endometriomas are detected many times more often by ultrasound in contrast to other forms of endometriosis. Despite severe dysmenorrhea in DE, with a mean score of 8 (6–9) points on the VAS, it doesnt reduce the time to diagnosis, whereas CPP was a more significant symptom and a reason for seeking medical help more quickly.
In order to improve endometriosis diagnosis, the PPI threshold was calculated using ROC analysis. A PPI score of ≥3.8 points suggested the presence of DE with a sensitivity of 44.59% and specificity of 92.52% (AUC 0.722 (0.04) [95% CI 0.651;0.786], p<0.001) (Fig. 4).
Discussion
One of the fundamental principles for the early diagnosis of endometriosis, aimed at minimizing the risk of progression to more severe forms, is accurate assessment of disease symptoms. However, the clinical manifestations of endometriosis are diverse and often extend beyond classical signs, such as dysmenorrhea, dyspareunia, CPP, and infertility. The association between these symptoms and the prevalence of endometriosis remains debatable. For instance, a study by Ashkenazi M.S. et al. involving 2964 patients revealed that dysmenorrhea was more likely linked to stage I of the disease, while CPP was predominantly observed in stages II, III, and IV. "Non-classical" pain and infertility were positively correlated with disease stage [29]. Another study by Zhao H. et al. using logistic regression analysis suggested that pain intensity ≥4 points on the VAS and tenderness in the sacrouterine ligament area could indicate stage IV disease with 87.2% sensitivity and 71.0% specificity (AUC 0.760 [95% CI 0.677–0.842]) [7]. In contrast, our study found that patients with DE, as opposed to patients with SUP and OMA, exhibited more severe dysmenorrhea, dyspareunia, CPP, and dyschezia, along with significantly lower SF-12 Health Assessment scores on both physical and mental scales. This is likely directly related to the heightened pain syndrome in this group of women.
Compared to assessing only the severity of dysmenorrhea or CPP, which may not always correlate with the severity and prevalence of endometriosis, PPI emerges as a more informative indicator. It considers less common pain manifestations, such as dysuria and dyschezia. Despite the statistical significance between the groups, distinguishing between SUP and DE based on dysmenorrhea severity alone (7 (5;8) VAS points for SUP; 8 (6;9) VAS points for DE) is challenging. However, the mean PPI value for DE was 1.5 times higher than SUP and twice higher than OMA (PPI 3.46 (1.86) points for DE; 2.35 (1.86) points for SUP; 1.75 (1.17) points for OMA). ROC analysis results indicated that with PPI≥3.8 points, DE can be assumed with 44.59% sensitivity and 92.52% specificity (AUC 0.722 [0.04] [95% CI 0.651;0.786], p<0.001).
Only 5% of the patients with endometriosis exhibited a highly probability of neuropathic component. However, no significant relationship was found between the PainDetect questionnaire score and the severity of dysmenorrhea, CPP, and other pain manifestations. A study by Konrad L. et al. reported comparable results, noting a neuropathic component of pain in only 4.2% of women with endometriosis, with no statistically significant difference from the control group (1.1%) [30]. Despite this, our study identified a significantly higher PainDetect score for severe endometriosis, in contrast to the findings of the aforementioned study. Literature suggests that the neuropathic component of pain may be associated with the activation of small sensory fibers in endometriotic lesions, leading to mixed pain syndrome in conjunction with inflammation [31, 32]. It may also result from changes in the central nervous system, specifically CS [33].
Central sensitization plays a crucial role in endometriosis CPP formation. In our study, significant sensitization was observed in every third patient with endometriosis-associated pelvic pain; CSI scores differed depending on the form of endometriosis, with a score ≥40 noted in every second patient with DE, every third patient with SUP, and every fifth patient with OMA. This observation is relevant when considering surgical treatment for pain, as a higher CSI score may not necessarily lead to a significant reduction in existing clinical symptoms [34]. Additionally, in DE, the more severe form of endometriosis and comorbidities associated with CS, such as migraine and IBS, are significantly more common than in women with SUP and OMA. A previous study demonstrated that women with endometriosis and migraine had higher rates of dysmenorrhea, CPP, dyspareunia, dyschezia, and higher mean PPI values than those without concomitant migraine [33].
Conclusion
In the outpatient examination stage of endometriosis diagnosis, evaluating the severity of pain syndrome is more crucial than merely confirming its presence. Identifying women at risk of developing endometriosis allows for timely and comprehensive examination, including pelvic MRI, aimed at identifying more severe forms of endometriosis during the initial outpatient visit. The calculation of PPI can aid in assuming the presence of deep endometriosis. This approach holds promise for reducing the time to diagnosis, allowing for a personalized approach to patient management.
The study results led to the conclusion that central sensitization significantly contributes to pain genesis in patients with endometriosis-associated pelvic pain, while the neuropathic component plays a lesser role.
References
- Signorile P.G., Cassano M., Viceconte R., Marcattilj V., Baldi A. Endometriosis: a retrospective analysis of cinical data from a cohort of 4,083 patients, with focus on symptoms. In vivo. 2022; 36(2): 874-83. https://dx.doi.org/10.21873/invivo.12776.
- Vercellini P., Fedele L., Aimi G., Pietropaolo G., Consonni D., Crosignani P.G. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum. Reprod. 2007; 22(1): 266-71. https://dx.doi.org/10.1093/humrep/del339.
- Abbott J., Hawe J., Hunter D., Holmes M., Finn P., Garry R. Laparoscopic excision of endometriosis: a randomized, placebo-controlled trial. Fertil. Steril. 2004; 82(4): 878-84. https://dx.doi.org/1010.1016/j.fertnstert.2004.03.046.
- Cetera G.E., Merli C.E.M., Facchin F., Viganò P., Pesce E., Caprara F., Vercellini P. Non-response to first-line hormonal treatment for symptomatic endometriosis: overcoming tunnel vision. A narrative review. BMC Womens Health. 2023; 23(1): 347. https://dx.doi.org/1010.1186/s12905-023-02490-1.
- Maddern J., Grundy L., Castro J., Brierley S.M. Pain in endometriosis. Front. Cell. Neurosci. 2020; 14: 590823. https://dx.doi.org/10.3389/fncel.2020.590823.
- Surrey E.S., Soliman A.M., Johnson S.J., Davis M., Castelli-Haley J., Snabes M.C. Risk of developing comorbidities among women with endometriosis: A retrospective matched cohort study. J. Womens Health (Larchmt). 2018; 27(9): 1114-23. https://dx.doi.org/10.1089/jwh.2017.6432.
- Zhao H., Zhang J., Bao ZL., Kong J., Wei W., Gu J.Q. A preoperative predictive model for stage IV endometriosis. J. Obstet. Gynaecol. 2023; 43(1): 2188072. https://dx.doi.org/1010.1080/01443615.2023.2188072.
- Schliep K.C., Mumford S.L., Peterson C.M., Chen Z., Johnstone E.B., Sharp H.T. et al. Pain typology and incident endometriosis. Hum. Reprod. 2015; 30(10): 2427-38. https://dx.doi.org/10.1093/humrep/dev147.
- Conroy I., Mooney S.S., Kavanagh S., Duff M., Jakab I., Robertson K. et al. Pelvic pain: What are the symptoms and predictors for surgery, endometriosis and endometriosis severity. Aust. N. Z. J. Obstet. Gynaecol. 2021; 61(5): 765-72. https://dx.doi.org/10.1111/ajo.13379.
- Chen C.X., Carpenter J.S., Ofner S., LaPradd M., Fortenberry J.D. Dysmenorrhea symptom-based phenotypes: A replication and extension study. Nurs. Res. 2021; 70(1): 24-33. https://dx.doi.org/10.1097/NNR.0000000000000477.
- Agarwal S.K., Antunez-Flores O., Foster W.G., Hermes A., Golshan S., Soliman A.M. et al. Real-world characteristics of women with endometriosis-related pain entering a multidisciplinary endometriosis program. BMC Womens Health. 2021; 21(1): 19. https://dx.doi.org/10.1186/s12905-020-01139-7.
- Khan K.N., Kitajima M., Fujishita A., Hiraki K., Matsumoto A., Nakashima M., Masuzaki H. Pelvic pain in women with ovarian endometrioma is mostly associated with coexisting peritoneal lesions. Hum. Reprod. 2013; 28(1): 109-18. https://dx.doi.org/10.1093/humrep/des364.
- Perelló M., Martínez-Zamora M.A., Torres X., Munrós J., Llecha S., De Lazzari E. et al. Markers of deep infiltrating endometriosis in patients with ovarian endometrioma: a predictive model. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017; 209: 55-60. https://dx.doi.org/10.1016/j.ejogrb.2015.11.024.
- Пронина В.А., Думановская М.Р., Чернуха Г.Е. Оптимизация принципов ранней диагностики эндометриоза на основе оценки коморбидности и клинической манифестации. Акушерство и гинекология. 2023; 4: 87-96. [Pronina V.A., Dumanovskaya M.R., Chernukha G.E. Principles of early diagnosis of endometriosis based on the assessment of comorbidity and clinical manifestations. Obstetrics and Gynecology. 2023; (4): 87-96. (in Russian)]. https://dx.doi.org/10.18565/aig.2023.9.
- Chapron C., Lafay-Pillet M.C., Santulli P., Bourdon M., Maignien C., Gaudet-Chardonnet A. et al. A new validated screening method for endometriosis diagnosis based on patient questionnaires. EClinicalMedicine. 2022; 44: 101263. https://dx.doi.org/10.1016/j.eclinm.2021.101263.
- Ballard K.D., Seaman H.E., de Vries C.S., Wright J.T. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case-control study--Part 1. BJOG. 2008; 115(11): 1382-91. https://dx.doi.org/10.1111/j.1471-0528.2008.01878.x.
- Fauconnier A., Drioueche H., Huchon C., Du Cheyron J., Indersie E., Candau Y. et al. Early identification of women with endometriosis by means of a simple patient-completed questionnaire screening tool: a diagnostic study. Fertil. Steril. 2021; 116(6): 1580-9. https://dx.doi.org/10.1016/j.fertnstert.2021.07.1205.
- Verket N.J., Falk R.S., Qvigstad E., Tanbo T.G., Sandvik L. Development of a prediction model to aid primary care physicians in early identification of women at high risk of developing endometriosis: cross-sectional study. BMJ Open. 2019; 9(12): e030346. https://dx.doi.org/10.1136/bmjopen-2019-030346.
- Ricci G., Castelpietra E., Romano F., Di Lorenzo G., Zito G., Ronfani L. et al. Case-control study to develop and validate a questionnaire for the secondary prevention of endometriosis. PLoS One. 2020; 15(3): e0230828. https://dx.doi.org/10.1371/journal.pone.0230828.
- Bazot M., Bharwani N., Huchon C., Kinkel K., Cunha T.M., Guerra A. et al. European Society of Urogenital Radiology (ESUR) guidelines: MR imaging of pelvic endometriosis. Eur. Radiol. 2017; 27(7): 2765-75. https://dx.doi.org/10.1007/s00330-016-4673-z.
- Méndez Fernández R., Barrera Ortega J. Magnetic resonance imaging of pelvic endometriosis. Radiologia. 2017; 59(4): 286-96. https://dx.doi.org/10.1016/j.rx.2017.02.002.
- Khashchenko E.P., Uvarova E.V., Fatkhudinov T.K., Chuprynin V.D., Asaturova A.V., Kulabukhova E.A. et al. Endometriosis in adolescents: diagnostics, clinical and laparoscopic features. J. Clin. Med. 2023; 12(4): 1678. https://dx.doi.org/10.3390/jcm12041678.
- Maciel C., Ferreira H., Djokovic D., Kyaw Tun J., Keckstein J., Rizzo S., Manganaro L. MRI of endometriosis in correlation with the #Enzian classification: applicability and structured report. Insights Imaging. 2023; 14(1): 120. 10.1186/s13244-023-01466-x.
- Manganaro L., Fierro F., Tomei A., Irimia D., Lodise P., Sergi M.E. et al. Feasibility of 3.0T pelvic MR imaging in the evaluation of endometriosis. Eur. J. Radiol. 2012; 81(6): 1381-7. https://dx.doi.org/10.1016/j.ejrad.2011.03.049.
- Thomeer M.G., Steensma A.B., van Santbrink E.J., Willemssen F.E., Wielopolski P.A., Hunink M.G. et al. Can magnetic resonance imaging at 3.0-Tesla reliably detect patients with endometriosis? Initial results. J. Obstet. Gynaecol. Res. 2014; 40(4): 1051-8. https://dx.doi.org/10.1111/jog.12290.
- Freynhagen R., Tölle T.R., Gockel U., Baron R. The painDETECT project - far more than a screening tool on neuropathic pain. Curr. Med. Res. Opin. 2016; 32(6): 1033-57. https://dx.doi.org/10.1185/03007995.2016.1157460.
- Neblett R., Hartzell M.M., Mayer T.G., Cohen H., Gatchel R.J. Establishing clinically relevant severity levels for the central sensitization inventory. Pain Pract. 2017; 17(2): 166-75. https://dx.doi.org/10.1111/papr.12440.
- Gandek B., Ware J.E., Aaronson N.K., Apolone G., Bjorner J.B., Brazier J.E. et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. International Quality of Life Assessment. J. Clin. Epidemiol. 1998; 51(11): 1171-8. https://dx.doi.org/10.1016/s0895-4356(98)00109-7.
- Ashkenazi M.S., Huseby O.L., Kroken G., Trocha M., Henriksson A., Jasiak H. et al. The clinical presentation of endometriosis and its association to current surgical staging. J. Clin. Med. 2023; 12(7): 2688. https://dx.doi.org/10.3390/jcm12072688.
- Konrad L., Fruhmann Berger L.M., Maier V., Horné F., Neuheisel L.M., Laucks E.V. et al. Predictive model for the non-invasive diagnosis of endometriosis based on clinical parameters. J. Clin. Med. 2023; 12(13): 4231. https://dx.doi.org/10.3390/jcm12134231.
- Mechsner S., Kaiser A., Kopf A., Gericke C., Ebert A., Bartley J. A pilot study to evaluate the clinical relevance of endometriosis-associated nerve fibers in peritoneal endometriotic lesions. Fertil. Steril. 2009; 92(6): 1856-61. https://dx.doi.org/10.1016/j.fertnstert.2008.09.006.
- Kajitani T., Maruyama T., Asada H., Uchida H., Oda H., Uchida S. et al. Possible involvement of nerve growth factor in dysmenorrhea and dyspareunia associated with endometriosis. Endocr. J. 2013; 60(10): 1155-64. https://dx.doi.org/10.1507/endocrj.ej13-0027.
- Багирова У.А., Чернуха Е.Г., Филатова Е.Г. Особенности болевого синдрома при генитальном эндометриозе и мигрени. Неврология, нейропсихиатрия, психосоматика. 2021; 13(1): 31-7. [Bagirova U.A., Chernukha E.G., Filatova E.G. Features of pain syndrome in genital endometriosis and migraine. Neurology, Neuropsychiatry, Psychosomatics. 2021; 13(1): 31-7. (in Russian)]. https://dx.doi.org/10.14412/2074-2711-2021-1-31-37.
- Orr N.L., Huang A.J., Liu Y.D., Noga H., Bedaiwy M.A., Williams C. et al. Association of central sensitization inventory scores with pain outcomes after endometriosis surgery. JAMA Netw Open. 2023; 6(2): e230780. https://dx.doi.org/10.1001/jamanetworkopen.2023.0780.
Received 01.12.2023
Accepted 07.02.2024
About the Authors
Veronika A. Pronina, Obstetrician-Gynecologist, PhD student, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(916)025-86-26, ver22595@yandex.ru, https://orcid.org/0000-0003-4566-4065Galina E. Chernukha, Dr. Med. Sci., Professor, Chief Researcher, Obstetrician-Gynecologist at the Department of Gynecological Endocrinology, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(985)999-60-00, c-galina1@yandex.ru, https://orcid.org/0000-0002-9065-5689
Elena G. Filatova, Neurologist, Dr. Med. Sci., Professor at the Department of Neurological Diseases, Sechenov University, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia (Sechenov University), 117997, Russia, Moscow, Trubetskaya str., 8-2, +7(903)148-76-13, eg-filatova@mail.ru,
https://orcid.org/0000-0001-9978-4180
Alina E. Solopova, Dr. Med. Sci., Leading Researcher at the Department of Radiology, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(985)432-68-52, a_solopova@oparina4.ru, Scopus Author ID: 24460923200; Researcher ID: P-8659-2015, https://orcid.org/0000-0003-4768-115X
Corresponding author: Galina E. Chernukha, c-galina1@yandex.ru



