Experience in ultrasound detection of placental separation
Aim. To investigate the prospects of using ultrasound imaging to detect placental separation.Mudrov V.A.
Materials and methods. The study prospectively analyzed the time of placental separation and total blood loss, depending on the method of detecting placental separation. The dynamic uterine ultrasound examination in the third stage of labor was considered a promising technique that objectively reflects the placenta complete separation. Results. The length of the third stage of labor was 12.0 (11.4; 12.5) and 9.0 (8.7; 9.8) minutes when the placental separation was identified by the standard and ultrasound assessment, respectively. Blood loss was 60.0 (55.8; 61.2) ml higher when the placental separation was assessed by standard signs compared with ultrasound examination. Conclusion. The study findings confirmed the feasibility of using ultrasound imaging to detect placental separation, which reduces total blood loss and length of the third stage of labor.
Keywords
Until recently, approaches to managing the third stage of labor included expectant management and active management [1]. Expectant management means waiting for the signs of separation of the placenta and its spontaneous delivery under the influence of uterine contractions and the force of gravity [1, 2]. Active management of the third stage of labor involves administering intravenous oxytocin, early umbilical cord cutting, transabdominal manual massage of the uterus, and controlled traction of the umbilical cord [1]. According to the WHO, active management of the third stage of labor helps reduce blood loss, duration of the third stage of labor, and the risk of postpartum hemorrhage [1, 3]. Meanwhile, early umbilical cord cutting leads to a newborn's hypovolemia and increases the risk of anemia, which excludes this manipulation from the routine application [1]. Given that controlled traction of the umbilical cord insignificantly affects the massive bleeding rate, untrained specialists should refrain from its use and limit the active management of labor to the routine administration of oxytocin [4]. The umbilical cord's forceful traction can lead not only to a rupture of the umbilical cord but also to iatrogenic uterine inversion, accompanied by bleeding and pain shock [4, 5].
Signs of placental separation after vaginal delivery supported by current clinical guidelines include signs of Schroeder, Alfeld, Klein, and Küstner–Chukalov [4]. Due to the widespread introduction of uterotonic drugs at the beginning of the third stage of labor, it is impossible to use the Schroeder sign. Meanwhile, the signs of Dovzhenko, Strassmann, Mikulich– Radetzky, Hohenbichler, and Rossier have not been widely used in practice [2, 3]. Placental separation is generally recognized not by one, but by a combination of two or more signs [2, 3]. The main disadvantage of assessing signs of placental separation is their subjectivity. This assessment's effectiveness directly depends on the obstetrician experience and knowledge, his/her attentiveness, visual acuity, and sometimes on the temperament of medical personnel. Placental infringement in the cervical os due to spasm can be hidden under the mask of Duncan placenta [2].
Meanwhile, failed attempts to extract the unseparated placenta are also a risk factor for bleeding [2]. Retained placenta after vaginal delivery is an important cause of postpartum hemorrhage due to the distention of the uterine cavity [2, 6]. Therefore, the search for diagnostic tools for detecting placental separation, which hypothetically may help reduce blood loss, is of great practical interest.
Krapp M. et al. proposed to detect placental separation by a cessation of color Doppler detected blood flow in basal plate vessels [7]. Cessation of blood flow between the basal placenta and myometrium following delivery is the sonographic sign of normal placental separation. The authors also divided the third stage of labor into the latent, contraction, detachment, and expulsion phases. The beginning of expulsion was recognized by the displacement of the placenta into the vagina. Meanwhile, the authors mainly positioned this technique as a method for diagnosing placental accreta and did not suggest its routine use to reduce the amount of postpartum blood loss [7]. According to a cohort study by Edwards H. et al., retention of the detached placenta in the uterine cavity is a strong predictor for an increase in postpartum blood loss and is a weak predictor of the third stage of labor prolongation [8].
In the presence of moderate and severe anemia, even insignificant blood loss during childbirth requires transfusion of donor red blood cells, which increases the risk of complications associated with allogeneic blood transfusions [9]. Besides, anemia is associated with an increase in the incidence of complications and the length of hospital stay after delivery, which leads to a rise in the cost of treatment [9]. Therefore, obstetrics is currently faced with the need to minimize postpartum blood loss in the context of preventing obstetric aggression [1].
Therefore, the present study aimed to investigate the prospects of using dynamic ultrasound to detect placental separation in the third stage of labor.
Materials and methods
The study prospectively analyzed 130 childbirths in the Perinatal Center of the Chita Regional Clinical Hospital in 2019–2020. The placental separation was determined by signs of Schroeder, Alfeld, Klein, and Kyustner– Chukalov in group 1(n=50), signs of Schroeder, Alfeld, Klein, Küstner–Chukalov, Dovzhenko, Strassmann, Mikulich–Radetzky, Hohenbichler, and Rossier in group 2 (n=50), and by ultrasound in group 3 (n=30). The groups are comparable in age, parity, and fetal weight. The exclusion criteria were operative delivery, early postpartum hemorrhage, chorioamnionitis, induced labor, polyhydramnios, multiple pregnancies, large fetus, anemia before childbirth, and placenta accreta spectrum. In general, patients at risk of developing postpartum hemorrhage were excluded from the study. All women underwent a general and special obstetric examination following clinical guidelines approved by the Ministry of Health of Russia [4, 5]. No budget funds were spent on this study; in each case, a separate informed consent was obtained; the work was conducted following the requirements of the Declaration of Helsinki of the World Medical Association (2013). Considering that several complications can accompany controlled cord traction, it was decided to refrain from its use and limit the active management of labor to the routine administration of oxytocin [4]. All women received 10 IU of intramuscular oxytocin within the first minute postpartum to reduce bleeding [4].
Ultrasound assessment of placental separation was carried out in the third stage of labor in the delivery room using a premium class portable ultrasound scanner MySono U5 Samsung Medison (Samsung Medison Bldg, Korea). The echographic examination was carried out in dynamic mode using a transabdominal convex probe with a frequency of 3.5–5.0 MHz in the probe's sagittal plane. The sensor's satisfactory position was confirmed by visualization of the uterus and placenta on the monitor screen. The separation of the placenta along the entire length and its displacement into the lower uterine segment was considered as the fact of complete placental separation (Figure).
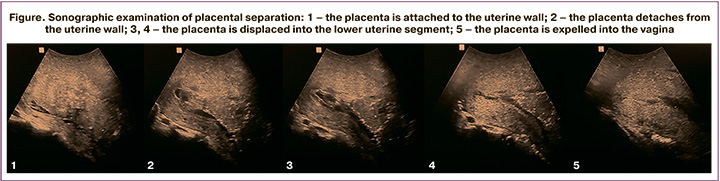
Postpartum blood loss was measured by BRASS-V blood collection drape and by weighing blood-soaked materials. Also, 10 minutes after the placenta's delivery, the length, width, and anteroposterior size of the uterine body were sonographically measured using the standard technique to determine uterine contractility [10]. A complete blood count was obtained on day 3 to verify the hemoglobin level.
Statistical analysis
Statistical analysis was performed according to recommendations of the International Committee of Medical Journal Editors (ICMJE) and Statistical Analyses and Methods in the Published Literature (SAMPL) guidelines [11, 12]. The normality of the distribution was tested by the Shapiro–Wilk test. Since the variables did not meet the normality assumption, quantitative variables were expressed as median (Me) and the quartiles Q1 and Q3. Kruskal–Wallis test was used for comparing numerical data between three groups followed by pairwise comparison using the Mann–Whitney U-test with Bonferroni's correction for significant differences. Categorical variables were compared by the χ2 test. Relative risk was calculated to determine the differences between standard and ultrasound assessment of placental separation. In all cases, p<0.05 was considered statistically significant [12]. Statistical analysis was carried out using the IBM SPSS Statistics Version 25.0 software package (International Business Machines Corporation, license No. Z125-3301-14, USA).
Results and discussion
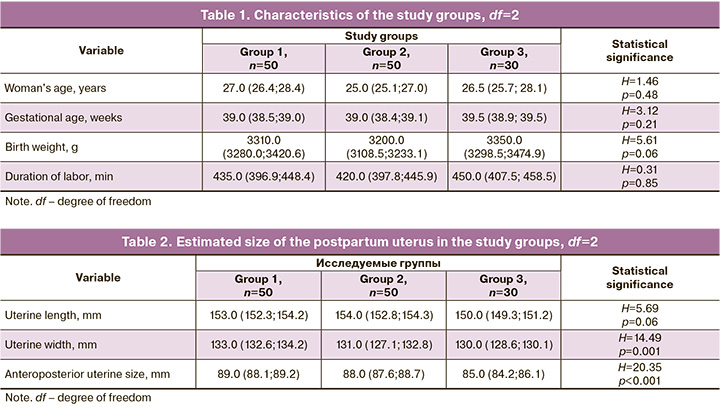
Groups did not differ significantly in age, gestational age, birth weight, and duration of labor (Table 1).
The number of primiparous women in group 1, 2, and 3 was 58% (29/50), 52% (26/50), and 56% (17/30), respectively (χ2 = 2.54, p=0.64). The absence of statistically significant differences between the study groups in the described parameters allows adequate assessment of feasibility to use ultrasound for detecting placental separation compared to the standard assessment.
The rate of false-positive results in detecting complete placental separation was 10% (5/50), 18% (9/50), and 0% (0/30) in groups 1, 2, and 3, respectively (χ2 = 6.37, p=0.04). The high rate of false-positive results in group 2 was since the signs of Hohenbichler and Strassmann primarily reflect the relationship between the blood flow of the uterus and the placenta, but do not reflect the fact of placental separation; signs of Rossier and Schroeder reflect the uterine contractile activity, contributing to placental separation, the effectiveness of their assessment is reduced against the background of the routine administration of oxytocin. Other signs of placental separation may be positive at the stage of partial placental separation when part of the placenta descends into the lower uterine segment or the vagina; meanwhile, complete separation has not yet occurred.
The rate of spontaneous placental delivery was 96% (48/50), 92% (46/50), and 87% (26/30) in groups 1, 2, and 3, respectively, (χ2 = 2.31, p=0.32). The most common cause of the absence of placental delivery was the diastasis of rectus abdominis muscles. After emptying the urinary bladder, the placenta was delivered using the Abuladze method [4]. A slightly lower spontaneous placental delivery rate in group 3 was probably due to the placenta's higher location (in the projection of the lower uterine segment). Meanwhile, this difference was not statistically significant (p=0.32) and requires further study.
The duration of the third stage of labor 12.0 (11.6; 12.5) minutes, 11.5 (11.4; 12.3) minutes, and 9.0 (8, 7; 9.8) minutes in groups 1, 2, and 3, respectively (H=21.94, df=2, p<0.001). Noteworthy it is not so much a decrease in the duration of the third stage of labor when using ultrasound in comparison with standard signs (U=355.5, p <0.001), but the absence of statistically significant differences between groups 1 and 2 (U = 1178.0, p = 0.57). This fact suggests that there is no need to determine several clinical signs. Determination of signs of Schroeder, Alfeld, Klein, Kustner–Chukalov, Dovzhenko, Strassmann, Mikulich–Radetzky, Hohenbichler, and Rossier increases the complexity of detecting placental separation. A decrease in the duration of the third period in this situation is associated not with an acceleration of the placenta separation, but with a high sensitivity of ultrasound examination (p<0.001). Assessment of placental separation signs takes a longer time than ultrasound examination, which hypothetically increases postpartum blood loss.
The intensity of myometrial retraction in the study groups was estimated by measuring uterine size by ultrasound 10 minutes after placental delivery (Table 2).
The absence of statistically significant differences in the uterine body length between the study groups is probably due to the more substantial tropism of oxytocin to the longitudinal muscle fibers and less to the circular and oblique myometrial muscle fibers. The transverse and anteroposterior dimensions of the uterus in groups 1 and 2, on average, were 3 mm longer than those of group 3 (p<0.001), despite a slightly higher mean birth in the study group (p=0.06), which, of course, indicates more intensive retraction processes with timely isolation of the placenta.
The total blood loss in group 1, 2, and 3 was 365.0 (353.7; 373.9), 350.0 (345.7; 364.7), and 300.00 (291, 9; 310.1) ml, respectively (H=22.03, df=2, p <0.001). The blood loss volume in group 3 was lower than in group 1 on average by 63 (61.8; 63.8) ml (U=317.0, p <0.001), and lower than in group 2 by 54 (53, 8; 54.6) ml (U=344.5, p <0.001). The higher blood loss volume in groups 1 and 2 is due to less intense contractions of the uterus having separated placenta in its cavity for a longer time. Intensive myometrial retraction promotes compression and deformation of venous vessels and retracts the spiral uterine arteries into the myometrium [2, 6]. Simultaneously, the blood clot formation begins, triggered by blood coagulation factors, and accelerated by placental tissue activators [2]. At the beginning of the thrombus formation process, the clots are loose, loosely connected to the vessels, easily detached, and washed out by the blood flow with insufficient retraction of the uterine myometrium, which explains the increase in blood loss with delayed delivery of the placenta even for a short time [2]. The total blood loss volumes in groups 1 and 2 did not differ significantly (U=1143.0, p=0.46), which confirms the lack of a need for determining several signs of placental separation. Meanwhile, the use of color Doppler for detecting complete placental separation of the does not seem appropriate, since attempts to deliver the placenta when it has not yet been completely separated can lead to the rupture of thin fetal membranes, which can also cause postpartum hemorrhage [4, 7].
The incidence of post-hemorrhagic anemia was 32% (16/50), 28% (14/50), and 17% (5/30) in group 1, 2, and 3, respectively, (χ2 = 2.29, p=0.32). The absence of statistically significant differences between the study groups is probably due to the small sample size. Considering the similarity of the studied indicators of groups 1 and 2, it was decided to combine these groups to determine the risk of post-hemorrhagic anemia using standard methods for assessing signs of placental separation as a whole. The absolute risk of developing post-hemorrhagic anemia in the postpartum period when using standard signs of placental separation was 0.17 when using ultrasound assessment – 0.3, relative risk RR = 1.8 (95% CI 0.77; 4.23), standard error of relative risk (S) – 0.44, the level of significance of this relationship p=0.17. This result is probably due to physiological blood loss in all study groups (less than 0.5–0.7% of the woman's body weight) [5].
Therefore, ultrasound detection of placental separation helps reduce the duration of the third stage of labor and postpartum blood loss due to intense myometrial retraction but does not affect the incidence of post-hemorrhagic anemia.
Conclusion
The present study's findings confirmed the feasibility of using ultrasound to detect placental separation, which reduces total blood loss and length of the third stage of labor. This imaging modality may be used in parturient women to prevent complications and reduce hospital stay length.
References
1. Радзинский В.Е. Акушерская агрессия. М.: Издательство журнала StatusPraesens; 2011. 688 с. [Radzinsky V.E. Obstetric aggression. Moscow: Izdatel'stvo zhurnala StatusPraesens; 2011. 688 p. (in Russian)].
2. Чернуха Е.А. Родовой блок. Руководство для врачей. М.: Триада-Х; 2005. 708с. [Chernukha E.A. The birth block. A guide for doctors. Moscow: Triada-X; 2005. 708p. (in Russian)].
3. Hofmeyr G., Mshweshwe N.T., Gulmezoglu A. Controlled cord traction for the third stage of labour. Cochrane Database Syst. Rev. 2015; (1): CD008020. https://dx.doi.org/10.1002/14651858.CD008020.pub2.
4. Клинические рекомендации (протокол лечения) № 15-4/10/2-3185«Оказание медицинской помощи при одноплодных родах в затылочном предлежании (без осложнений) и в послеродовом периоде», утвержденные Министерством здравоохранения Российской Федерации 6 мая 2014 г. [Clinical recommendations (treatment protocol) No. 15-4/10/2-3185 "Provision of medical care for single-fetal delivery in the occipital (without complications) and postpartum period," approved by the Ministry of Health of the Russian Federation on May 6, 2014. (in Russian)].
5. Клинические рекомендации (протокол лечения) № 15-4/10/2-2535«Профилактика, алгоритм ведения, анестезия и интенсивная терапия при послеродовых кровотечениях», утвержденные Министерством здравоохранения Российской Федерации 26 марта 2019 г. [Clinical recommendations (treatment protocol) No. 15-4/10/2-2535 "Prevention, management algorithm, anesthesia and intensive care for postpartum bleeding," approved by the Ministry of Health of the Russian Federation on March 26, 2019. (in Russian)].
6. Малыбаева Е.Р. Этиология и частота встречаемости послеродовых гипотонических кровотечений. Современные проблемы науки и образования. 2013; 2: 22. Доступно по: http://www.science-education.ru/ru/article/ view?id=8639 [Malybaeva E.R. Etiology and frequency of postpartum hypotonic bleeding. Current problems of science and education. 2013; 2. (in Russian)].
7. Krapp M., Baschat A.A., Hankeln M., Gembruch U. Gray scale and color Doppler sonography in the third stage of labor for early detection of failed placental separation. Ultrasound Obstet. Gynecol. 2000; 15(2): 138-42. https://dx.doi. org/ 10.1046/j.1469-0705.2000.00063.x.
8. Edwards H.M., Svare J.A., Wikkelso A.J., Lauenborg J., Langhoff-Roos J. The increasing role of a retained placenta in postpartum blood loss: a cohort study. Arch. Gynecol. Obstet. 2019; 299(3): 733-40. https://dx.doi.org/10.1007/ s00404-019-05066-3.
9. Клинические рекомендации (протокол лечения) № 15-4/10/2-3798«Кровесберегающие технологии в акушерской практике», утвержденные Министерством здравоохранения Российской Федерации 29 мая 2014 г. [Clinical recommendations (treatment protocol) No. 15-4/10/2-3798 "Blood-saving technologies in obstetric practice," approved by the Ministry of Health of the Russian Federation on May 29, 2014. (in Russian)].
10. Мерц Э. Ультразвуковая диагностика в акушерстве и гинекологии. Т.1. Акушерство. Пер. с англ. Гус А.И., ред. 2-е изд. М.: МЕДпресс-информ; 2011. 720c. [Gus A.I., ed. Ultrasound diagnostics in obstetrics and gynecology. Volume 1: Obstetrics. Eberkhard Merts. Moscow: MEDpress-inform; 2011. 720p. (in Russian)]
11. ICMJE. Uniform requirements for manuscripts submitted to biomedical journals: writing and editing for biomedical publication. J. Pharmacol. Pharmacother. 2010; 1(1): 42-58.
12. Lang T., Altman D. Basic statistical reporting for articles published in biomedical journals: The “Statistical analyses and methods in the published literature” or The SAMPL Guidelines. Int. J. Nurs. Stud. 2014; 52(1): 1-11. https://dx.doi. org/ 10.1016/j.ijnurstu.2014.09.006.
Received 21.04.2020
Accepted 11.06.2020
About the Authors
Viktor A. Mudrov, Ph.D., Associate Professor at the Department of Obstetrics and Gynecology, Faculty of Medicine and Dentistry, Chita State Medical Academy of Minzdrav of Russia. Tel.: +7(914)513-53-46. E-mail: mudrov_viktor@mail.ru. ORCID: 0000-0002-5961-5400. 39A Gorky str., Chita, 672090, Russia.For citation: Mudrov V.A. Experience in ultrasound detection of placental separation. Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2020; 10: 78-82 (in Russian)
https://dx.doi.org/10.18565/aig.2020.10.78-82



