Maternity care and therapeutic approaches for fetal bradyarrhythmia
Aim. To investigate the causes of and therapeutic approaches for different types of fetal bradyarrhythmia.Khodzhaeva Z.S., Potapova A.A., Klimenchenko N.I., Timoshina I.V., Bokeriya E.L., Kosheleva N.M.
Materials and methods. We examined 15 women with fetal bradycardia at 18–36 weeks of gestation.
Group I included nine pregnant women with moderate bradycardia (fetal heart rate 86–110 bpm). Six women with severe bradycardia (fetal heart rate 45–78 bpm) made up group II. The study methods included special laboratory examination and statistical analysis. Therapeutic approaches were based on indications and comprised hydroxychloroquine, therapeutic plasmapheresis, immunoglobulin therapy, and pulse corticosteroid therapy.
Results. Group II patients were statistically significantly more likely to have autoimmune diseases and higher titers of autoantibodies to SSA (Ro) and antinuclear antibodies. In the setting of specific therapy, 33.3% of patients showed regression of bradyarrhythmia to grade 1–2 AV block. In 66.7% of patients, there was no heart failure progression or the development of non-immune fetal hydrops. In group I, 88.8% of patients required no specific therapy. The gestational age at delivery in both groups was 38.4 (1.1) weeks. Thirty-three percent of the neonates in group I had multiple cardiovascular and pulmonary malformations. Transient cardiac arrhythmias were observed in 44% of group I neonates. Four out of 6 newborns in group II required pacemaker implantation.
Conclusion. Early diagnosis (up to 19–20 weeks of gestation) of fetal heart rate abnormalities, timely and comprehensive examination of the pregnant woman contributes to the early initiation of therapy and the prevention of irreversible damage to the fetal cardiac conduction system.
Keywords
Fetal arrhythmias are diagnosed in 1–3% of pregnancies. Depending on the heart rate (HR), fetal arrhythmias are classified into tachy- and bradyarrhythmias [1, 2].
Among all fetal cardiac arrhythmias cases, the most severe types are malignant arrhythmias, which are found in 1:5000–20,000 pregnancies. The perinatal mortality of neonates with cardiac arrhythmias and non-immune fetal hydrops researches to 30% and increases to 100% in the presence of heart defects [1–3].
Factors for developing fetal bradyarrhythmias are categorized into fetal (about 25%) and maternal (about 75%). Of most significant interest is the study of maternal factors associated with the development of fetal arrhythmia and the feasibility of their therapeutic correction during pregnancy [4].
The present study is aimed to investigate the causes and pregnancy outcomes in patients with different types of fetal bradyarrhythmia (FB).
Materials and methods
We examined 15 pregnant women diagnosed with FB at 18 to 37 weeks gestation who were managed at the V.I. Kulakov NMRC for OG&P from 2018 to 2019. The Research Ethics Committee of the V.I. Kulakov NMRC for OG&P approved this study. All participants provided signed informed consent to take part in the study.
The inclusion criterion was the presence of FB in singleton pregnancy according to the ultrasound findings (heart rate <110 bpm).
The exclusion criteria were maternal diseases, which exclude the possibility of efferent therapy, immunoglobulin therapy, and glucocorticoid pulse therapy.
Clinical evaluation included immunology laboratory tests (anti-Ro/SSA and anti-La/SSB antibodies, antibodies against double-stranded DNA (dsDNA), anti-nuclear antibodies), serial fetal echocardiography, ultrasound fetometry, and Doppler cardiac ultrasound, antenatal cardiotocography, as well as consultations of related specialists. According to the indications, patients were administered hydroxychloroquine, therapeutic plasmapheresis, immunoglobulin therapy, and glucocorticoid pulse therapy. Additional treatment courses were administered based on the dynamic assessment of maternal and fetal state and maternal serum autoantibody titers.
Statistical analysis
Statistical analysis was performed using IPM SPSS Statistics, version 22, and Microsoft Office Excel 2007. Quantitative were expressed as means (M) and standard deviation (SD). Categorical variables were compared using Fisher’s exact test. The distribution of continuous variables was tested for normality using the generalized D'Agostino-Pearson test. Between-group differences in continuous variables were assessed by Student’s t-test. Differences were considered statistically significant at p<0.05.
 Results
Results
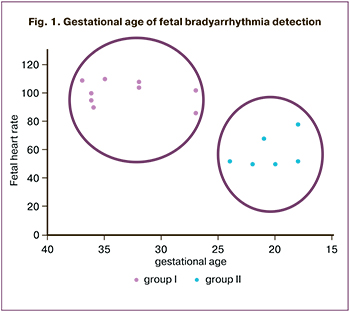
In 6 out of 15 (40%) pregnant women, fetal arrhythmias were diagnosed in less than 26 weeks of pregnancy, in the remaining 9 (60%) at the end of the II, beginning of the III trimester, and in 26% beyond 36 weeks' gestation. Depending on FB severity, pregnant women were divided into two groups. A group of moderate bradycardia (n=9) included patients with fetal heart rate 86–110 bpm and atrioventricular (AV) conduction 1:1 registered at ≥ 27 weeks of gestation. Group II (n=6) comprised patients with severe bradycardia, registered at 18–26 weeks, and all AV block cases (Fig. 1).
The age of the study participants ranged from 19 to 40 and averaged 30.3 (5.3) years. In groups I and II, it was 31.3 (5.6) and 28.8 (4.0) years, respectively. There were no statistically significant differences in age between these groups. All women lived in the same climatic and geographical conditions, mainly in the Central Federal District. No statistically significant differences were found regarding their socio-economic status.
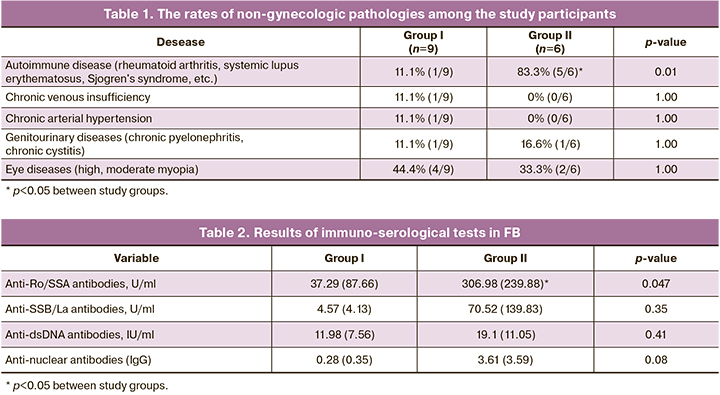
The incidence of autoimmune diseases was statistically significantly higher in group II (p=0.01) (Table 1). Moreover, 4 out of 6 (66.6%) pregnant women with severe FB were diagnosed with the onset of an autoimmune disease.
There were no statistically significant differences between the groups in the rates of early gestational toxemia, threatened miscarriage, and vaginal dysbiosis during the 1st trimester of pregnancy. No statistically significant differences were observed during the II and III trimesters of pregnancy in the incidence of gestational diabetes mellitus threatened preterm birth and ARVI.
The rates of ultrasound-detected fetal growth restriction, oligohydramnios, and polyhydramnios were comparable between the groups (p≥0.05).
Comparative analysis of laboratory test findings (table 2) showed that the titer of anti-SSA/Ro antibodies was statistically significantly higher in group II.
Considering the probable maternal origin of FB, we used basic therapy for a rheumatological autoimmune disease to reduce the general inflammatory response and prevent fibrosis. After a consultative interdisciplinary discussion, patients were administered hydroxychloroquine and oral fluorinated glucocorticoids (methylprednisolone and/or dexamethasone). A course of therapeutic plasmapheresis was carried out, consisting of three sessions, followed by intravenous pulse glucocorticoid (dexamethasone at a dosage of 32 mg) against the background of ongoing oral glucocorticoids therapy. In patients with rapid progression of fetal arrhythmias, intravenous human immunoglobulin at a dosage of 1 g/kg was used as a combination therapy for high autoantibody titers.
The therapeutic approaches are presented in Table 3. Due to the progression of fetal AV block grade and an increase in the titer of anti-SSA/Ro and anti-SSB/LA antibodies, 4 out of 6 pregnant women (66.7%) were administered a repeat course of plasmapheresis. Against the background of combination therapy with dynamic control, high autoantibody titers persisted, but 2 out of 6 pregnant women (33.3%) showed AV block regression to grades 1–2. No progression of heart failure and non-immune hydrops was observed in the remaining four patients (66.7%), making it possible to prolong the pregnancy to a full term in all 6 cases.
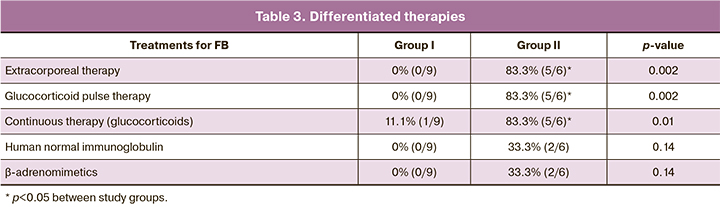
Two patients (33.3%) with severe FB (group II), who had fetal heart rate below 55 bpm, received selective β2-adrenomimetics (clenbuterol). Subsequently, their newborns required pacemaker implantation on the first day of life (Table 3).
In group I, 8 out of 9 patients (88.8%) did not require specific therapy due to transient FB episodes according to fetal state monitoring.
The delivery mode choice was based on FB severity. Two out of 9 (22.2%) pregnant women in the group I and one out of 6 pregnant women (16.7%) in group II with a stable state of the fetus and fetal heart rate above 95-100 bpm had a vaginal delivery. In group I, 44.4% (4 out of 9) of pregnant women started labor spontaneously. However, two of them were delivered by cesarean section due to the development of acute fetal hypoxia, confirmed by scalp lactate testing. The remaining patients (55.5 and 83.3%, respectively) underwent planned abdominal delivery due to severe fetal bradycardia with grade 2–3 AV block. The median gestational age at delivery in both groups was 38.4 (1.1) weeks. There were no significant differences in gestational ages and delivery modes between the study groups.
Analysis of neonatal outcomes showed no statistically significant differences in birth weight (group I – 3133.6 (350.9) g, group II – 3176 (272.9) g), body length (group I – 50.4 ( 2.1) cm, group II – 50.8 (1.7) cm) and Apgar scores at the 1st and 5th minutes (group I – 8 and 9 points, group II – 7 and 8 points, respectively).
Three out of 9 (33%) newborns of group I had multiple cardiovascular and pulmonary malformations (one left lung cystic-adenomatous malformation, one multiple interatrial communication, left ventricular myocardium trabecularity of non-compact type, and one aortic coarctation). In 4 out of 9 (44%) newborns, cardiac arrhythmias persisted after birth in the form of sinus bradycardia, incomplete right bundle branch block, or WPW (Wolff-Parkinson-White) syndrome. Only 2 out of 9 (22%) newborns had no evidence of neonatal arrhythmias.
In group I, none of the newborns required pacemaker installation.
In group II, 4 out of 6 newborns (66.7%) required cardiac surgery. These children were diagnosed in utero with arrhythmias persisting in the first hours of life as grade 3 AV block with a heart rate of 50–60 bpm. They were transferred to a cardiac surgery hospital to install a permanent pacemaker during the first day. In one case, pacemaker implantation resulted in ineffective ventricular stimulation. This newborn died on the second day of life due to congenital endocardial fibroelastosis of the right and left heart, severe arrhythmogenic myocardial dysfunction, and progressive cardiopulmonary insufficiency despite maximum doses cardiotonic and vasopressor therapy.
Two newborns (33.3%) in group II, who had a marked positive effect of maternal antenatal immunosuppressive therapy, were followed during the first month of life for the right bundle branch blockade and grades 1 and 2 AV block (Mobitz 1, Mobitz 2).
 Therapy for newborns was determined by the type of arrhythmia and heart rate. Glucocorticoids were required in 28% of children from group II and 10% from group I. In group I, 40% of newborns did not require specific therapy, and they remained under close follow-up. The need for antibiotic treatment in both groups was comparable and amounted to 20%.
Therapy for newborns was determined by the type of arrhythmia and heart rate. Glucocorticoids were required in 28% of children from group II and 10% from group I. In group I, 40% of newborns did not require specific therapy, and they remained under close follow-up. The need for antibiotic treatment in both groups was comparable and amounted to 20%.
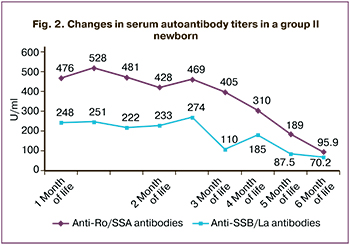
In newborns with severe bradyarrhythmia, a change in the serum Ro/La antibody titers was observed. For instance, during the first six months of life of newborn S., the titers of anti-Ro and anti-La antibodies decreased by 5 and 3.5 times, respectively (Fig. 2).
Discussion
FB is rare and challenging to diagnose fetal heart conduction abnormality, especially before 20 weeks of pregnancy. Early diagnosis of fetal cardiac arrhythmias, timely and comprehensive examination contributes to the early treatment initiation and the prevention of irreversible damage to the fetal cardiac conduction system, significantly affecting neonatal outcomes. Differential diagnosis of the causes of FB is extremely important, which determines the antenatal management strategy.
According to several authors, more than 85% of all fetal AV block develops from 16 to 28 weeks of gestation, which is consistent with our data [5]. Severe bradycardia, represented exclusively by AB block, was diagnosed at 18 to 26 weeks (group II), and moderate FB was registered at a gestational age of more than 27 weeks (group I).
The above-described significant differences in the time of diagnosis (Fig. 1), the severity of FB, and autoantibody titers (antinuclear and anti-SSA, Fig. 2) between the groups allow us to conclude that moderate and severe bradycardia have different etiopathogenesis. Fetal heart rhythm abnormalities in pregnant women of group I was observed at later stages of pregnancy and were not associated with maternal immune status. In 77.7% (7 out of 9) of cases, they were caused by the fetal heart block, including congenital heart anomalies, canalopathy, and WPW syndrome. The early development of FB is associated with severe and sometimes irreversible damage to the fetal heart conduction system due to participation in the pathogenesis of the mother's autoimmune mechanisms. Thus, 83.3% (5 out of 6) cases of FB in group II were due to the maternal factors of impaired heart electrical impulse generation and conduction. The transplacental transition of the above-described autoantibodies against nuclear receptors results in a stable inflammatory reaction in the fetal myocardium.
Our findings of similarities in the pregnancy course in the groups are consistent with other studies reporting that anti-SSA antibodies' presence does not affect complications and outcomes of pregnancy except the risk of developing fetal grade III AV block [6].
Unfortunately, today there is no unified approach to the management of FB. Attempts are being made to use various glucocorticoid treatment regimens, drugs with anti-inflammatory properties, immunoglobulins, and extracorporeal technics. These treatment modalities suppress the development of autoimmune-mediated fetal bradycardia due to a decrease in maternal titer of anti-Ro and anti-La antibodies binding to cardiomyocytes and leading to apoptosis. Due to the small number of observations, convincing evidence on FB therapy is lacking.
Nevertheless, some treatment modalities described in the literature have confirmed effectiveness in the treatment and prevention of irreversible damage to the fetal cardiac conduction system [7–9]. However, the prediction and prevention of postnatal blockage progression remain to be determined in further studies.
One of the main questions regarding the timing and mode of delivery is that the assessment of the fetus with FB is challenging and practically impossible in case of persistent rhythm disturbance. In most cases, we chose the operative delivery due to the difficulties of cardiotocographic monitoring during labor and the possible additional load on the fetal cardiovascular system during vaginal delivery. Our findings are also consistent with the 2015 systematic review, where 75% of pregnancies with fetal AV block were delivered by Caesarean section [5]. Spontaneous childbirth became possible in pregnant women in group II with regression of AV block to grade 1 during immunosuppressive therapy.
A study by J.P. Buyon et al. reported high neonatal mortality (up to 30%) in newborns with grade 3 AV block and subsequent irreversible morbidity. Therefore, up to 64–70% of newborns with complete AV block require pacemaker implantation in the first two weeks of life. According to our data, 4 out of 6 newborns in group II required cardiac surgery.
The decrease in serum Ro/La antibody titers of newborns noted in our work over the first six months of life proves their transplacental transition and is confirmed by the literature data that this type of FB is a model of passively acquired autoimmune disease [10]. Our results showed that 88.8% of moderate FBs did not require specific therapy and needed only close follow-up, which is consistent with the literature [11–13].
Two out of 9 (22.2%) pregnant women in the group I had successful vaginal deliveries due to stable rhythm during follow-up and the absence of fetal heart failure progression. A high rate of emergency intrapartum cesarean section (44.4% – 4 out of 9 pregnant women) in this group was due to the onset of fetal disorders according to cardiotocography and Doppler ultrasound. This complication was probably caused by an additional load on the fetal cardiovascular system when women went into labor.
Three newborns with cardiovascular and pulmonary defects required dynamic electrocardiographic monitoring without specific therapy.
Conclusion
Timely differential diagnosis of FB is an essential issue in modern perinatal care. The combined use of ultrasound and laboratory examination helps diagnose fetal rhythm and conduction disorders, determining their causes, and may be used to develop appropriate management strategy and guide drug and extracorporeal treatment planning.
The present study findings demonstrate the feasibility of a special immunological examination in diagnosing FB and fetal cardiac screening sonography from 18 to 28 weeks of pregnancy.
References
- Yuan S., Xu Z. Fetal arrhythmias: prenatal evaluation and intrauterine therapeutics. Ital. J. Pediatr. 2020; 46: 21. https://dx.doi.org/10.1186/s13052-020-0785-9.
- Бокерия Е.Л. Перинатальная кардиология: настоящее и будущее. Часть II: нарушение ритма сердца и проводимости. Российский вестник перинатологии и педиатрии. 2019; 64 (4): 6-10. [Bockerija E.L. Perinatal cardiology: the present and the future. Part II: cardiac arrhythmias and conduction. Rossiyskiy Vestnik Perinatologii i Pediatrii (Russian Bulletin of Perinatology and Pediatrics). 2019; 64(4): 6-10. (in Russian)]. https://doi.org/10.21508/1027-4065-2019-64-4-6-10.
- Strasburger J.F. Fetal arrhythmias. Prog. Pediatr. Cardiol. 2000; 11(1): 1‐17. https://dx.doi.org/10.1016/s1058-9813(00)00031-x.
- Brito-Zerón P., Izmirly P.M., Ramos-Casals M., Buyon J.P., Khamashta M.A. The clinical spectrum of autoimmune congenital heart block. Nat. Rev. Rheumatol. 2015; 11(5): 301-12. https://dx.doi.org/10.1038/nrrheum.2015.29.
- Buyon J.P., Clancy R.M., Friedman D.M. Autoimmune associated congenital heart block: integration of clinical and research clues in the management of the maternal/foetal dyad at risk. J. Intern. Med. 2009; 265(6): 653-62. https://dx.doi.org/10.1111/j.1365-2796.2009.02100.x.
- Brucato A., Cimaz R., Caporali R., Ramoni V., Buyon J. Pregnancy outcomes in patients with autoimmune diseases and anti-Ro/SSA antibodies. Clin. Rev. Allergy Immunol. 2011; 40(1): 27-41. https://dx.doi.org/10.1007/s12016-009-8190-6.
- Izmirly P.M., Costedoat-Chalumeau N., Pisoni C.N., Khamashta M.A., Kim M.Y., Saxena A. et al. Maternal use of hydroxychloroquine is associated with a reduced risk of recurrent anti-SSA/Ro-antibody-associated cardiac manifestations of neonatal lupus. Circulation. 2012; 126(1): 76-82. https://dx.doi.org/10.1161/CIRCULATIONAHA.111.089268.
- Saxena A., Izmirly P.M., Mendez B., Buyon J.P., Friedman D.M. Prevention and treatment in utero of autoimmune-associated congenital heart block. Cardiol. Rev. 2014; 22(6): 263-7. https://dx.doi.org/10.1097/CRD.0000000000000026.
- Zhou K.Y., Hua Y.M. Autoimmune-associated congenital heart block: a new insight in fetal life. Chin. Med. J. (Engl.). 2017; 130(23): 2863-71. https://dx.doi.org/10.4103/0366-6999.219160.
- Кошелева Н.М., Алекберова З.С. Неонатальная волчанка. Современная ревматология. 2015; 9(4): 92-7. https://dx.doi.org/10.14412/1996-7012-2015-4-92-97. [Kosheleva N.M., Alekberova Z.S. Neonatal lupus. Modern rheumatology. 2015; 9 (4): 92-7. (in Russian)]. https://dx.doi.org/10.14412/1996-7012-2015-4-92-97.
- Bravo-Valenzuela N.J., Rocha L.A., Machado Nardozza L.M., Araujo Júnior E. Fetal cardiac arrhythmias: current evidence. Ann. Pediatr. Cardiol. 2018; 11(2): 148-63. https://dx.doi.org/110.4103/apc.APC_134_17.
- Hofbeck M., Ulmer H., Beinder E., Sieber E., Singer H. Prenatal findings in patients with prolonged QT interval in the neonatal period. Heart. 1997; 77(3): 198‐204. https://dx.doi.org/110.1136/hrt.77.3.198.
- Mitchell J.L., Cuneo B.F., Etheridge S.P., Horigome H., Weng H.Y., Benson D.W. Fetal heart rate predictors of long QT syndrome. Circulation. 2012; 126(23): 2688‐95. https://dx.doi.org/110.1161/CIRCULATIONAHA.112.114132.
Received 22.06.2020
Accepted 10.12.2020
About the Authors
Zulfiya S. Khodzhaeva, Dr. Med. Sci., Professor, Deputy Director of Obstetrics Institute, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.E-mail: zkhodjaeva@mail.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Alyona A. Potapova, Ph.D. Student at the Department of High Risk Pregnancy, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
E-mail: doc.PotapovaAA@yandex.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Nataliya I. Klimenchenko, PhD, Senior Researcher at the Department of Obstetrics and Extragenital Pathology, V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia. E-mail: natalite@list.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Irina V. Timoshina, Ph.D., Researcher at the Department of High Risk Pregnancy, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
E-mail: timoshinairina@yandex.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Ekaterina L. Bokeriya, Dr. Med. Sci., Professor, Head of the 2nd Department of Pathology of Newborn and Preterm Babies, V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia. E-mail: e_bokeriya@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Nadezhda M. Kosheleva, Ph.D., Rheumatologist, Senior Researcher at the Laboratory of Vascular Rheumatology, V.A. Nasonova Research Institute of Rheumatology.
E-mail: nadkosheleva@yandex.ru. 115522, Russia, Moscow, Kashirskoye shosse, 34A.
For citation: Khodzhaeva Z.S., Potapova A.A., Klimenchenko N.I., Timoshina I.V., Bokeriya E.L., Kosheleva N.M. Maternity care and therapeutic approaches for fetal bradyarrhythmia.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 3: 74-80 (in Russian)
https://dx.doi.org/10.18565/aig.2021.3.74-80



