Molecular genetic predictors and ovarian reserve in women with deep infiltrating endometriosis
Objective: To investigate molecular genetic predictors and ovarian reserve in patients with infiltrating extragenital endometriosis. Materials and methods: This is a case-control study including 70 patients of reproductive age with deep infiltrating endometriosis (study group) and 50 fertile women (control group). All patients underwent testing for ovarian reserve. The molecular genetic analysis included testing for polymorphic variants of genes encoding proteins involved in the regulation of apoptosis [C-KIT: 2600 G>A, KITLG: 80441 C>T, TP53: Ex4+119 G>C (Arg72Pro)] and angiogenesis ((VEGF-A: +12143 C>А, VEGF-A: -2578 C>A, VEGF-A: -634 G>C, VEGF-A: +936 C>T). Results: The AMH level was significantly lower in patients with deep infiltrating endometriosis [2.4 (2.0) ng/ml] than in the control group [3.8 (3.2) ng/ml], p<0.05. Antral follicle counts were also significantly lower in women in the study group [8.3 (4.5)] than in the control group [11.8 (4.1)], p<0.001. Women in the study group had a statistically significantly higher frequency of the polymorphic allele Ex4+119 G>C of the TP53 gene [OR 2.43 (95% CI1.12; 5.27)], p<0.03. Carriage of the polymorphic allele +12143 C>А of the VEGF-A gene in a homo- or heterozygous state increases the risk of deep infiltrating endometriosis [OR 2.18 (95% CI 1.03; 4.61)], p<0.05. Trilocus model constructed by multivariate analysis suggested the involvement of a combination of single nucleotide polymorphisms of genes regulating apoptosis (C-KIT: 2600 G>A, TP53: Ex4+119 G>C) and angiogenesis (VEGF-A: -2578 C>A) in the pathogenesis of endometriosis. Conclusion: Deep infiltrating endometriosis without visible affection of the ovaries may be associated with a significant decline in ovarian reserve. Dysregulation of apoptosis and angiogenesis in deep infiltrating endometriosis may be a critical mechanism undelaying diminished ovarian reserve due to the premature activation of primordial follicles and their early depletion.Melkozerova O.A., Okulova E.O., Mikhelson A.A., Tretyakova T.B.
Keywords
Endometriosis affects 5–15% of women of reproductive age. One of the most common reasons for patients with endometriosis to seek medical care is infertility, which affects 25 to 50% of women in this patient category. Endometriosis is diagnosed in 58% of women undergoing laparoscopy as the final stage of the examination for infertility. The etiology and pathophysiology of endometriosis are not well understood. It has been assumed that a multifactorial effect of endometrial morphological and genetic alterations of the reproductive system may have a role [1, 2].
Among factors causing female infertility or subfertility, the most important is the depletion of the pool of residual ovarian follicles. Recent studies have confirmed a decline in ovarian reserve in patients with genital endometriosis, regardless of the location of endometriotic heterotopias [3, 4]. The mechanism of a decrease in ovarian reserve in deep infiltrating endometriosis has not yet been established.
In recent years, the common properties of endometriosis, especially its infiltrating forms, with cancer have been widely discussed. They included uncontrolled growth and multifocal endometrioid implantation, reduced apoptosis, lymph nodes metastasis and the spread inside and outside the abdominal cavity, penetration into surrounding tissues, stimulation of neoangiogenesis, chronic inflammation, and oxidative stress [5–8].
In endometriotic implants, genetic and epigenetic mutations have been identified that are involved in malignant transformation. Enhanced angiogenesis and decreased apoptosis, necessary for cell growth, are the most important signs of a malignant tumor, also found in the tissues of deep infiltrating endometriosis [9, 10].
In recent years, dysregulated apoptosis in endometriosis has been considered one of the causes of the reduced ovarian reserve due to the premature activation of primordial follicles and their early depletion [11].
This study aimed to investigate molecular genetic predictors and ovarian reserve in patients with infiltrating extragenital endometriosis.
Materials and methods
This case-control study included 120 patients of reproductive age who were managed at the Department of Gynecology of the Ural Research Institute of Maternity and Child Care, Ministry of Health of Russia, from 2019 to 2021. The control-to-case ratio was 1: 1.4. The study group consisted of 70 patients of reproductive age who underwent surgery for deep infiltrating endometriosis. In all patients of the study group, the diagnosis was verified histologically. The control group included 50 fertile patients of reproductive age without extragenital endometriosis who underwent uterine plastic surgery. The criteria for matching the groups were gender, age, anthropometric data, social status, and the forthcoming organ-sparing pelvic surgery without intervention on the adnexa.
The study protocol was approved by the local ethics committee (protocol No. 5 dated 10/08/2019). All patients signed written informed consent to participate in this study and for the publication of their data.
Baseline assessment included detailed medical and gynecological history, menstrual and reproductive functions, and clinical evaluation of presenting disease.
Ovarian reserve in patients of both groups was evaluated on days 2–5 of the menstrual cycle. It included blood levels of anti-Müllerian hormone (AMH), follicle-stimulating hormone (FSH), and estradiol determined by the Beckman Coulter (USA) Access 2 enzyme-linked immunosorbent assay using reagents of the same manufacturer following the instructions. Antral follicle counts were determined by transvaginal ultrasonography (US) performed in the early follicular phase on a Voluson 8 ultrasound system (General Electric, USA).
The reduced ovarian reserve was defined according to the Bologna criteria developed by The ESHRE Consensus, i.e., antral follicle count by transvaginal ultrasonography <5–7 follicles or AMH<0.5–1.1 ng/ml [12].
To construct a predictive model of the disease, all patients underwent molecular genetic testing for polymorphic variants of genes encoding proteins involved in the regulation of apoptosis [C-KIT: 2600 G>A, KITLG: 80441 C>T, TP53: Ex4+119 G>C (Arg72Pro)] and angiogenesis (VEGF-A: +12143 C>А, VEGF-A: -2578 C>A, VEGF-A: -634 G>C, VEGF-A: +936 C>T) using the set of reagents from TestGene LLC (Russia). DNA was isolated from 0.5 ml of venous blood taken in a test tube with ethylenediamminetetraacetate (EDTA) as an anticoagulant using Test GS-Genetics reagents (DNA-Technology LLC, Russia). The amount of isolated genomic DNA was estimated using a set of reagents to control sampling for polymerase chain reaction (PCR) (DNA-Technology LLC, Russia). For the study, we took at least 1.0 ng of genomic DNA per reaction.
Genetic testing was performed using allele-specific real-time PCR with the recording of the melting curves of the amplification products. PCR results were analyzed automatically by a DT-96 detecting amplifier produced by DNA-Technology LLC (Russia).
Statistical analysis
Statistical analysis was performed using Microsoft Excel (2010), SPSS Statistics version 22.0 (IBM Microsoft, USA), and Multifactor Dimensionality Reduction 2.0 beta 8 statistical software. The sample size was calculated by a power calculator for binary outcome using data on the number of patients with deep infiltrative endometriosis carrying polymorphisms of genes regulating apoptosis and angiogenesis [13]. Assuming a significance level of p<0.05, a 95% confidence interval, and a study power of 80%, the total sample size of 120 patients was required. Quantitative variables showing normal distribution and equal variance were expressed as means (M) and standard deviation (SD). Between-group differences in continuous variables were assessed by analysis of variance (ANOVA). Variables not meeting normality assumptions were reported as the median (Me) and interquartile range (Q1; Q3). Between-group differences for frequencies of genotypes and alleles were assessed by the χ2 test. The strength of associations was assessed in terms of odds ratio (OR) with a 95% confidence interval (CI). A test for the deviation from the Hardy-Weinberg equilibrium was performed in both groups was performed using the χ2 test using the Hardy–Weinberg equilibrium software.
Several risk models have been used to estimate the impact of polymorphic genetic markers. The general (additive) model assumes that the penetrance (expected value of a trait) in heterozygotes lies between the penetrance values for both homozygotes. In the general model analysis, the frequencies of the "wild" homozygous, heterozygous, and polymorphic genotypes were assessed separately. The multiplicative model assumes that penetrance depends on the number of copies of the predisposing allele; in the studied groups, the frequency of each variant allele (“wild” or polymorphic) was estimated separately. The dominant model assumes that the polymorphic allele is dominant, and its significance is manifested in hetero- and homozygous carriage; the total frequency of heterozygotes and polymorphic homozygotes was estimated. The recessive model assumes that the polymorphic allele is recessive and that the effect on penetrance is manifested only for polymorphic homozygotes; in the studied groups, the frequency of polymorphic homozygotes and the total frequency of heterozygotes and “wild” homozygotes were compared [14, 15].
Intergenic interactions were assessed using the bioinformatics multifactor dimensionality reduction (MDR) method, which allows evaluating all two-factor, three-factor, and four-factor SNP combination models, choosing the best models with the lowest prediction error and the highest reproducibility. The final level of significance p for the best n-locus model was estimated by the Monte Carlo procedure (1000 simulations). Differences were considered statistically significant at p<0.05.
Results and discussion
The mean age of women was 33 (5.16) years in the study group and 33 (3.44) years in the control group, p>0.05. No statistically significant differences were found in the anthropometric parameters; the mean body mass index was 22.54 (3.57) and 23.40 (3.91) kg/m2 in the study and control group, respectively, p>0.05.
Most of the patients in the study group (56/70, 80%) and in the control group (36/50, 72%), p>0.05, had higher education. Clinical and demographic characteristics of the patients of the study groups are presented in Table 1.
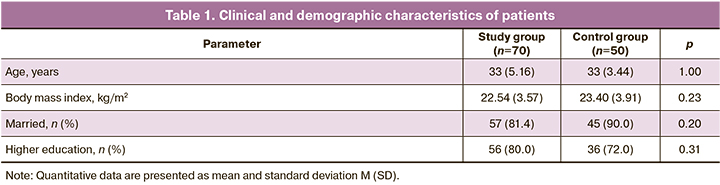
Comparison of comorbidities showed that 7/70 (10%) patients with deep infiltrating endometriosis had anemia, while none of the patients in the control group had anemia, p=0.022. A smoking habit was reported by 7/70 (10.0%) women in the study group and 5/50 (10%) in the control group, p>0.05.
Age at menarche, duration of the menstrual cycle, and duration of menstrual bleeding were comparable in the groups, p> 0.05. Patients in the study group were more likely to have painful menstruations (39/70, 55.7%) than patients in the control group (14/50, 28.0%), p=0.003.
The mean age of initiation of sexual relations was also comparable (p> 0.05). There were no statistically significant differences in the frequency of using combined oral contraceptives (p>0.05).
At least one past pregnancy was reported by 37/70 (52.9%) patients of the study group, and in 29/70 (41.4%), it ended in childbirth, while 50/50 (100%) women in the control group had a history of childbirth (p<0.001). The incidence of past medical abortions was significantly lower in patients with deep infiltrating endometriosis (7/70, 10.0%) than in patients in the control group (17/50, 34.0%), p=0.011. There were no statistically significant differences in the incidence of spontaneous miscarriages, missed miscarriages, and ectopic pregnancies in patients of both groups, p> 0.05.
Half of the patients with deep infiltrating endometriosis (34/70, 48.6%) and 7/50 (14.0%) patients in the control group had infertility, p<0.001. At the same time, primary endometriosis-associated infertility was more common (24/34, 70.6%) than secondary (10/34, 29.4%).
Cervical ectopy was observed in 12/70 (17.1%) study group patients versus 2/50 (4.0%) in the control group, p=0.028. In 6/70 (8.6%) patients, deep infiltrating endometriosis was concurrent with internal genital organ congenital malformations; in the control group patients, congenital malformations of the genital organs were not observed (p=0.034). Uterine fibroids were also more common in the study group (21/70, 30%) than among controls (5/50, 10%), p=0.009. There were no statistically significant differences in the incidence of other gynecological diseases in the two groups.
There were no statistically significant differences in the number of patients with a history of ovarian surgery in the study group [21/70 (30.0%)] and the control group [8/50 (16.0%)], p>0.05. In the study group, 17/70 (24.3%) patients had a history of surgery for ovarian endometriomas, on average 5.6 (0.51) years ago. А history of surgical excision of deep infiltrating endometriotic lesions reported 7/70 (10%) patients in the study group on average 3.0 (0.36) years ago. History of hysteroscopic surgery was statistically significantly more common in the study group (20/70, 28.6%) than in the control group (6/50, 12.0%), p=0.03. There were no significant differences between the two groups regarding the history of other gynecological operations.
Patients with deep infiltrating endometriosis were consistently more likely to suffer from chronic pelvic pain (60/70, 85.7%) than patients in the control group (6/50, 12.0%), p<0.001. Also, patients in the study group experienced dyspareunia significantly more often (14/70, 20%) than patients in the control group (2/50, 4%), p=0.012. Dyschezia and dysuria were found in 4/70 (5.7%) and 2/70 (2.8%) patients of the study, respectively, while no patients in the control group reported these symptoms.
Assessing ovarian reserve shoved that the level of AMH at the preoperative stage was lower in patients with infiltrating extragenital endometriosis than in patients in the control group with a mean difference of 1.3 ng/ml [2.4 (2.0) ng/ml in the study group vs. 3.8 (3.2) ng/ml in the control group], p=0.02.
The mean FSH level did not differ statistically significantly between the groups, amounting to 8.1 (8.7) mIU/ml in the study group and 7.2 (8.7) mIU/ml in the control group p>0.05. There were also no statistically significant differences in the mean serum estradiol level between the study group [150.3 (123.7) pg /ml] and the control group (168.4 (112.5) pg/ml), p>0.05.
Antral follicle counts measured by transvaginal ultrasound were statistically significantly lower in the study group – 8.3 (4.5) than in the control group – 11.8 (4.1), p<0.001. Results of ovarian reserve tests are presented in Table 2.
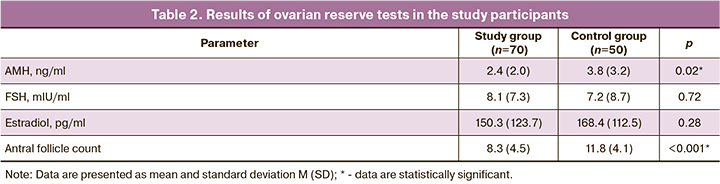
Our findings indicate a decrease in the ovarian reserve in patients of reproductive age with an infiltrating form of extragenital endometriosis. The mechanism of a reduction in ovarian reserve in deep infiltrating endometriosis is not fully understood.
Results of molecular genetic testing for polymorphic variants of genes encoding proteins involved in the regulation of apoptosis [C-KIT: 2600G>A, KITLG: 80441 C>T, TP53: Ex4+119 G>C (Arg72Pro)] and angiogenesis [VEGF-A: +12143 C>А, VEGF-A: -2578C>A, VEGF-A: -634 G>C, VEGF-A: +936C>T] showed that distribution of alleles and genotypes by polymorphic variants of the studied genes in both groups were consistent with Hardy–Weinberg equilibrium (Table 3)
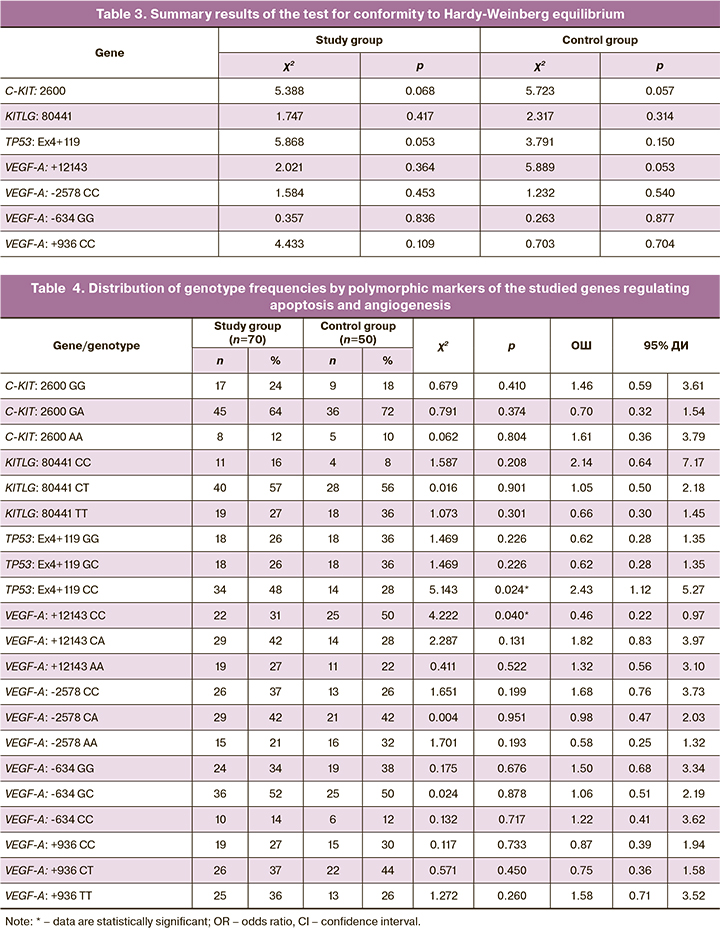
Analyzing the distribution of genotypes by polymorphic markers of the studied genes showed that the variant of the TP53: Ex4+119 CC apoptosis regulator gene was identified significantly more often in patients with deep infiltrating endometriosis than in patients in the study group (OR=2.43 (95% CI 1.12; 5.27); p<0.05) (Table 4).
The distribution of allele frequencies by polymorphic loci of the studied genes (Table 5) showed statistically significant differences in the frequency of the Ex4 + 119 G> C polymorphic allele of the TP53 gene (Arg72Pro). In women of the study group, the variant Ex4+119 C allele of the TP53 gene was recorded statistically significantly more often than in the control group patients (OR=1.87 (95% CI 1.1; 3.15); p=0.018). There were no statistically significant differences in the frequency of alleles for polymorphic markers of other studied genes between the groups.
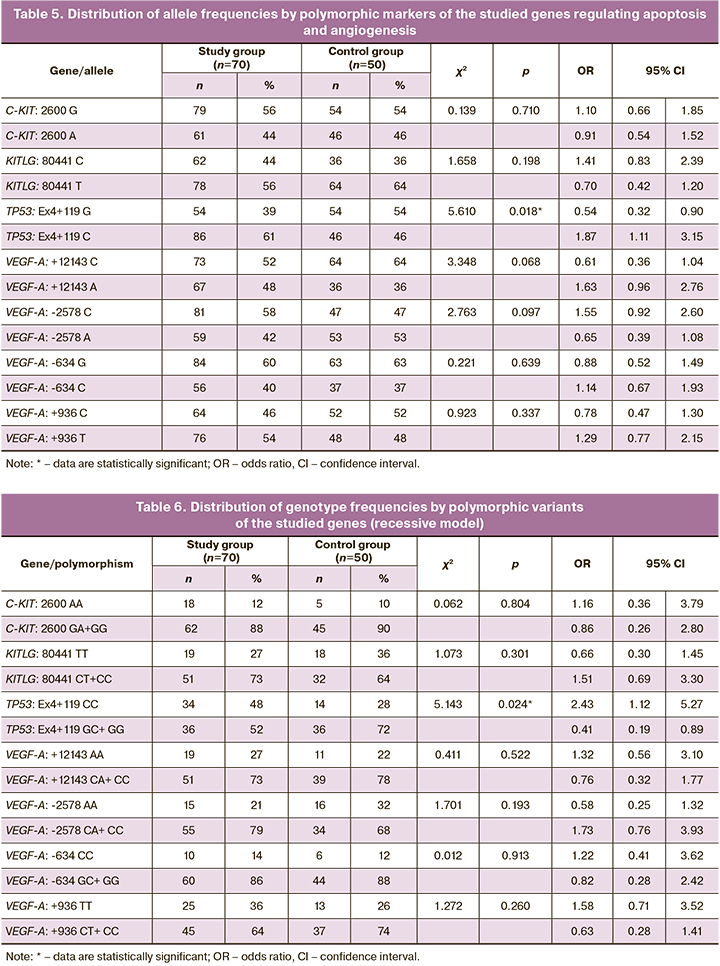
To assess the possible impact of the genotype on the risk of developing deep infiltrating endometriosis, we applied recessive and dominant models of the distribution of genotype frequencies for the studied polymorphic markers of the studied genes in patients of both groups.
Analyzing the recessive model of genotype frequencies distribution (Table 6) showed that women in the study group had a statistically significantly higher frequency of the polymorphic marker Ex4+119 CC of the TP53 gene than in the control group. The Ex4+119 CC genotype of the TP53 gene can be regarded as the genotype of the risk for developing deep infiltrating endometriosis since its carriage increases the risk of developing this disease (OR=2.43 (95% CI 1.12; 5.27); p<0.03). On the contrary, the presence of the variant allele Ex4+119 G in the genotype in a homo- or heterozygous state reduces the odds of developing deep infiltrating endometriosis (OR=0.41 (95% CI 0.19; 0.89); p<0.03).
One of the critical tumor suppressor genes is the TP53 gene that limits the likelihood of genetically unstable cells. The p53 protein regulates a wide range of cellular processes that eliminate potentially dangerous cells prone to malignant transformation. TP53 is a transcription factor for over 300 genes responsible for activating vital functions such as replication, DNA repair, apoptosis, antioxidant protection, etc. In human populations, the p53 protein activity is modified by genetic polymorphism. The most significant is the point replacement of guanine with cytosine in the 72nd codon of the 4th exon (Ex4+119G>C, Arg72Pro, rs 1042522). In contrast, the G and C alleles encode proteins differing in biochemical and physiological properties, which have different efficiencies in maintaining the cell's genetic homeostasis in a genotoxic environment settings [16].
Many studies have confirmed the association between the Arg72Pro polymorphism of the TP53 gene and the risk of developing breast and endometrial cancers [17].
The association between the TP53 Arg72Pro polymorphism and the risk of ovarian cancer has been extensively studied, but the results were contradictory. Zhang A. et al. (2017) conducted a meta-analysis that included 24 published studies with 10113 patients. The combined results showed no significant association between the Arg72Pro polymorphism of the TP53 gene and the risk of ovarian cancer [18].
Studies of TP53 gene polymorphisms have found their wide application in oncology, and the relationship between polymorphisms of this gene and the risk of endometriosis remains controversial [19].
Meta-analysis by Li J. et al. (2015) including 14 studies from eight countries reported a positive association between TP53 Arg72Pro (rs1042522) polymorphism and the risk of endometriosis, especially among the Asian population [in the dominant model OR=0.746 (95% CI 0.585; 0.952), in in the recessive model OR=0.650 (95% CI 0.510; 0.829); in the allelic analysis OR=0.762 (95% CI 0.654; 0.888)] [20].
Yan Y. et al. (2015), in a meta-analysis that included 15 case-control studies, also confirmed that the carriage of the Arg72Pro polymorphism of the TP53 gene increases the risk of developing endometriosis [19].
Vagnini L.D. et al. (2015), in a study on 605 infertile patients of reproductive age, found that the carriage of the rs4648551 A>G polymorphism of the TP73 gene, encoding the p73 isoform of the p53 protein is associated with a reduction in ovarian reserve with a decrease in the blood AMH levels and antral follicle counts [21].
Besides reducing apoptosis, the pathogenesis of endometriosis and malignant neoplasms is associated with the activation of neoangiogenesis. In recent years, research has been increasingly focused on the role of vascular endothelial growth factor (VEGF), a key regulator of angiogenesis, in developing endometriosis.
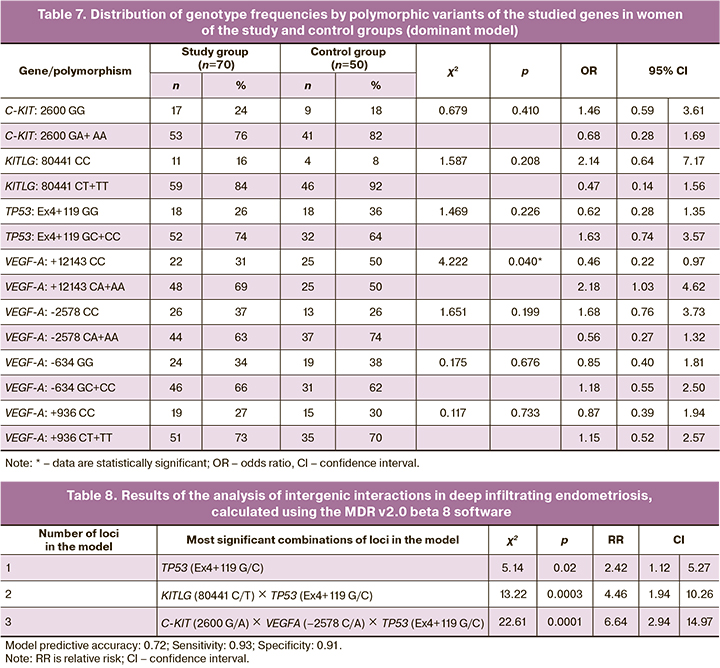
The results of the analysis of the dominant model of the distribution of genotype frequencies (Table 7) revealed the significance of the polymorphic marker VEGF-A: +12143 C>А in the risk of developing deep infiltrating endometriosis. The presence of the variant allele +12143 A in the genotype in a homo- or heterozygous state increases the risk of developing this pathology (OR=2.18 (95% CI 1.03; 4.61); p<0.05). VEGF-A genotype: +12143 CС, on the contrary, exerts a protective effect against the development of deep infiltrating endometriosis (OR=0.46 (95% CI 0.22; 0.97); p<0.05).
Recent studies have demonstrated an increased level of VEGF in the peritoneal fluid of patients with endometriosis and receptors to VEGF (VEGFR1) in the ectopic endometrium [22–24]. Moreover, the level of VEGF expression in patients with endometriosis directly correlates with the grade of the disease [25, 26]. At the same time, studies of polymorphic variants of the VEGF gene have conflicting results [27].
A study by Cardoso J.V. et al. (2017) involving 293 patients with endometriosis and 223 control patients demonstrated a positive correlation between single nucleotide substitutions -2578 C> A and -1154G> A of the VEGF gene and the risk of developing endometriosis. At the same time, the VEGF + 405G> C and VEGFR2 1192 C> T polymorphisms were associated with a reduced risk of developing the disease. Combined analysis of VEGF-VEGFR2 genotypes revealed the role of intergenic interactions in predisposition to endometriosis [28].
In a study by Rashidi B.H. et al. (2019), including 100 patients with endometriosis and 200 control subjects, no association was found between the -2578 A/C and +936 C/T polymorphisms of the VEGF gene and the risk of endometriosis. Also, the authors did not find a relationship between the expression of micro-RNA VEGF and the risk of developing this disease [29].
Later Pergialiotis V. et al. (2020), in a meta-analysis based on 20 studies, also did not reveal the effect of single nucleotide polymorphisms -460C/T, +405G/C, +936C/T, -2578A/C, and -1154G/A of the VEGF gene on the risk of developing and the severity of endometriosis [30].
In their study, Liu P. et al. (2020) demonstrated that patients in the early stages of premature ovarian failure have an altered cytokine profile characterized by an increase in chemokines and growth factors, including VEGF [31].
The inconsistency of the data obtained by different authors dictates the need for further study of the role of VEGF gene polymorphisms in the development of endometriosis and their effect on the ovarian reserve in this disease.
It is believed that individual genetic variants make a relatively minor contribution to the formation of the pathological phenotype. Therefore, to understand the critical links in the pathogenesis of the disease, it is necessary to analyze the intergenic and gene-environmental interactions that play a role in the formation of the clinical phenotype of the disease.
A modern approach for modeling intergenic interactions implies using the bioinformatics multifactor dimensionality reduction (MDR) method. According to multivariate analysis, a three-locus model C-KIT (2600 G/A) × VEGFA (-2578 C/A) × TP53 (Ex4+119 G/C) was formed, characterized by a sensitivity of 70% and a specificity of 74% (Table 8).
We identified 13 genotypes associated with increased risk and 12 genotypes associated with a reduced risk of deep infiltrating endometriosis (Figure).
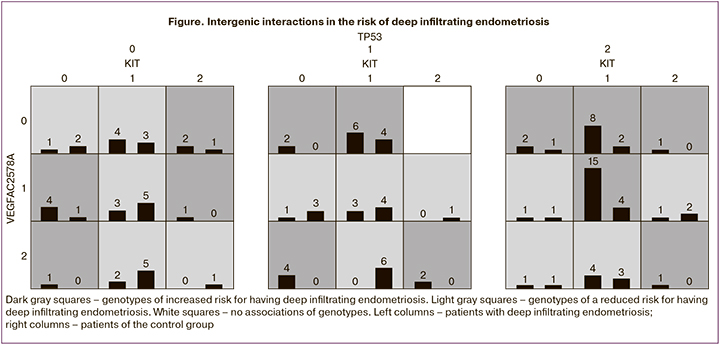
According to this predictive model, a combination of single nucleotide polymorphisms of genes regulating apoptosis (C-KIT: 2600 G>A, TP53: Ex4+119 G>C) and angiogenesis (VEGF-A: -2578 C>A) plays a role in the pathogenesis of deep infiltrating endometriosis. The sensitivity and specificity of the method were 93% and 91%, respectively. To predict the presence of deep infiltrating endometriosis in patients with the disease manifesting as pelvic pain, dyspareunia, or infertility, a molecular genetic study is performed to determine single nucleotide polymorphisms of the C-KIT, TP53 genes that regulate apoptosis, and the VEGF-A gene that regulates angiogenesis. The presence of a combination of single nucleotide polymorphisms of the C-KIT genes: 2600 G>A, TP53: Ex4+119 G>C, VEGF-A: -2578 C>A indicates a high risk for developing deep infiltrating endometriosis associated with reduced ovarian reserve.
C-KIT encodes the c-kit tyrosine kinase receptor involved in activating the antiapoptotic PI3K/AKT/mTOR signaling pathway. This is one of the universal intracellular signaling pathways responsible for suppressing apoptosis, cell growth, and proliferation. This signaling pathway plays a significant role in the progression of endometriosis, activating proliferation and suppressing apoptosis of endometriotic cells [32].
The signaling pathway of phosphatidylinositol 3 kinase plays an essential role in activating primordial follicles, which is emphasized in studies on transgenic animals [33].
Analysis of recessive and dominant models of the distribution of genotype frequencies for polymorphic variants of genes activating the PI3K/AKT/mTOR signaling pathway (C-KIT: 2600 G>A, KITLG: 80441 C>T) showed that these markers are not predictive of the risk of developing deep infiltrating endometriosis (Tables 4, 5). However, the combination of the 2600 G>A polymorphism of the C-KIT gene with the Ex4+119 G>C polymorphisms of the TP53 gene and -2578 C>A of the VEGF-A gene, according to the analysis of intergenic interactions, plays a significant role in the development of this disease. These findings demonstrate some molecular genetic mechanisms of combined dysregulation of apoptosis and angiogenesis, which underlie the reduction of ovarian reserve in deep infiltrating endometriosis in patients of reproductive age.
Conclusion
Deep infiltrating endometriosis without visible affection of the ovaries may be associated with a significant decline in ovarian reserve in patients of reproductive age.
We identified an association between polymorphic alleles of genes regulating apoptosis [TP53: Ex4+119 G>C (Arg72Pro) and angiogenesis (VEGF-A: +12143 C>А)], which determines an increased risk of developing deep infiltrating endometriosis in women of reproductive age, and a diminished ovarian reserve.
The analysis of intergenic interactions enables us to establish the types of genotypes associated with a high and low risk of deep infiltrating endometriosis and obtain a three-locus model that allows for determining a genetic predisposition to this pathology with a sensitivity of 93% and a specificity of 91%.
Dysregulation of apoptosis and angiogenesis in deep infiltrating endometriosis may be one of the essential mechanisms underlying a reduction in the ovarian reserve due to the premature activation of primordial follicles and their early depletion.
References
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Эндометриоз. 2020. 60c. [Ministry of Health of the Russian Federation. Clinical guidelines. Endometriosis. 2020. (in Russian)].
- Адамян Л.В., Салимова Д.Ф., Кондратович Л.М. Патогенетические аспекты эндометриоз-ассоциированного бесплодия. Проблемы репродукции. 2015; 21(6): 90-6. [Adamyan L.V., Salimova D.F., Kondratovich L.M. Pathogenetic aspects of endometriosis-associated infertility. Problems of Reproduction. 2015; 21(6): 90-6. (in Russian)]. https://doi.org/10.17116/repro201521682-88.
- Ashrafi M., Arabipoor A., Hemat M., Salman-Yazdi R. The impact of the localisation of endometriosis lesions on ovarian reserve and assisted reproduction techniques outcomes. J. Obstet. Gynaecol. 2019; 39(1): 91-7. https://dx.doi.org/10.1080/01443615.2018.1465898.
- Romanski P.A., Brady P.C., Farland L.V., Thomas A.M., Hornstein M.A. The effect of endometriosis on the antimüllerian hormone level in the infertile population. J. Assist. Reprod. Genet. 2019; 36(6): 1179-84. https://dx.doi.org/10.1007/s10815-019-01450-9.
- Муфтайдинова Ш.К., Буралкина Н.А., Файзуллин Л.З. Эндометриоз и рак. Акушерство и гинекология. 2021; 3: 12-7. [Muftaydinova Sh.K., Buralkina N.A., Faizullin L.Z. Endometriosis and cancer. Obstetrics and Gynecology. 2021; 3: 12-7. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.3.12-17.
- Wilson R.B. Hypoxia, cytokines and stromal recruitment: parallels between pathophysiology of encapsulating peritoneal sclerosis, endometriosis and peritoneal metastasis. Pleura Peritoneum. 2018; 3(1): 20180103. https://dx.doi.org/10.1515/pp-2018-0103.
- Králíčková M., Laganà A.S., Ghezzi F., Vetvicka V. Endometriosis and risk of ovarian cancer: what do we know? Arch. Gynecol. Obstet. 2020; 301(1): 1-10. https://dx.doi.org/10.1007/s00404-019-05358-8.
- Башмакова Н.В., Мелкозерова О.А., Михельсон А.А., Акулова Е.О. Роль средовых факторов в патогенезе бесплодия, ассоциированного с генитальным эндометриозом (обзор литературы). Проблемы репродукции. 2019; 25(5): 42-8. [Bashmakova N.V., Melkozerova O.A., Mikhelson А.А. et al. The role of environmental factors in the pathogenesis of infertility associated with genital endometriosis (literature review). Problems of Reproduction. 2019; 25(5): 42-8. (in Russian)]. https://doi.org/10.17116/repro20192505142.
- Kajiyama H., Suzuki S., Yoshihara M., Tamauchi S., Yoshikawa N., Niimi K. et al. Endometriosis and cancer. Free Radic. Biol. Med. 2019; 133: 186-92. https://dx.doi.org/10.1016/j.freeradbiomed.2018.12.015.
- Мелкозерова О.А., Башмакова Н.В., Окулова Е.О. Генетические и эпигенетические механизмы бесплодия, ассоциированного с генитальным эндометриозом. Акушерство и гинекология. 2019; 8: 26-32. [Melkozerova O.A., Bashmakova N.V., Okulova E.O. Genetic and epigenetic mechanisms of infertility associated with genital endometriosis. Оbstetrics and gynecology. 2019; 8: 26-32. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.8.26-32.
- Kitajima M., Dolmans M.M., Donnez O., Masuzaki H., Soares M., Donnez J. Enhanced follicular recruitment and atresia in cortex derived from ovaries with endometriomas. Fertil. Steril. 2014; 101(4): 1031-7. https://dx.doi.org/10.1016/j.fertnstert.2013.12.049.
- Ferraretti A.P., La Marca A., Fauser B.C., Tarlatzis B., Nargund G., Gianaroli L., ESHRE working group on Poor Ovarian Response Definition. ESHRE consensus on the definition of 'poor response' to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum. Reprod. 2011; 26(7): 1616-24. https://dx.doi.org/10.1093/humrep/der092.
- Sealed Envelope Ltd. 2012. Power calculator for binary outcome superiority trial. Available at: https://www.sealedenvelope.com/power/binary-superiority
- Clarke G.M., Anderson C.A., Pettersson F.H., Cardon L.R., Morris A.P., Zondervan K.T. Basic statistical analysis in genetic case-control studies. Nat. Protoc. 2011; 6(2): 121-33. https://dx.doi.org/10.1038/nprot.2010.182.
- Zang Y., Zhang H., Yang Y., Zheng G. Robust genomic control and robust delta centralization tests for case-control association studies. Hum. Hered. 2007; 63(3-4): 187-95. https://dx.doi.org/10.1159/000099831.
- Khan M., Khalil A., Rashid H. Evaluation of the p53 Arg72Pro polymorphism and its association with cancer risk: a HuGE review and meta-analysis. Genet. Res. (Camb.). 2015; 97: e7. https://dx.doi.org/10.1017/S0016672315000075.
- Diakite B., Kassogue Y., Dolo G., Wang J., Neuschler E., Kassogue O. et al. p.Arg72Pro polymorphism of P53 and breast cancer risk: a meta-analysis of case-control studies. BMC Med. Genet. 2020; 21(1): 206. https://dx.doi.org/10.1186/s12881-020-01133-8.
- Zhang A., Shi T.Y., Zhao Y., Xiang J., Yu D., Liang Z. et al. No association between TP53 Arg72Pro polymorphism and ovarian cancer risk: evidence from 10113 subjects. Oncotarget. 2017; 8(68): 112761-9. https://dx.doi.org/10.18632/oncotarget.22603.
- Yan Y., Wu R., Li S., He J. Meta-analysis of association between the TP53 Arg72Pro polymorphism and risk of endometriosis based on case-control studies. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015; 189: 1-7. https://dx.doi.org/10.1016/j.ejogrb.2015.03.015.
- Li J., Chen Y., Mo Z., Li L. TP53 Arg72Pro polymorphism (rs1042522) and risk of endometriosis among Asian and Caucasian populations. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015; 189: 73-8. https://dx.doi.org/10.1016/j.ejogrb.2015.03.026.
- Vagnini L.D., Renzi A., Oliveira-Pelegrin G.R., Canas Mdo C., Petersen C.G., Mauri A.L. et al. The TP73 gene polymorphism (rs4648551, A>G) is associated with diminished ovarian reserve. PLoS One. 2015; 10(3): e0120048. https://dx.doi.org/10.1371/journal.pone.0120048.
- Barreta A., Sarian L.O., Ferracini A.C., Costa L.B.E., Mazzola P.G., de Angelo Andrade L. et al. Immunohistochemistry expression of targeted therapies biomarkers in ovarian clear cell and endometrioid carcinomas (type I) and endometriosis. Hum. Pathol. 2019; 85: 72-81. https://dx.doi.org/10.1016/j.humpath.2018.10.028.
- Sekiguchi K., Ito Y., Hattori K., Inoue T., Hosono K., Honda M. et al. VEGF receptor 1-expressing macrophages recruited from bone marrow enhances angiogenesis in endometrial tissues. Sci. Rep. 2019; 9(1): 7037. https://dx.doi.org/10.1038/s41598-019-43185-8.
- Li C., Zhao H.L., Li Y.J., Zhang Y.Y., Liu H.Y., Feng F.Z. et al. The expression and significance of leukemia inhibitory factor, interleukin-6 and vascular endothelial growth factor in Chinese patients with endometriosis. Arch. Gynecol. Obstet. 2021; 304(1): 163-70. https://dx.doi.org/10.1007/s00404-021-05980-5.
- Tang T., Lai H., Huang X., Gu L., Shi H. Application of serum markers in diagnosis and staging of ovarian endometriosis. J. Obstet. Gynaecol. Res. 2021; 47(4): 1441-50. https://dx.doi.org/10.1111/jog.14654.
- Huang Y., Zhang T., Chen L., Yu M., Liu Q., Zhou C. et al. Elevated expressions of SHP2 and GAB2 correlated with VEGF in eutopic and ectopic endometrium of women with ovarian endometriosis. Gynecol. Endocrinol. 2020; 36(9): 813-8. https://dx.doi.org/10.1080/09513590.2020.1787378.
- Méar L., Herr M., Fauconnier A., Pineau C., Vialard F. Polymorphisms and endometriosis: a systematic review and meta-analyses. Hum. Reprod. Update. 2020; 26(1): 73-102. https://dx.doi.org/10.1093/humupd/dmz034.
- Cardoso J.V., Abrão M.S., Vianna-Jorge R., Ferrari R., Berardo P.T., Machado D.E. et al. Combined effect of vascular endothelial growth factor and its receptor polymorphisms in endometriosis: a case-control study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017; 209: 25-33. https://dx.doi.org/10.1016/j.ejogrb.2016.10.046.
- Rashidi B.H., Sarhangi N., Aminimoghaddam S., Haghollahi F., Naji T., Amoli M.M. et al. Association of vascular endothelial growth factor (VEGF) Gene polymorphisms and expression with the risk of endometriosis: a case-control study. Mol. Biol. Rep. 2019; 46(3): 3445-50. https://dx.doi.org/10.1007/s11033-019-04807-6.
- Pergialiotis V., Fanaki M., Bellos I., Stefanidis K., Loutradis D., Daskalakis G. The impact of vascular endothelial growth factor single nucleotide polymorphisms in the development and severity of endometriosis: a systematic review of the literature. J. Gynecol. Obstet. Hum. Reprod. 2020; 22: 101732. https://dx.doi.org/10.1016/j.jogoh.2020.101732.
- Liu P., Zhang X., Hu J., Cui L., Zhao S., Jiao X. et al. Dysregulated cytokine profile associated with biochemical premature ovarian insufficiency. Am. J. Reprod. Immunol. 2020; 84(4): e13292. https://dx.doi.org/10.1111/aji.13292.
- Fabregues F., Ferreri J., Calafell J.M., Moreno V., Borrás A., Manau D. et al. Pregnancy after drug-free in vitro activation of follicles and fresh tissue autotransplantation in primary ovarian insufficiency patient: a case report and literature review. J. Ovarian Res. 2018; 11(1): 76. https://dx.doi.org/10.1186/s13048-018-0447-3.
- Rehnitz J., Alcoba D.D., Brum I.S., Hinderhofer K., Youness B., Strowitzki T. et al. FMR1 and AKT/mTOR signalling pathways: potential functional interactions controlling folliculogenesis in human granulosa cells. Reprod. Biomed. Online. 2017; 35(5): 485-93. https://dx.doi.org/10.1016/j.rbmo.2017.07.016.
Received 08.10.2021
Accepted 08.11.2021
About the Authors
Oxana A. Melkozerova, Dr. Med. Sci., Deputy of Director for Science, Ural Research Institute of Maternity and Child Care, Ministry of Health of the Russian Federation, +7(343)371-24-27, +7(922)219-45-06, abolmed1@mail.ru, http://orcid.org/0000-0002-4090-0578, 620028, Russia, Ekaterinburg, Repina str., 1.Ekaterina O. Okulova, Ph.D. Student of the Department of Reproductive Functions Preservation, Ural Research Institute of Maternity and Child Care,
Ministry of Health of the Russian Federation, +7(343)371-24-27, cat93_07@mail.ru, http://orcid.org/0000-0002-3035-2862, 620028, Russia, Ekaterinburg, Repina str., 1.
Anna A. Mikhelson, Dr. Med. Sci., Head of the Department of Reproductive Functions Preservation, Head of the Department of Gynecology, Ural Research Institute of Maternity and Child Care, Ministry of Health of the Russian Federation, +7(343)371-24-27, ann_lukach@list.ru, https://orcid.org/0000-0003-1709-6187,
620028, Russia, Ekaterinburg, Repina str., 1.
Tatyana B. Tretyakova, Ph.D., Senior Researcher at the Department of Biochemical Research with Genetics Group, Head of the Genetics Laboratory, Ural Research Institute of Maternity and Child Care, Ministry of Health of the Russian Federation, +7(343)371-08-78, tbtretyakova@yandex.ru, http://orcid.org/0000-0002-5715-7514,
620028, Russia, Ekaterinburg, Repina str., 1.
Corresponding author: Ekaterina O. Okulova, cat93_07@mail.ru
Authors' contributions: Melkozerova O.A., Tretyakova T.B. – conception and design of the study; Okulova E.O., Mikhelson A.A., Melkozerova O.A. – data collection and analysis; Okulova E.O. – statistical analysis; Okulova E.O., Melkozerova O.A. – manuscript drafting; Melkozerova O.A., Tretyakova T.B., Mikhelson A.A. – manuscript editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The study was financed from the federal budget within the framework of the state order for the implementation of research No. 0056-00128-21-05.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Melkozerova O.A., Okulova E.O., Mikhelson A.A., Tretyakova T.B. Molecular genetic predictors and ovarian reserve in women with deep infiltrating endometriosis.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 11: 175-186 (in Russian)
https://dx.doi.org/10.18565/aig.2021.11.175-186



