В настоящее время концепция «стерильной матки», а также парадигма, что плод развивается в стерильной среде, широко обсуждается [1]. Поэтому роль микроорганизмов в поддержании гомеостаза в полости матки заслуживает внимания. Данные последних обзоров литературы сфокусированы на корреляции между комменсальной колонизацией матки, проблемами бесплодия и репродуктивными исходами [2–5]. Известно, что бактерии оказывают влияние на иммунную систему [6, 7]. Таким образом, если микроорганизмы влияют на иммунную систему еще до беременности, то это может в значительной степени сказываться на рецептивном потенциале эндометрия. Успех имплантации эмбриона во многом зависит от состояния эндометрия в момент «окна имплантации» – короткого периода, во время которого происходят анатомические и молекулярные изменения, необходимые для нидации эмбриона. Ухудшение восприимчивости эндометрия во время менструального цикла ведет к нарушению плацентации и бесплодию [8–10].
В силу того, что бактерии могут играть важную роль в морфологических изменениях, к примеру, клеток слизистой оболочки, не вызывает сомнения возможность негативного влияния деструктивных последствий воздействия микроорганизмов на процессы децидуализации. В то же время комменсальная микробиота может обеспечить защиту от патогенных видов микроорганизмов и тем самым способствовать благополучию в полости матки [11, 12].
В связи с этим цель нашего исследования – оценить влияние микробиоты полости матки на успешность имплантации эмбриона у женщин при использовании вспомогательных репродуктивных технологий (ВРТ).
Материалы и методы
В исследование включены 80 пациенток, обратившихся в ФГБУ «Национальный медицинский исследовательский центр акушерства, гинекологии и перинатологии имени академика В.И. Кулакова» Минздрава России с жалобами на бесплодие и имевшие в анамнезе одну или более безуспешных попыток ЭКО.
Критерии включения в исследование: возраст от 23 до 37 лет, одна и более неудачных попыток ЭКО с переносом эмбрионов «хорошего» качества. Критерии исключения: наличие у пациенток интерстициальной и/или субсерозной миомы матки более 4 см, наружный генитальный эндометриоз (III–IV стадия), внутриматочная патология (внутриматочная перегородка, субмукозная миома, полип эндометрия, «тонкий» эндометрий, хронический эндометрит), тяжелые формы патозооспермии у супруга (3–4 степень).
Проведено культуральное исследование дистального отдела эмбриокатетеров после переноса эмбриона. Особое внимание уделяли соблюдению правил асептики при проведении забора материала из полости матки, чтобы максимально исключить возможность контаминации матки микрофлорой влагалища и цервикального канала. Предварительно шейку матки обрабатывали стерильным ватным тампоном.
Учитывая, что в матке преимущественно может содержаться минимальное количество микроорганизмов (нулевая гипотеза), дистальный фрагмент эмбриокатетера предварительно помещали в пробирку со средой накопления (1 мл), используемой для гемокультур (Oxoid, Великобритания). После 48 ч культивирования в анаэробном боксе (Jouan, Франция) в атмосфере трехкомпонентной газовой смеси (N2 – 80%; CO2 – 10%; Н2 – 10%) состав микробиоты исследовали методом культуромики, с использованием расширенного набора селективных и неселективных питательных сред. Для выделения факультативно-анаэробных микроорганизмов использовали колумбийский агар (Oxoid, Великобритания), маннит-солевой агар (Himedia, Индия), среду CHROMagar, Франция (для выявления и дифференциации Streptococcus agalactiae), энтерококковый агар (ФГУН «ГИЦПМиБ», Оболенск, Россия), агар Эндо-ГРМ (Оболенск), декстрозный агар Сабуро (Oxoid, Великобритания). Лактобациллы культивировали на среде Лактобакагар (ФГУН «ГИЦПМиБ», Оболенск, Россия), строгие анаэробы – на агаре для бифидобактерий (Himedia, Индия), прередуцированном агаре Шедлера с необходимыми добавками, основном агаре для анаэробов, перфрингенс агаре (Oxoid, Великобритания).
Видовую идентификацию выделенных микроорганизмов проводили с помощью времяпролетного MALDI-TOF масс-спектрометра AutoFlex III c программным обеспечением Maldi BioTyper версия 3.0 (Bruker Daltoniks, Германия).
Пациентки были разделены на 2 группы: I – пациентки с переносом эмбриона в цикле стимуляции (n=50); II – с переносом эмбриона в криоцикле (n=30). Всем женщинам проводили селективный перенос 1 эмбриона «хорошего» качества на 5-й сутки культивирования. Пациенткам I группы проводили стимуляцию суперовуляции со 2–3-го дня менструального цикла по протоколу с антагонистом гонадотропин-рилизинг-гормона. В качестве триггера овуляции использовался хорионический гонадотропин человека в стандартной дозе 9000 МЕ.
Перенос эмбриона в криоцикле осуществляли без заместительной гормональной терапии, в естественном овуляторном цикле. Поддержку посттрансферного периода проводили препаратом дидрогестерона в дозе 20 мг в cутки в криоцикле и 40 мг в сутки – в цикле стимуляции суперовуляции.
Сравнительный анализ полученных результатов проводили у женщин как в циклах стимуляции суперовуляции, так и в криоциклах в зависимости от наступления беременности или ее отсутствия.
Результаты
Результаты культурального исследования эмбриокатетеров после завершения процедуры ЭКО (расценивали как «микробиота матки») представлены в таблицах 1 и 2.

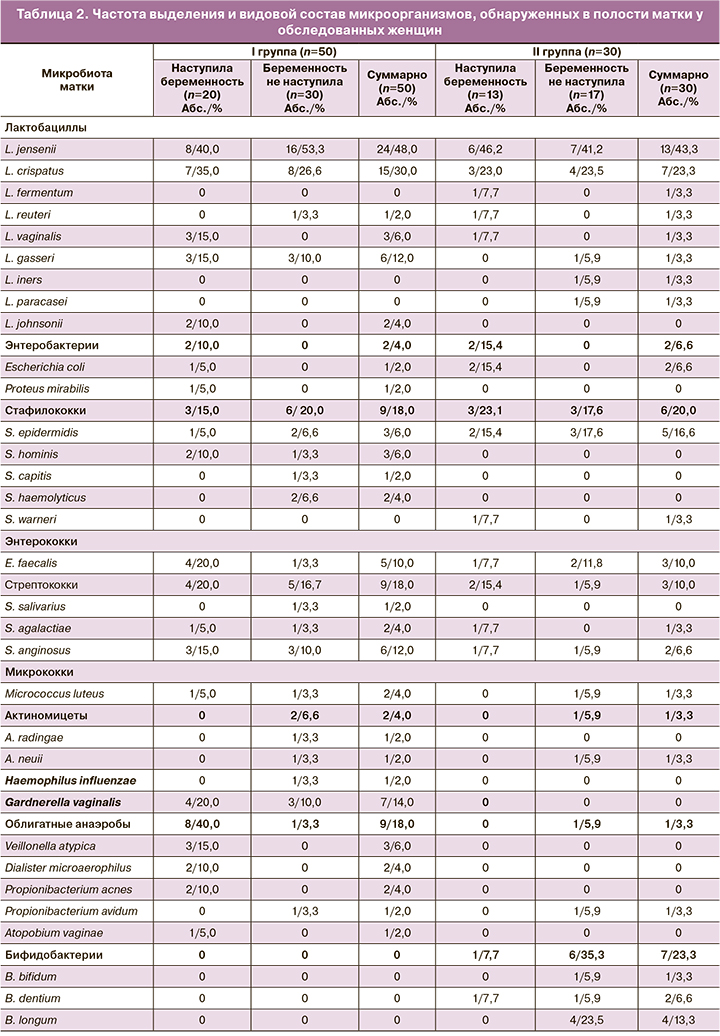
Установлено, что полость матки у женщин I и II групп была стерильной только в 12,0 и 13,3% случаев соответственно (табл. 1). У подавляющего большинства женщин отмечен рост микроорганизмов как в монокультуре (у 28,0% в I группе и у 30,0% – во II группе), так и в ассоциациях различных видов микроорганизмов (у 60,0 и 56,6% женщин соответственно). Наиболее представленной частью микробиоты в обеих группах были лактобациллы, которые выделяли с частотой 78,0 и 66,6% соответственно. В 26,0% случаев в I группе и 20,0% – во II группе эти микроорганизмы присутствовали в монокультуре, в 14,0 и 13,3% – выделены ассоциации разных видов лактобацилл, а в 38,0 и 20,0% – ассоциации лактобацилл с условно-патогенными микроорганизмами (УПМ). Второй по частоте встречаемости составляющей микробиоты матки были УПМ: в I группе – у 48,0% женщин и во II – у 40,0%. В монокультуре УПМ соответственно выделены у 2,0 и 3,3% женщин, ассоциации только УПМ – у 8,0 и 6,6% женщин и ассоциации УПМ с лактобациллами и бифидобактериями соответственно у 38,0 и 29,9% женщин.
Следует отметить, что у пациенток I группы УПМ в ассоциациях с лактобациллами встречались почти в два раза чаще, чем во II группе (38,0 и 20,0% соответственно), а бифидобактерии, напротив, выявляли только у пациенток II группы (23,3%).
Видовой состав выделенных из полости матки микроорганизмов оказался разнообразным (табл. 2): у обследованных женщин выделено 147 штаммов микроорганизмов (97 и 50 штаммов соответственно в I и II группах), относящихся к 11 родам и 33 видам бактерий. Доминирующими в обеих группах были лактобациллы (9 видов), из которых 2 вида – Lactobacillus jensenii (L. jensenii) и Lactobacillus crispatus (L. crispatus) занимали ведущую позицию (78,0 и 66,6% в сравниваемых группах соответственно). Обращает на себя внимание, что высеваемость облигатно-анаэробных бактерий в I и II группах соответственно составила 18,0 и 3,3%; G. vaginalis обнаружена только в I группе (14,0%), а бифидобактерии, напротив, обнаружены только во II группе (23,3%).
Что касается частоты наступления беременности в сравниваемых группах, то беременность наступила у 40,0% женщин I группы и у 43,3% женщин II группы.
В I группе у женщин с наступившей беременностью доминирующими были лактобациллы (у 85,0% женщин): L. jensenii (40,0%), L. crispatus (35,0%). Среди УПМ (50,0%) наиболее часто встречались G. vaginalis и Enterococcus faecalis (E. faecalis) (по 20,0%) и Streptococcus anginosus (S. anginosus) (15,0%). У женщин с отсутствием беременности (60,0%) частота выделения лактобацилл оказалась ниже, чем у женщин с наступившей беременностью (73,3%), но превалировали также L. jensenii (53,3%) и L. crispatus (26,6%). В составе УПМ (46,6%) наиболее часто встречались G. vaginalis (10,0%) и факультативно-анаэробные микроорганизмы: S. anginosus (10,0%), Staphylococcus haemolyticus (S. haemolyticus) (6,6%). Обращает на себя внимание обнаружение Actinomyces sp. (6,6%) и редко выделяемого из половых органов вида Haemophilus influenzae (3,3%).
Во II группе беременность наступила у 13 женщин (43,3%). Доминировали лактобациллы (у 69,2% женщин), из них наиболее часто встречались L. jensenii (46,2%) и L. crispatus (23,0%). Среди УПМ (38,5%) наиболее часто выделяли факультативные анаэробы: Escherichia coli (E. coli) (15,4%) и Staphylococcus epidermidis (S. epidermidis) (15,4%). У женщин с отсутствием беременности (56,7%) частота выделения лактобацилл составила 64,7% с преобладанием L. jensеnii (41,2%) и L. crispatus (23,5%). В составе УПМ (41,2%) также чаще высевали факультативные анаэробы: S. epidermidis (17,6%) и E. faecalis (11,8%); у 5,9% женщин обнаружен Actinomyces neuii. У 35,3% женщин, у которых не наступила беременность, полость матки была колонизирована бифидобактериями (B. bifidum, B. dentium, B. longum).
Обсуждение
Полученные нами данные о микробной колонизации полости матки согласуются с результатами исследований Mitchell M. et al., Cicinelli E. et al., [13–18], предполагающих, что полость матки нестерильна. В то же время необходимо отметить, что в соответствии с общепринятой методикой переноса эмбриона, исключающей предварительную деконтаминацию шейки матки и цервикального канала антисептиком, не представлялось возможным полностью исключить вероятность контаминации проводника, а возможно, и эмбриокатетера микрофлорой экзо- и эндоцервикса. Однако большинство исследователей в аналогичной ситуации не акцентируют внимание на этом аспекте работы.
В исследовании Mitchell M. et al. [13] изучены образцы из полости матки 58 женщин, полученные после гистерэктомии. Микробная обсемененность полости матки выявлена у 55 (95%) пациенток, из них у 52 обнаружен только один вид микроорганизмов. Наиболее распространенными были: Lactobacillus iners (выделен у 45% женщин из полости матки и у 61% – из отделяемого влагалища) и L. crispatus (у 33% – из полости матки, у 56% – из отделяемого влагалища). В нашем исследовании наиболее представленной частью микроорганизмов были лактобациллы (L. jensenii и L. crispatus), которые занимали ведущую позицию у женщин с переносом эмбриона в цикле стимуляции (78,0%) и у женщин с переносом в криоцикле (66,6%).
В исследовании Moreno I. et al. [19] оценивалось влияние микробиоты эндометрия на репродуктивные исходы. Показано, что группа пациенток со снижением количества лактобацилл (менее 10%) и преобладанием условно-патогенной микрофлоры (более 90%) в эндометрии, по сравнению с группой с преобладанием лактобацилл (более 90%), имела достоверно более низкую частоту имплантации (23,1% против 60,7%), наступления беременности (33,3% против 70,6%), прогрессирующей беременности (13,3% против 58,8%) и живорождения (6,7% против 58,8%). Проведенное нами исследование показало, что из 33 забеременевших женщин наилучшие результаты имплантации отмечены при колонизации матки только лактобациллами как в стимулированном, так и в криоцикле (42,4%).
Микробиом эндометрия у бесплодных пациенток оценен в исследовании Tao X. et al. [20]. В исследование включены образцы микробиоты полости матки, полученные у 70 пациенток, которым проводилась программа ЭКО. Из 70 образцов были отобраны 33, в которых содержалось более 90% лактобацилл и 50 образцов, в которых обнаружено 70% лактобацилл. Помимо лактобацилл, выявлены УПМ: Corynebacterium spp. – у 40 женщин, Bifidobacterium spp. – у 15, Staphylococcus spp. – у 38 и Streptococcus spp. – у 38 женщин. В нашем исследовании у женщин с наступившей беременностью при переносе эмбриона в цикле стимуляции суперовуляции среди УПМ (50%) наиболее часто встречались такие виды, как G. vaginalis и E. faecalis (по 20%) и S. anginosus (15%). У женщин с отсутствием беременности в составе УПМ (46,6%) наиболее часто выделяли G. vaginalis (10%) и факультативно-анаэробные микроорганизмы: S. anginosus (10%), S. haemolyticus (6,6%). У забеременевших женщин с переносом эмбриона в криоцикле среди УПМ (38,5%) наиболее часто обнаруживали факультативные анаэробы E. coli (15,4%) и S. epidermidis (15,4%). У женщин этой группы с отсутствием беременности в составе УПМ (41,2%) также наиболее часто выявляли факультативные анаэробы: S. epidermidis (17,6%) и E. faecalis (11,8%), у 5,9% женщин обнаружен Actinomyces neuii. У 35,3% женщин полость матки колонизирована бифидобактериями (B. bifidum, B. dentium, B. longum).
Проведенное нами исследование показало, что при обсеменении матки УПМ в ассоциации с лактобациллами суммарная частота наступления беременности составила 36,4%, а при колонизации только УПМ – 9,1% из расчета на перенос эмбриона в полость матки. При отсутствии колонизации эндометрия (стерильная полость матки) беременность наступила также лишь в 9,1% случаев. Вероятнее всего, существует взаимосвязь между влиянием гормонального фона и бактериальной обсемененностью полости матки в стимулированных и в криоциклах. Данная работа носит пилотный характер, необходимы дальнейшие исследования на более крупной выборке женщин.
Заключение
Полученные нами данные позволяют предположить наличие функциональной системы «микробиота–эндометрий», которая характеризуется преобладанием лактобацилл. В результате воздействия различных неблагоприятных факторов гомеостаз полости матки может быть нарушен, что в последующем сказывается на репродуктивных исходах. Таким образом, возможно, при подготовке к переносу эмбриона в полость матки целесообразна своевременная коррекция нарушений микробиоты нижних отделов полового тракта с использованием пробиотиков, содержащих лактобациллы. В то же время высокая частота выделения бифидобактерий у женщин с отсутствием беременности требует уточнения возможного отрицательного влияния этих микроорганизмов на имплантацию на большей популяции женщин.



