The clinical diagnostic value of maternal autoantibodies against extractable nuclear antigens in fetal bradyarrhythmia
Autoantibodies against extractable nuclear antigens in mothers with autoimmune diseases (AID) or asymptomatic carriers may cause fetal bradyarrhythmia to the point of congenital heart block (CHB).Menzhinskaya I.V., Khodzhaeva Z.S., Bokeria E.L., Klimenchenko N.I., Potapova A.A., Van'ko L.V., Krechetova L.V., Kosheleva N.M.
Aim. To investigate the clinical diagnostic value of maternal autoantibodies against extractable nuclear antigens in fetal bradyarrhythmia.
Materials and methods. The study included pregnant women with fetal bradyarrhythmia (group 1, n=15) and with regular fetal heart rhythm, who were divided into groups without (group 2, n=20) and with (group 3, n=10) AID. The women were tested for serum antinuclear antibodies (ANA), IgG antibodies against SSA/Ro, SSB/La, Sm, and double-stranded DNA using an enzyme immunoassay.
Results. IgG antibodies against SSA/Ro and ANA were detected in 46.5% and 40% of women in group 1, which was 36.2 and 12.7 times more often than in group 2. These antibodies had a high diagnostic value in fetal atrioventricular heart block. Among pregnant women with fetal bradyarrhythmia, anti-SSA/Ro antibodies were detected more often than anti-SSB/La antibodies. Specific treatment of maternal AID contributed to the regular fetal heart rate.
Conclusion. The detection of autoantibodies against SSA/Ro in asymptomatic carriers with fetal bradyarrhythmia and preventive CHB therapy can improve fetal outcomes.
Keywords
Antibodies against nuclear antigens are a large group of antibodies that react with nucleic acids and associated proteins. Antibodies against extractable nuclear antigens interact with water-soluble complexes of ribonucleoproteins (SSA/Ro, SSB/La, Sm, RNP/ Sm, Scl-70, Jo-1, etc.) in nuclei and cytoplasmic compartment. They have important implications in the diagnosis of various diseases, including systemic lupus erythematosus (SLE), scleroderma, Sjögren’s syndrome, and other systemic connective tissue diseases [1]. Antibodies to double-stranded DNA bind to native DNA molecules and are specific for SLE [2]. They can be detected in women years before the first clinical manifestations of autoimmune diseases and serve as biomarkers of the disease onset and activity.
These antibodies play an important role during pregnancy, when maternal anti-Ro and anti-La antibodies cross the placenta and might affect cardiac fetal development by damaging fetal conduction tissues [3–5]. It is important to note that fetal heart rhythm disturbances often are the first symptom of autoimmune disease requiring maternal examination [6]. From 50% to 80% of all cases of fetal bradyarrhythmia are associated with maternal autoimmune disease, such as Sjögren’s syndrome, SLE, rheumatoid arthritis, mixed or undifferentiated connective tissue diseases or with asymptomatic carriage of anti-Ro and anti-La antibodies [7].
Pathogenetic mechanisms of fetal anti-SSA/ Ro-antibody-mediated bradyarrhythmia include immune complex deposits, inflammation with subsequent development of fibrosis, myocardial calcification leading to blockage of signal conduction at the atrioventricular (AV) node [4, 5].
Two percent of infants born to anti-SSA/Ro and SSB/La-positive mothers had autoimmune-mediated congenital heart block (CHB) diagnosed in utero, which is a manifestation of severe neonatal lupus [3, 5]. The case fatality rate in infants with autoimmune CHB reaches 30%, and in babies, with concomitant cardiomyopathy, it is 100% [7]. According to some authors, in the first year of life, 70% of newborns with CHB need to be implanted with permanent cardiac pacemakers, and the vast majority of them (up to 75-80%) require this operation in the first two weeks of life [5].
Maternal anti-Ro and anti-La antibodies cross the placenta as early as week 11 of gestation and starting from weeks 16–18, clinical manifestations of autoimmune CHB can be observed in the form of a reduction in ventricular rate to 50–70 bpm while maintaining a regular atrial rate [5]. According to ultrasound data, in second-degree AV block some atrial impulses are completely blocked, and third-degree AV block is characterized by the presence of a complete atrioventricular dissociation, when atrial and ventricular activation are independent of each other [8, 9].
Complete AV block (also known as third-degree AV block) is the most severe and often irreversible manifestation of CHB. Often, autoimmune-mediated damage to the heart also results in endocardial fibroelastosis, dilated cardiomyopathy, and valvular disease that are associated with significantly higher perinatal morbidity and mortality [7, 10].
Based on the preceding, the present study aimed to investigate the clinical diagnostic value of maternal autoantibodies against extractable nuclear antigens in fetal bradyarrhythmia.
Materials and methods
The study group (group 1) included pregnant women with fetal bradyarrhythmia (n = 15) at 16 to 36 weeks’ gestation. Comparison groups (groups 2 and 3) comprised pregnant women of similar gestational age with normal fetal heart rhythm who did not have (group 2, n=20) and had (group 3, n=10) autoimmune diseases. All women underwent laboratory and instrumental examinations and were managed at the V.I. Kulakov NMRC for OG&P of Minzdrav of Russia and the V.A. Nasonova Research Institute of Rheumatology. Clinical evaluation included a weekly echocardiographic examination of the fetus, ultrasound fetometry, serial Doppler ultrasound of the fetus, and daily antenatal cardiotocography. Consultations with related specialists were held.
In group 1, 13 of 15 women (86.7%) had no established diagnosis of autoimmune connective tissue disease at the time of registration for antenatal care. At 18 weeks of gestation, 9 (60%) and 6 (40%) of them were diagnosed with fetal sinus bradycardia and second-degree or third-degree AV block, respectively. Four (26.7%) pregnant women with fetal heart AV block were suspected of having the debut of autoimmune disease including SLE (n=2), Sjögren’s syndrome (n=1), SLE and Sjögren’s syndrome (n=1). In one pregnant SLE patient, who did not receive immunosuppressive therapy due to an allergic reaction to hydroxychloroquine, a fetal heart rhythm disturbance in the form of sinus bradyarrhythmia was detected at 36 weeks. The second patient with rheumatoid arthritis, who discontinued specific therapy and visited the Center at 23 weeks of gestation for fetal heart rhythm disturbance, was diagnosed with the 2-d degree AV block. Besides, 2 (13.3%) women were diagnosed with dilated cardiomyopathy.
No statistically significant differences were observed between both groups regarding the rates of respiratory, gastrointestinal, urinary, and cardiovascular diseases.
Group 3 included 10 pregnant women with a normal fetal heart rate and a diagnosed autoimmune diseases including SLE (n=6), Sjögren’s syndrome (n=5), rheumatoid arthritis (n=3), systemic scleroderma (n=2) and ankylosing spondylitis (n=1). Somatic comorbidities included myopia (n=3), autoimmune thyroiditis, gallstone disease, duodenal ulcer, urolithiasis, pyelonephritis, thrombophlebitis, one case of each disease.
Women of these three groups did not significantly differ in parity, obstetric and gynecological history, and complications of the current pregnancy.
The study was approved by the Research Ethics Committee of the Center. Written informed consent was obtained from all patients enrolled in the study.
The participants were tested for serum antinuclear antibodies (ANA), IgG antibodies against SSA/ Ro, SSB/La, Sm, and double-stranded DNA using enzyme-linked immunosorbent assay (ELISA) kits (ORGENTEC Diagnostika, Germany). Analyses were carried out according to the manufacturer's instructions. The ANA level was evaluated by the calculated positivity index (PI); the result was considered positive at a PI level of more than 1.2.
Statistical analysis was performed using the Microsoft Office Excel 2010, Statistica for Windows (version 10) and MedCalc (version 12) software. The distribution of continuous variables was tested for normality using the Kolmogorov-Smirnov test and the Shapiro-Wilk W-test. Data with non-normal distribution were reported as the median (Me) with a 95% confidence interval (95% CI). The statistical significance of between-group differences for continuous variables was tested with the Mann– Whitney test; categorical variables were compared by the χ2 test. The relationship between the independent variables and the dependent binary variable was evaluated using logistic regression and ROC analysis (Receiver Operating Characteristics). Differences between the groups were considered statistically significant at p<0.05.
Results and discussion
Eight (53.3%) mothers with fetal bradyarrhythmia (5 with AV heart block and in 3 with sinus bradycardia) had elevated levels of autoantibodies.
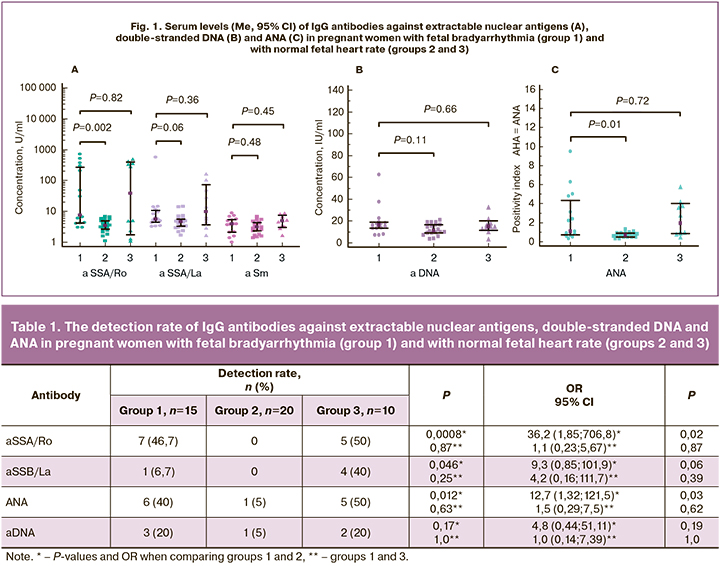
IgG antibodies against SSA/Ro and ANA were detected in 46.7% and 40% of women in group 1, which was significantly more often than in group 2 (Table 1). The risk of detecting IgG antibodies against SSA/Ro and ANA in pregnant women with fetal bradyarrhythmia was 36.2 and 12.7 times higher, respectively, than in group 2 among women with regular fetal heart rate. Moreover, in one pregnant woman with an increased level (30.1 U/ ml) of antibodies against SSA/Ro (normal range 0–25 U/ml), the ANA PI was 1.0 and corresponded to the normal range. This discrepancy in results may be due to the different sensitivity of tests for antibody detection. It is important to note that in the group with fetal bradyarrhythmia, antibodies to the SSA / Ro antigen were detected significantly more often than to SSB/La (p=0.015). No anti-Sm antibodies were found in any of the three groups.
It should be noted that the majority of women in group 1 (86.7%) were not diagnosed with the rheumatic disease at the time of registration for antenatal care. Four pregnant women with fetal AV block and one patient with fetal bradycardia, positive for antibodies to SSA / Ro (n=5), ANA (n=5) and antibodies to double-stranded DNA (n=2) were suspected of having the debut of Sjögren’s syndrome and/or SLE. These results are consistent with the data of a systematic review (2015), demonstrating the high prevalence of asymptomatic carriage of anti-SSA/Ro and anti-SSB/La antibodies in mothers of children with CHB [10]. These antibodies can be detected several years before the diagnosis of SLE or Sjögren’s syndrome, and autoimmune CHB may be one of the first indirect signs of rheumatic disease in women of childbearing age.
Two mothers with normal fetal heart rate in group 2 were found to have low titers of antibodies to DNA or ANA, which can be considered as a carriage of antibodies. The prevalence of this condition in healthy people without clinical manifestations of autoimmune diseases is 10-15%. In these cases, antibodies re-testing and follow-up are recommended for timely diagnosis of a possible autoimmune disorder. Besides, these antibodies can be detected in first-degree relatives of patients suffering from rheumatic diseases, in patients with thyroid autoimmune diseases, liver and infectious diseases, while taking some medications, including antiarrhythmic, hypotensive, antiepileptic drugs and antidepressants. However, the influence of these factors in seropositive patients in group 2 was excluded.
In group three, 70% of women with autoimmune diseases, including SLE (60%), Sjögren’s syndrome (50%), rheumatoid arthritis (30%), systemic scleroderma (20%) and ankylosing spondylitis (10%), were positive for IgG antibodies against SSA/Ro, SSB/La, double-stranded DNA, and ANA (Table 1). IgG antibodies to SSB/La were detected in patients with rheumatic diseases more often than in group 1 (p=0.046).
The median level of IgG antibodies to the SSA/Ro antigen and PI ANA were significantly higher in pregnant women with fetal bradyarrhythmia than in group 2 with normal fetal heart rate (Fig. 1). The levels of autoantibodies of other specificities in these groups did not significantly differ. There were no significant differences between groups 1 and 3 in the median levels of the antibodies.
At 26.3 (1.9) weeks of gestation, 6 (40%) pregnant women in group 1 were diagnosed with fetal second-degree or third-degree AV block. Five of them had high levels of anti-SSA/ Ro and ANA antibodies, and anti-SSB/La and anti-DNA antibodies were found in one patient each. Median levels of serum IgG antibodies against SSA/Ro and ANA in mothers with fetal AV block (332.9 [3.0; 721] and 4.9 [0.9; 9.5], respectively, were higher than in mothers with fetal sinus bradycardia (5.9 [3.0; 47.2] and 0.7 [0.3; 3.1]) (Fig. 2). The levels of antibodies against Ro/SSA in 3 newborns with CHB were equal to or were lower than the level of antibodies in their mothers. Mean levels of antibodies in children and mothers were 362.7 (104.5) U /ml and 496.7 (127.3) U/ml, respectively, and did not differ significantly (p = 0.23).
At the same time, in pregnant women with fetal sinus bradycardia, the median level of anti-SSA/Ro antibodies was higher than that in group 2 with normal fetal heart rate (3.5 [1.1; 6.8]; p = 0.024). Median ANA levels in these women did not significantly differ from that in group 2 (0.7 [0.3; 1.3]; p=0.68).
The results are consistent with data from other studies showing higher serum levels of anti-SSA/Ro and anti-SSB/La antibodies in mothers with autoimmune fetal CHB than in mothers of healthy children [10]. The titer of maternal anti-SSA/Ro antibodies is believed to be predictive in assessing the risk of developing autoimmune CHB [11]. According to a systematic literature review, the presence of maternal anti-La antibodies in the absence of anti-Ro antibodies is uncommon. It is associated with <1% of reported cases of autoimmune CHB. Therefore, these antibodies are not recommended as a biomarker for predicting autoimmune CHB [10].
Mothers with children suffering from CHB may have other autoantibodies including anti-DNA and anti-Sm antibodies in mothers with SLE, rheumatoid factor in mothers with rheumatoid arthritis or Sjögren’s syndrome, and anti-RNP antibodies in mothers with mixed connective tissue disease [10]. The feasibility of additional testing for these antibodies of mothers with fetal CHB, negative for anti-SSA/Ro and SSB/La antibodies is being considered.
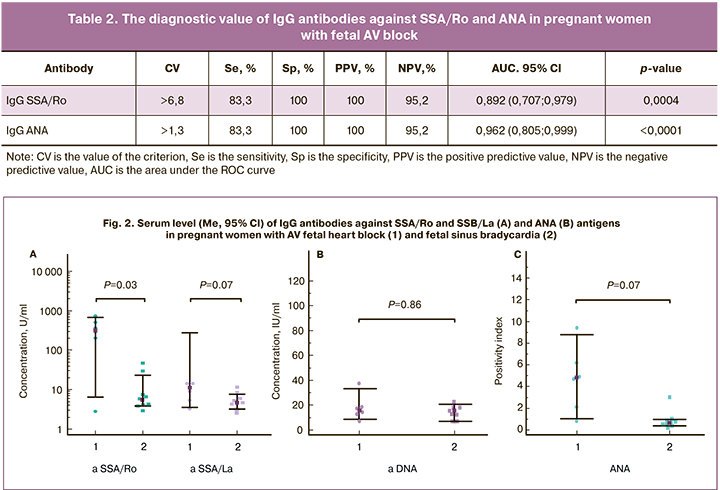
According to the ROC analysis, testing for IgG antibodies against SSA/Ro and ANA in pregnant women with fetal AV block was characterized by high sensitivity, specificity, the area under the ROC curve (AUC), and positive and negative predictive values (table 2); the accuracy of both tests was 96.2%.
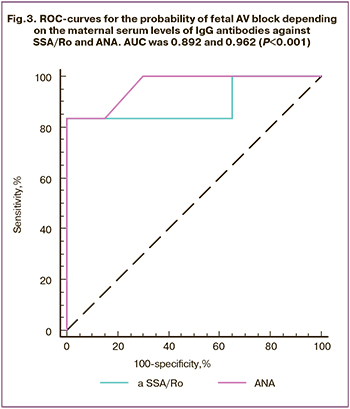 The constructed ROC curves made it feasible to classify mothers as having or not having fetal AV block based on the level of IgG antibodies against SSA/Ro and ANA (Fig. 3). In contrast, the area under the curves did not significantly differ (p=0.87). The predictive ability of the model as measured by AUC was very good (AUC> 0.8) when based on IgG antibodies to SSA/Ro as a predictor and excellent (AUC> 0.9) when based on ANA. According to logistic regression analysis, with a combination of IgG antibodies to SSA/Ro and ANA, the AUC increased to 0.967 (95% CI 0.811; 0.999) (p<0.0001), the accuracy of the test, in this case, was 96.2%.
The constructed ROC curves made it feasible to classify mothers as having or not having fetal AV block based on the level of IgG antibodies against SSA/Ro and ANA (Fig. 3). In contrast, the area under the curves did not significantly differ (p=0.87). The predictive ability of the model as measured by AUC was very good (AUC> 0.8) when based on IgG antibodies to SSA/Ro as a predictor and excellent (AUC> 0.9) when based on ANA. According to logistic regression analysis, with a combination of IgG antibodies to SSA/Ro and ANA, the AUC increased to 0.967 (95% CI 0.811; 0.999) (p<0.0001), the accuracy of the test, in this case, was 96.2%.
Anti-SSA/Ro antibodies also had high diagnostic accuracy for the diagnosis of fetal sinus bradycardia with AUC 0.767 (95% CI 0.573; 0.903; p=0.005), the sensitivity of 55.6%, the specificity of 85%, PPV of 62.5%, NPV of 80.9%, and test accuracy of 79, 3% The predictive ability of the model, in this case, was rated as good (AUC> 0.7).
Maternal anti-SSA/Ro autoantibodies were found to have the ability to recognize one or two of its isoforms (Ro 52 kD and Ro 60kD). The specific Ro52 epitope corresponding to amino acids 200-239 and designated p200 is considered as a serological biomarker of an increased risk of developing autoimmune CHB [4]. Tonello M. et al. (2016) reported that the women testing positive for anti-p200 +anti-Ro52+anti-Ro60 antibodies were more susceptible to CHB than those with other antibody profiles [12]. Identification of a high level of antibodies may be useful for selecting mothers with an increased risk of fetal CHB to monitor them with fetal heart echocardiography and choose appropriate prenatal care [13].
The pronounced damaging effect of anti-Ro/SSA antibodies to the fetal conduction tissues is associated with the massive trans-placental passage of maternal antibodies and the activity of cardiac organogenesis. There is increasing evidence suggesting that anti-Ro/ SSA antibodies may trigger rhythm disturbances through an inhibiting cross-reaction with several cardiac ionic channels, particularly the calcium channels (L-type and T-type), but also the potassium channel [14]. Different expression of these channels and their involvement in the cardiac electrophysiology during lifespan might account for the occurrence of age-related differences. Thus, fetal cardiomyocytes are characterized by low expression of L-type calcium channels. Therefore, anti-Ro/SSA antibodies may produce a significant reduction in the calcium influx through L-type channels and cause critical inhibition of the electrical activity of cardiomyocytes. Besides, fetal cardiomyocytes readily undergo apoptosis. Apoptosis was found to be exaggerated more than 30-fold in the septal tissue of CHB-affected fetal hearts.
Systemic corticosteroids were the first drugs proposed as the first-line therapy for rheumatic diseases, and are considered as a potential therapeutic agent for the treatment of CHB [15]. According to published data, dexamethasone and betamethasone contribute to a decrease in maternal serum autoantibody titers but do not directly affect the myocardium and the fetal heart conduction system. Fluorinated glucocorticoids monotherapy was not found to significantly improve pregnancy outcomes for infants but help reduce fetal pericardial or pleural effusions, ascites, and hydrops fetalis [16, 17]. At the same time, a combination of glucocorticoids and intravenous immunoglobulin for women with fetal AV block and concomitant cardiomyopathy and/or endocardial fibro-elastosis at 23 weeks of gestation, a positive result was obtained with 80% neonatal survival during prospective observation with an average follow-up of 2.9 years without the need for heart transplantation [18]. However, further studies are required to optimize the treatment regimen and confirm its effectiveness.
Recently, the use of hydroxychloroquine, which is known to inhibit Toll-like receptor signaling, has been proposed as a potential preventive therapy for autoimmune CHB [19]. Anti-SSA/Ro antibody-positive women with SLE receiving hydroxychloroquine throughout pregnancy were shown to have a decreased risk of having a child with cardiac manifestations of neonatal lupus. Further studies have confirmed that the use of hydroxychloroquine reduces the recurrence rate of CHB in subsequent high-risk pregnancies. [20] Data obtained by Martínez-Sanchez et al. (2017) on the lower prevalence of fetal CHB in mothers taking hydroxychloroquine, confirmed that this is a promising approach to the prevention of CHB [21].
Currently, there exists no standard guide for the treatment of autoimmune CHB. The use of efferent therapy methods was shown to be effective in removing anti-SSA/Ro and SSB/La antibodies associated with the pathogenesis of CHB [22]. Combination therapy, includingplasmapheresis, intravenousimmunoglobulins, and betamethasone, was found to be effective and safe in patients with CHB [23].
Management strategy for autoimmune-mediated fetal bradyarrhythmia included the use of plasmapheresis, systemic corticosteroid pulse therapy, corticosteroids (Metipred), and hydroxychloroquine (Plaquenil) as the first-line therapy. The optimal number of courses of plasmapheresis and pulse therapy with dexamethasone was determined on a case-by-case basis and depended on the degree of fetal AV block and the dynamics of the autoantibody titers during specific treatment. Four pregnant women required a second course of plasmapheresis due to the progression of fetal bradyarrhythmia and increasing titers of anti-SSA/ Ro and SSB/La antibodies. In two cases, despite high titers of ANA and anti-SSA/Ro antibodies, the use of plasmapheresis and specific immunosuppressive therapy resulted in the regression of AV block and restoration of sinus rhythm. In other cases, there was no progression of fetal heart rhythm disturbances and the development of non-immune hydrops fetalis, which helped prolong pregnancy to full term.
A significant contribution to understanding the issue of prevention of autoimmune-mediated fetal bradyarrhythmia was made by the results of an immunological examination of pregnant women with rheumatic diseases in group 3. Since these women underwent pregravid care, in most cases, their pregnancy occurred against the background of persistent remission of an autoimmune disease. From an early stage, they were closely monitored by a rheumatologist and obstetrician-gynecologist. They received the necessary immunosuppressive therapy in the form of monotherapy or a combination of systemic glucocorticoids and hydroxychloroquine, starting from the pregravid state, throughout the gestation, which probably which helped to avoid irreversible damage to the fetal heart conductive system.
Conclusion
Mothers with fetal bradyarrhythmia have a higher detection rate and titers of IgG autoantibodies against SSA/Ro and ANA than in pregnant women with normal fetal heart rate. These antibodies are an independent risk factor for fetal bradyarrhythmia and have a high diagnostic value for fetal AV block. In fetal bradyarrhythmia, maternal anti-SSA/Ro antibodies are detected significantly more often than anti-SSB/La antibodies. Specific treatment of rheumatic disease in mothers results in the conversion of fetal bradycardia to the normal fetal heart rhythm. Testing for anti-SSA/ Ro autoantibodies in asymptomatic carriers with fetal bradyarrhythmia allows identification of women at risk for developing autoimmune CHB, and preventive therapy can improve pregnancy outcomes for infants. Studies in this direction are now ongoing.
References
- Ling M., Murali M. Antinuclear antibody tests. Clin. Lab. Med. 2019; 39(4): 513-24. https://dx.doi.org/10.1016/j.cll.2019.07.001.
- Vasquez-Canizares N., Wahezi D., Putterman C. Diagnostic and prognostic tests in systemic lupus erythematosus. Best Pract. Res. Clin. Rheumatol. 2017; 31(3): 351-63. https://dx.doi.org/10.1016/j.berh.2017.10.002.
- Кошелева Н.М., Алекберова З.С. Неонатальная волчанка. Современная ревматология. 2015; 4: 92-8. [Kosheleva N.M., Alekberova Z.S. Neonatal lupus. Modern rheumatology. 2015; 4: 92-8. (in Russian)].
- Ambrosi A., Wahren-Herlenius M. Congenital heart block: evidence for a pathogenic role of maternal autoantibodies. Arthritis Res. Ther. 2012; 14(2): 208. https://dx.doi.org/10.1186/ar3787.
- Wainwright B., Bhan R., Trad C., Cohen R., Saxena A., Buyon J., Izmirl P. Autoimmune-mediated congenital heart block. Best Pract. Res. Clin. Obstet. Gynaecol. 2020; 64: 41-51. https://dx.doi.org/10.1016/j.bpobgyn.2019.09.001.
- Бокерия Е.Л. Перинатальная кардиология: настоящее и будущее. Часть II: нарушение ритма сердца и проводимости. Российский вестник перинатологии и педиатрии. 2019; 64(4): 6-10. [Bokeria E.L. Perinatal cardiology: present and future. Part II: heart rhythm and conduction disorders. Rus. Vestn. perinatol. and pediatr. 2019; 64 (4): 6-10. (in Russian)].
- Zhou K.Y., Hua Y.M. Autoimmune-associated congenital heart block: a new insight in fetal life. Chin. Med. J. 2017; 130(23): 2863-71. https://dx.doi.org/10.4103/0366-6999.219160.
- Сафонова И.Н. Фетальные аритмии: антенальная ультразвуковая дифференциальная диагностика, прогнозирование постнатальных результатов и перинатальная практика. SonoAceUltrasound. 2014; 26: 17-29. [Safonova I.N. Fetal arrhythmias: antenal ultrasound differential diagnosis, prediction of postnatal results and perinatal practice. SonoAceUltrasound. 2014; 26: 17-29. (in Russian)].
- Беспалова Е.Д., Суратова О.Г., Бокерия Е.Л., Бартагова М.Н., Гасанова Р.М., Тюменева А.И. Диагностика и лечение кардиальной патологии у плода. Бокерия Л.А., ред. М.: Издательство НЦССХ им. А.Н. Бакулева; 2015. 244 с. [Bespalova E.D., Suratova O.G., Bokeria E.L., Bartagova M.N., Gasanova R.M., Tyumeneva A.I. Diagnosis and treatment of cardiac abnormalities in the fetus. Edited by Bokeria L.A. Moscow: Publishing house of the Bakulev CVSRC; 2015. 244 p. (in Russian)].
- Brito-Zerón P., Izmirly P.M., Ramos-Casals M., Buyon J.P., Khamashta M.A. The clinical spectrum of autoimmune congenital heart block. Nat. Rev. Rheumatol. 2015; 11(5): 301-12. https://dx.doi.org/10.1038/nrrheum.2015.29.
- Jaeggi E., Laskin C., Hamilton R., Kingdom J., Silverman E. The importance of the level of maternal anti-Ro/SSA antibodies as a prognostic marker of the development of cardiac neonatal lupus erythematosus a prospective study of 186 antibody-exposed fetuses and infants. J. Am. Coll. Cardiol. 2010; 55(24): 2778-84. https://dx.doi.org/10.1016/j.jacc.2010.02.042.
- Tonello M., Ruffatti A., Favaro M., Tison T., del Ross T., Calligaro A. et al. Maternal autoantibody profiles at risk for autoimmune congenital heart block: a prospective study in high-risk patients. Lupus Sci. Med. 2016; 3(1): e000129. https://dx.doi.org/10.1136/lupus-2015-000129.
- Kan N., Silverman E.D., Kingdom J., Dutil N., Laskin C., Jaeggi E. Serial echocardiography for immune-mediated heart disease in the fetus: results of a risk-based prospective surveillance strategy. Prenat. Diagn. 2017; 37(4): 375-82. https://dx.doi.org/10.1002/pd.5021.
- Lazzerini P.E., Capecchi P.L., Laghi-Pasini F. Anti-Ro/SSA antibodies and cardiac arrhythmias in the adult: facts and hypotheses. Scand. J. Immunol. 2010; 72(3): 213-22. https://dx.doi.org/10.1111/j.1365-3083.2010.02428.x.
- Brucato A., Tincani A., Fredi M., Breda S., Ramoni V., Morel N. et al. Should we treat congenital heart block with fluorinated corticosteroids? Autoimmun. Rev. 2017; 16(11): 1115-8. https://dx.doi.org/10.1016/j.autrev.2017.09.005.
- Van den Berg N.W., Slieker M.G., van Beynum I.M., Bilardo C.M., de Bruijn D., Clur S.A. et al. Fluorinated steroids do not improve outcome of isolated atrioventricular block. Int. J. Cardiol. 2016; 225: 167-71. https://dx.doi.org/10.1016/j.ijcard.2016.09.119.
- Izmirly P.M., Saxena A., Sahl S.K., Shah U., Friedman D.M., Kim M.Y. et al. Assessment of fluorinated steroids to avert progression and mortality in anti-SSA/Ro-associated cardiac injury limited to the fetal conduction system. Ann. Rheum. Dis. 2016; 75(6): 1161-5. https://dx.doi.org/10.1136/annrheumdis-2015-208311.
- Trucco S.M., Jaeggi E., Cuneo B., Moon-Grady A.J., Silverman E., Silverman N. et al. Use of intravenous gamma globulin and corticosteroids in the treatment of maternal autoantibody-mediated cardiomyopathy. J. Am. Coll. Cardiol. 2011; 57(6): 715-23. https://dx.doi.org/10.1016/j.jacc.2010.09.044.
- Izmirly P.M., Kim M.Y., Llanos C., Le P.U., Guerra M.M., Askanase A.D. et al. Evaluation of the risk of anti-SSA/Ro-SSB/La antibody-associated cardiac manifestations of neonatal lupus in fetuses of mothers with systemic lupus erythematosus exposed to hydroxychloroquine. Ann. Rheum. Dis. 2010; 69(10): 1827-30. https://dx.doi.org/10.1136/ard.2009.119263.
- Izmirly P.M., Costedoat-Chalumeau N., Pisoni C.N., Khamashta M.A., Kim M.Y., Saxena A. et al. Maternal use of hydroxychloroquine is associated with a reduced risk of recurrent anti-SSA/Ro-antibody-associated cardiac manifestations of neonatal lupus. Circulation. 2012; 126(1): 76-82.https://dx.doi.org/10.1161/CIRCULATIONAHA.111.089268.
- Martínez-Sánchez N., Pérez-Pinto S., Robles-Marhuenda Á., Arnalich-Fernández F., Martín Cameán M., Hueso Zalvide E., Bartha J.L. Obstetric and perinatal outcome in anti-Ro/SSA-positive pregnant women: a prospective cohort study. Immunol. Res. 2017; 65(2): 487-94. https://dx.doi.org/10.1007/s12026-016-8888-5.
- Tonello M., Ruffatti A., Marson P., Tison T., Marozio L., Hoxha A. et al. Plasma exchange effectively removes 52- and 60-kDa anti-Ro/SSA and anti-La/SSB antibodies in pregnant women with congenital heart block. Transfusion. 2015; 55(7): 1782-6. https://dx.doi.org/ 10.1111/trf.13046.
- Ruffatti A., Cerutti A., Favaro M., Del Ross T., Calligaro A., Hoxha A. et al. Plasmapheresis, intravenous immunoglobulins and bethametasone - a combined protocol to treat autoimmune congenital heart block: a prospective cohort study. Clin. Exp. Rheumatol. 2016; 34(4): 706-13.
Received 29.05.2020
Accepted 11.06.2020
About the Authors
Irina V. Menzhinskaya, Dr.Med.Sci., Leading Researcher at the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.Tel.: +7(495)438-11-83. E-mail: i_menzinskaya@oparina4.ru. 4, Oparina str., Moscow, 117997, Russian Federation.
Zulfiya S. Khodzhaeva, Dr.Med.Sci.,, Professor, Deputy Director for Research of the Institute of Obstetrics, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Tel.: +7(495)438-07-88. Email: z_khodzhaeva@oparina4.ru. 4, Oparina str., Moscow, 117997, Russian Federation.
Ekaterina L. Bokeria, Dr.Med.Sci., Professor, Head of the 2nd Department of Pathology of Newborns and Premature Babies, Adviser to the Director, V.I. Kulakov NMRC
for OG&P of Minzdrav of Russia. Tel.: +7(495)438-27-05. E-mail: e_bokeriya@oparina4.ru. 4, Oparina str., Moscow, 117997, Russian Federation.
Natalia I. Klimenchenko, Ph. D., Senior Researcher at the Department of Obstetric and Extragenital Pathology, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Tel.: +7(495)438-06-74. E-mail: n_klimenchenko@oparina4.ru. 4, Oparina str., Moscow, 117997, Russian Federation.
Alena A. Potapova, Ph.D. Student at the V.I. Kulakov NMRC for OG&P of Minzdrav of Russia. Tel.: +7(495)438-07-88. E-mail: doc.PotapovaAA@yandex.ru.
4, Oparina str., Moscow, 117997, Russian Federation.
Ludmila V. Van’ko, Dr.Med.Sci., Professor, Leading Researcher at the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Tel.: +7(495)438-11-83. E-mail: LVanko@oparina4.ru. 4, Oparina str., Moscow, 117997, Russian Federation.
Lubov V. Krechetova, Dr.Med.Sci., Head of the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Tel.: +7(495)438-11-83. E-mail: l_krechetova@oparina4.ru. 4, Oparina str., Moscow, 117997, Russian Federation.
Nadezhda M. Kosheleva, Ph.D., Senior Researcher at the Laboratory of Systemic Rheumatic Diseases with a Group of Hemorheological Disorders, V.A. Nasonova Research Institute of Rheumatology. Tel.: +7(499)614-39-65. E-mail: nadkosheleva@yandex.ru. 34A, Kashirskoe shosse, Moscow, 115522, Russian Federation.
For citation: Menzhinskaya I.V., Khodzhaeva Z.S., Bokeria E.L., Klimenchenko N.I., Potapova A.A., Van’ko L.V., Krechetova L.V., Kosheleva N.M. The clinical diagnostic value of maternal autoantibodies against extractable nuclear antigens in fetal bradyarrhythmia.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2020; 7: 53-60 (in Russian)
https://dx.doi.org/10.18565/aig.2020.7.53-60



