Investigation of the efficacy of indole-3-carbinol on a rat model of endometriosis (experimental study)
Objective. To investigate the pharmacological activity of a drug with the active substance indole-3-carbinol on a rat model of endometriosis.Kiselev V.I., Ashrafyan L.A., Pronin S.M., Gerfanova E.V., Kuznetsov I.N., Drukh V.M., Udut V.V., Churin A.A., Pchelintseva O.I.
Materials and methods. An experiment was carried out on on pubertal female rats (n = 60). According to pathology modeling and therapy, the animals were divided into groups. Group 1 consisted of falsely operated animals receiving starch solution. Group 2 included falsely operated ones given indole-3-carbinol at a dose of 111 mg/kg. In Groups 3 to 5, endometriosis was modelled by autologous transplantation of endometrial tissue into the peritoneal cavity. Group 3 animals received 1% starch solution; Groups 4 and 5 had the active substance at doses of 37 and 111 mg/kg, respectively. Reactions to pain were studied in the animals and implants were also histologically assessed at 30 and 60 days.
Results. The untreated animals with induced pathology showed a more pronounced pain response than falsely operated animals. Histological estimation revealed that endometrial hyperplasia tended to reduce in the groups of females that had received the active substance compared with that in the control groups.
Conclusion. The investigation showed that indole-3-carbinol administered to the animals effectively decreased the size of an endometrioid focus and the degree of endometrioid heterotopies, thereby indicating the therapeutic effect on endometriosis-induced pain syndrome.
Keywords
Endometriosis is one of the most relevant problems in modern medicine. High prevalence of endometriosis and its increased incidence (up to 50%) in reproductiveaged women have been the subject of interest for doctors searching for new treatment approaches for women with endometriosis [1]. The underlying causes of endometriosis are numerous fundamental biological mechanisms; however, this issue remains understudied [2]. Inflammation, the changes in the hormonal status and hormonal receptors, genetic and epigenetic factors are prerequisites for the development of endometriosis [3–6]. The multifactorial nature of endometriosis complicates the search for effective therapies [2].
The processes of reducing apoptosis, proliferation, invasion and neoangiogenesis play a key role in the development of endometriosis. The course of the disease and clinical manifestations directly depend on the ongoing molecular processes [7].
Taking into consideration the proliferative and invasive processes which occur in endometrioid lesions, particular attention should be paid to the study of pharmacological compounds of non-hormonal origin that affect several pathogenetic links of endometriosis: normalization of the estrogen balance, pathological cell proliferation, inhibition of neoangiogenesis, as well as the ability to stimulate selective cell apoptosis [8]. One of such therapeutic agents is indole-3-carbinol.
Indole-3-carbinol (I3C) is a well-known plantderived molecule that has a wide range of biological properties against actively proliferating mammalian cells. Of particular interest is the antitumor activity of I3C which was studied in detail on various experimental models. As a result, the molecule was included in the list of compounds for drug prevention of malignant neoplasms of various localization [9].
Thus, there is high effectiveness of I3C therapy in combination with Tamoxifen in breast cancer, regardless of the estrogen status of the disease. The synergistic effect of therapy is achieved due to the impact on various signaling pathways [10]. The remarkable therapeutic potential of I3C results from its multifactorial action against proliferating cells. In particular, I3C inhibits the main signaling pathways that support aggressive cell division [11], induces apoptosis of cells under metabolic stress, inhibits pathological angiogenesis [12, 13], and has epigenetic activity by modulating the expression of histone deacetylases, DNA-methyltransferases, and microRNA synthesis in target cells [14–16]. On a systemic level, I3C regulates the metabolism of estrogens by restoring the physiological ratio of 16-alpha and 2-hydroxymetabolites, thus normalizing the cell division in hormone-sensitive tissues of the female reproductive system [9].
It should be noted that I3C is prone to oligomerization and quickly turns into diindolylmethane (DIM) under the influence of acidic environment of the stomach. Some authors ascribe therapeutic effects of I3C to DIM as a product of its bioconversion [17].
It is also noteworthy that DIM has proved to be able to inhibit the activity of hypoxia-inducible factor (HIF)1a, which, along with vascular endothelial growth factor (VEGF), is a key molecular target involved in the pathological growth of new vessels. It has been shown that in vitro in tumor cells under hypoxia, the level of HIF-1a, as well as its transcriptional activity, decreases in the presence of DIM. Moreover, DIM suppresses the expression of HIF-1a-responsive endogenous genes, which leads to inhibition of the expression of key hypoxia-responsive factors, such as VEGF, enolase-1, glucose transporter-1, phosphofructokinase, etc. A decrease in the level of HIF-1a in hypoxic tumor cells is accompanied by an increase in the rate of its enzymatic and proteosomal degradation, as well as a decrease in the rate of gene transcription [18].
All these mechanisms of action of I3C and DIM are highly likely to be implemented in the treatment of endometriosis, based on current understanding of its pathogenesis [19]. Using I3C as the basis, a drug for the treatment of hyperplastic processes in the mammary gland has been developed and registered [20]. Due to the above factors, the study was intended to evaluate the therapeutic effect of I3C on an experimental model of endometriosis.
The purpose of our research was to study the pharmacological activity of the drug with the active substance indolcarbinol on a model of endometriosis in rats.
This study is aimed at studying the possibility of using the drug for the treatment of endometriosis, as well as pain caused by this pathology.
Experimental drugs
The preparation produced by JSC “MiraxBioPharma” was used as an experimental drug; it contains the substance indolcarbinol (indol-3-carbinol) of 200 mg per capsule, the control drug is 1% starch solution.
Animal handling
For the experiment, 60 female outbred rats were obtained from the nursery of R&D “Home of Pharmacy”, Saint Petersburg. The animals were kept in standard conditions in accordance with the rules of proper laboratory practice, in standard transparent plastic cages, in groups of 6 species of the same gender, with free access to food and water.
The animals were kept under controlled environmental conditions (19–25°C and relative humidity of 30–70%). The light mode was 12 hours of light and 12 hours of darkness. The air exchange mode was installed, providing a change of about 15 room volumes per hour.
Ethical support
The study was reviewed by the bioethical Commission of JSC R&D “Home of Pharmacy” and approved for conducting No. BEK 4.61/17, dated 06.10.2017.
Materials and Methods
Before the study, the animals were randomly divided into 5 groups with 12 species in each group. Animals of Groups 1 and 2 underwent a false operation, during which they were performed an incision of the abdominal wall, exposure of the uterine horn (for 2 minutes), layerby-layer wound closure, and treatment of the suture with an antiseptic. Animals in Group 1 received a 1% starch solution, rats in Group 2 received an active drug at a dosage of 111 mg/kg per day. Animals in Groups 3, 4, and 5 underwent surgery to simulate endometriosis by autologous endometrial tissue transplantation into the abdominal cavity. Animals in Group 3 received a 1% starch solution, rats in Group 4 received an active drug at a dosage of 37 mg/kg per day, animals in Group 5 received an active drug at a dosage of 111 mg/kg per day. Therapy began after the emergence of animals from anesthesia. In the postoperative period, animals were intramuscularly administered Ketonal for pain relief at a dose of 5 mg/kg once a day, for 5 days [21].
The course of therapy was 30 days for 50% of animals, and 60 days for the other 50% of animals. The drug or control substance was administered daily, once a day, intragastrically.
Pain sensitivity was assessed 2, 4, and 8 weeks after the initiation of the therapy by measuring the pressure (in grams) required to elicit a pain response (withdrawal/ avoidance) when the sensor was applied to the abdominal wall. Threshold measurement on the abdominal wall was carried out using Electronic von Frey anesthesiometer (IITC Inc., Life Science Instruments, Woodland Hills, CA, USA). Five repeated measurements were made at 5-second intervals. When analyzing the data, the average for 5 measurements was calculated.
On the 30th and 60th day of therapy, animals were euthanized using a CO2 chamber without taking into account the estrous cycle. Euthanized animals were performed autopsy followed by morphometric assessment of the implant size: length, width, height and area of the endometriosis focus. Isolated implants with adjacent tissue and intact uterine horn were fixed in 10% formalin for subsequent histological examination.
Histological signs of endometriosis were evaluated using the following criteria of semiquantitative score assessment:
- cyst severity: 0 – no cyst; 1 – minor cyst; 2 – moderate cyst; 3 – large cyst;
- inflammatory infiltration: 0 – absent; 1 – mild; 2 – moderate; 3 – severe;
- vascularization: 0 – absent; 1 – mild; 2 – moderate; 3 – severe;
- presence of glandular structures: 0 – absence; 1 – a small number of glands; 2 – a moderate number of glands; 3 – a significant number of glands.
- type of epithelium: 0 – no epithelium; 1 – poorly preserved epithelium; small, flattened cells; 2 – mildly preserved epithelium, dominated by cubic cells; 3 – well-preserved epithelium layer; large, prismatic cells;
- hyperplasia of the endometrium/inner wall of the endometrioid focus: 0 – absence; 1 – mild; 2 – moderate; 3 – severe.
Statistical analysis
The obtained data were analyzed using the Statistica 10.0 software (StatSoft, USA). Nonparametric criteria were used for evaluating indicators.
Morphometric characteristics of endometriosis are presented in the study in the form of arithmetic mean (M) and standard deviation (SD). The data are quantitative, the distribution is non-normal. Paired comparison of groups was performed using the Mann-Whitney criterion (significance level p < 0.05).
Indicators of histological assessment were analyzed using rank analysis of variations in Kruskal–Wallis test. Data are presented as a median (Me) and quartiles Q1 and Q3 in the format Me (Q1;Q3). Statistically significant differences between the groups were evaluated by posteriori comparison procedures using the Mann-Whitney test (significance level p < 0.05).
Results
The death of animals in study groups was not registered during the study.
The results obtained during the evaluation of visceral pain sensitivity showed that in the group with induced pathology, the pain response to mechanical stimulation was significantly higher than in the falsely operated animals: the rats responded to less pressure. After 4 weeks of therapy, there was a decrease in the severity of the pain response, the effect was observed in both groups receiving the drug, but statistical significance was noted only in the group receiving the drug at a maximum dose of 111 mg/kg (p < 0.05). The analysis of the data obtained after 8 weeks of therapy showed similar results with the only difference that a statistically significant analgesic effect was observed already after giving the minimum dose of 37 mg/kg.
Thus, during the whole experiment a more pronounced pain response was observed in the group of animals with induced pathology that did not receive therapy compared to the falsely operated animals. After two weeks of therapy with the experimental drug, animals receiving the preparation at the maximum dose did not show any difference in pain sensitivity in comparison with the animals of the falsely operated groups. After 4 and 8 weeks of therapy, the analgesic effect was observed in all groups of animals with induced pathology that received the active drug.
Pathomorphological data
An intact uterine horn and an area of the peritoneum with an implant (a focus of endometriosis) were subjected to pathoanatomical and histological examination. The pathoanatomical study included a macroscopic assessment of endometriosis with morphometry.
In a histological study, the endometrial condition of the intact uterine horn and the degree of endometriosis were evaluated using semiquantitative assessment in points, from 0 to 3: where 0 – no changes; 1 – minor changes; 2 – moderate changes; 3 – severe changes.
Assessment of the endometrial condition of the intact uterine horn in experimental animals
Pathoanatomical examination of the uteri of falsely operated rats did not reveal any pathological changes. In rats receiving the experimental drug at a dose of 111 mg/ kg, a macroscopic study showed a decrease in the thickness of the uterine horns and the degree of vascular congestion of the organ, which was especially noticeable on the 60th day of the experiment.
The histological structure of the endometrium of the intact uterine horn in animals with the induced pathology also corresponded to the normal indicators. The endometrium is formed by a single-layer prismatic ciliated epithelium consisting of secretory and ciliated cells and its own plate formed by loose connective tissue containing numerous uterine glands. On the 30th day after the administration of the experimental drug, the group of falsely operated animals treated with the active drug at the dose of 111 mg/kg (Group 2) showed a significant decrease in the degree of endometrial proliferation, which was manifested by the reduction in the number of glandular structures, decrease in height of the epithelium and the degree of hyperplasia of the endometrium (Figure 1) compared to falsely operated animals treated with control substance (Group 1) (Figure 2). On the 60th day of the experiment, the same trend continued, but the signs of decreased endometrial proliferation in falsely operated animals receiving the active drug at a dose of 111 mg/kg were less pronounced.
Assessment of the development of endometrioid heterotopias
Macroscopic study of female rats in experimental groups with the induced pathology showed that most animals had an active growth of heterotopia and the formation of cysts of different sizes at the site of implants. The size of the cysts varied within 2–4×2– 6×2–10 mm, exceeding the initial size of the implants. A dense vascular network was clearly visualized.
Morphometric characteristics of endometriosis foci are shown in Table 1.
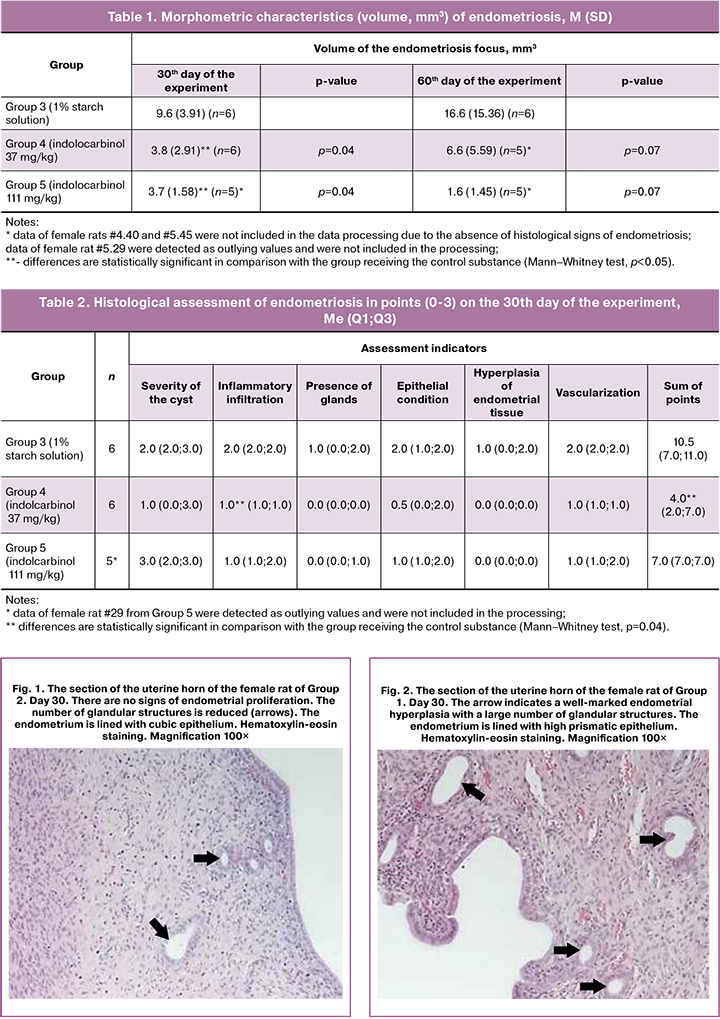
The animals receiving the active drug demonstrated a statistically significant decrease in the volume of endometriosis on the 30th day of the experiment (p < 0.05). On the 60th day, there was also a tendency to decrease the volume of the endometriosis focus in the groups receiving the experimental drug, but it did not reach statistical significance (p = 0.07). In general, the results of morphometry indicate the presence of a therapeutic effect of the drug in terms of reducing the volume of the endometrioid focus both 30 days and 60 days after the initiation of the therapy.
Histologically, the foci of endometriosis in most cases were characterized by a cystic structure. Mononuclear infiltration with a predominance of lymphocytes, macrophages and histiocytic cells was observed with varying degrees of severity. The wall of the cysts was formed by connective tissue elements with a small number of poorly differentiated myofibroblast-like cells. In some cases, hyperplasia of the internal wall of the endometrioid focus was detected. The contents of cysts were represented by an unstructured weakly basophilic component with a small number of mononuclear cells and desquamated epithelial cells. In the cyst wall, a few glandular structures reminding tubular glands of the endometrium were also detected. The inner wall of the endometrioid focus was lined with a single-layer epithelium of various degrees of preservation and differentiation. In female rats receiving the control substance, prismatic epithelium prevailed which is similar in structure to the eutopic endometrial epithelium of the intact uterine horn (in Fig. 3, 4). In animals receiving the active drug at doses of 37 mg/kg and 111 mg/kg, there was a noticeable decrease in the height of the epithelium, in most cases the cysts were lined with poorly preserved flattened epithelium, hyperplasia of the cyst wall was almost unobservable, and there was a significant decrease in the volume and degree of vascularization of the endometrioid focus (Fig. 5–8).
In some cases, there were signs of complete resorption of endometriosis with the detection of small quantities of poorly differentiated cells, as well as elements of loose connective and granulation tissue at the site of attachment of the implant, which was more often observed in animals receiving indolcarbinol at doses of 37 mg/kg and 111 mg/kg on the 60th day of the experiment.
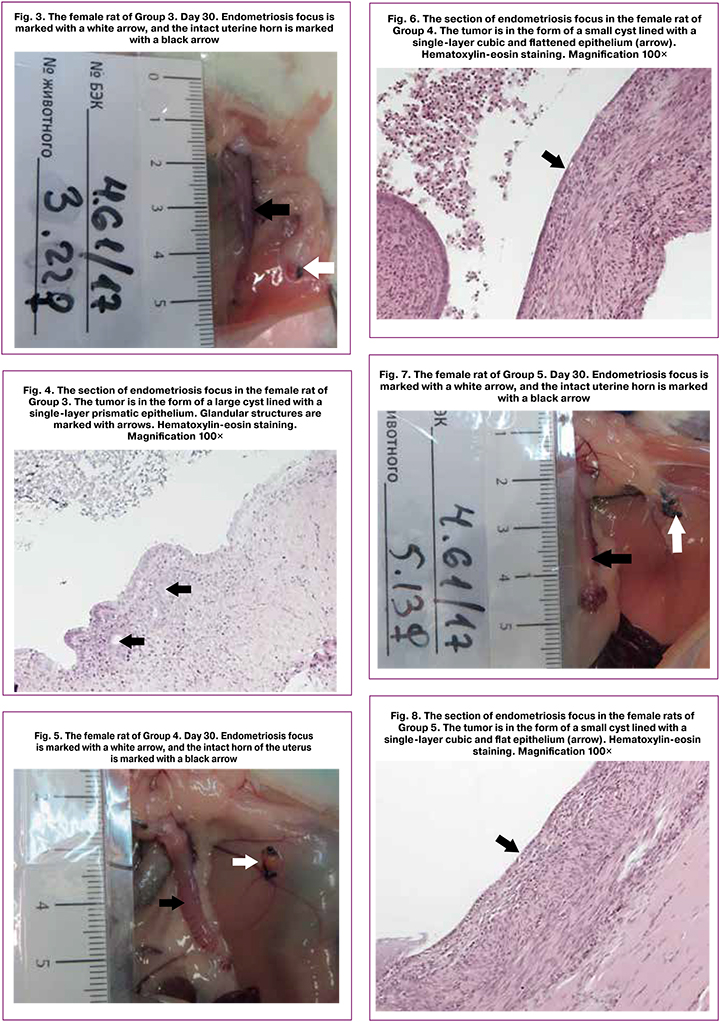
Data on the histological assessment of endometrioid heterotopias are presented in points (0–3) and shown in Table 2. The development of pathology and effectiveness of the treatment were evaluated in points on the basis of the state of the endometrial epithelium in implants according to a modified Keenan scale [22]:
0 points – no epithelial cells;
1 point – single epithelial cells;
2 points – moderately expressed epithelial layer;
3 points – well-marked epithelial layer.
When comparing the groups, a statistically significant decrease in the points for these indicators was found in the group of female rats receiving indolcarbinol at a dose of 37 mg/kg, in relation to the group of animals receiving the control substance (p<0.05, see Table 2).
On the 60th day of the experiment, a statistically significant decrease in points for the assessed parameters was found in the group of animals receiving indolcarbinol at a dose of 111 mg/kg, in relation to the group receiving the control substance.
Thus, according to the results of histological evaluation of the intact uterine horn, there was a tendency to reduce the degree of endometrial hyperplasia in the groups of female rats who received the active drug, compared to the control groups.
The results of morphometry of endometrioid foci show therapeutic effect of indolcarbinol when administered at doses of 37 mg/kg and 111 mg/kg: a decrease in the volume of the endometrioid focus was observed both 30 days and 60 days after the initiation of therapy. The therapeutic effectiveness of the drug is also confirmed by the results of histological evaluation of endometrioid heterotopias: the sum of points for the assessed indicators was lower in the groups receiving therapy.
Discussion
Endometriosis is a multifactorial disease. Nowadays, its conservative treatment is based on long-term hormonal therapy, which prevents its further development by reducing the effect of estrogen. Combined synthetic estrogen-gestagen drugs, progestins (drugs without an estrogen component), antigestagens, antigonadotropins, gonadotropin-releasing hormone agonists, antiestrogens, aromatase inhibitors are used for this purpose [23].
Since estrogens are key factors in maintaining endometrial homeostasis, any impairment of synthesis and metabolism of estrogens leads to the development of pathological changes in the endometrium. Changes in the functional activity of estrogen receptors (ER)β are considered to be an important link in the pathogenesis of endometriosis. It has been shown that there is an increase in the expression of ERß in endometriosis, regardless of the location of heterotopia (ovaries or abdominal cavity) [24]. Therefore, one of the possible therapeutic actions of the drug may be its antiestrogen effect [25].
In addition to antiestrogenic effect, indolcarbinole is known to have antiproliferative effects. The effectiveness of I3C (the active substance of the drug) has been shown for the prevention of breast cancer, uterine corpus cancer and cervical cancer [26, 27]. The development of endometriosis foci is associated with the functioning of a system of insulin-like growth factors that induce cell proliferation [28, 29]. There is a high expression of insulin-like growth factors (IGF1 and IGF2) in foci of endometriosis of any localization. Using the cultures of various cancer cell lines, it was found that I3C reduces proliferative activity by suppressing various links of intracellular intermediary, including IGF [30–32]. Antiproliferative effect of I3C can also be one of the leading factors determining the effectiveness of the drug, which was revealed in an endometriosis model in rats. However, identification of the exact mechanisms that mediate the detected therapeutic activity of the drug requires a further study.
Thus, the study showed that indolcarbinol when administered to animals in two doses (37 mg/kg and 111mg/kg) effectively reduced the size of the focus of endometriosis, as well as the severity of endometrioid heterotopias. Due to the reduction of pathological tissue in animals, a decrease in the endometriosis-induced pain syndrome was also observed.
The authors express their gratitude to the staff of the JSC R&D “Home of Pharmacy” for their assistance in conducting the research.
References
- Маржевская В.В., Присяжная Т.С., Жамойдик В.И., Берлев И.В., Малек А.В. Молекулярно-генетические основы эндометриоза: диагностический потенциал наследуемых и экспрессируемых факторов. Журнал акушерства и женских болезней. 2018; 67(3): 64-73. [Marzhevskaya V.V., Prisyazhnaya T.S., Zhamoidik V.I., Berlev I.V., Malek A.V. Molecular genetic bases of endometriosis: the diagnostic potential of inheritable and expressed factors. Zhurnal Akusherstva i Zhenskikh Bolezney (Journal of Obstetrics and Women’s Diseases). 2018; 67 (3): 64-73. (in Russian)].
- Адамян Л.В., ред. Клинические рекомендации по ведению больных. Эндометриоз: диагностика, лечение и реабилитация. М.; 2013. [Adamyan L.V. (ed.). Clinical practice guidelines for the management of patients. Endometriosis: diagnosis, treatment, and rehabilitation. Moscow; 2013. (in Russian)].
- Zhou W.J., Yang H.L., Shao J., Mei J., Chang K.K., Zhu R., Li M.Q. Anti-inflammatory cytokines in endometriosis. Cell. Mol. Life Sci. 2019; 76(11): 2111-32. https://dx.doi.org/10.1007/s00018-019-03056-x.
- Bulun S.E., Monsivais D., Kakinuma T., Furukawa Y., Bernardi L., Pavone M.E.,Dyson M. Molecular biology of endometriosis: from aromatase to genomic abnormalities. Semin. Reprod. Med. 2015; 33(3): 220-4. https://dx.doi.org/10.1055/s-0035-1554053.
- Donnez J. Introduction: From pathogenesis to therapy, deep endometriosis remains a source of controversy. Fertil. Steril. 2017; 108(6): 869-71. https://dx.doi.org/10.1016/j.fertnstert.2017.10.015.
- Киселев В.И., Пальцев М.А. Регуляция активности генов и новые лекарственные средства. Вестник Российской академии наук. 2016; 86(6): 512-8. [Kiselev V.I., Paltsev M.A. Gene activity regulation and new drugs. Vestnik Rossiyskoi Akademii Nauk (Bulletin of the Russian Academy of Sciences). 2016; 86 (6): 512-8. (in Russian)].
- Clemenza S., Sorbi F., Noci I., Capezzuoli T., Turrini I., Carriero C. et al. From pathogenesis to clinical practice: Emerging medical treatments for endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2018; 51: 92-101. https://dx.doi.org/10.1016/j.bpobgyn.2018.01.021.
- Aggarwal B.B., Ichikawa H. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle. 2005; 4(9): 1201-15. https://dx.doi.org/10.4161/cc.4.9.1993.
- Fujioka N., Fritz V., Upadhyaya P., Kassie F., Hecht S.S. Research on cruciferous vegetables, indole-3-carbinol, and cancer prevention: A tribute to Lee W. Wattenberg. Mol. Nutr. Food Res. 2016; 60(6): 1228-38. https://dx.doi.org/10.1002/mnfr.201500889.
- Cover C.M., Hsieh S.J., Cram E.J., Hong C., Riby J.E., Bjeldanes L.F., Firestone G.L. Indole-3-carbinol and tamoxifen cooperate to arrest the cell cycle of MCF-7 human breast cancer cells. Cancer Res. 1999; 59(6):1244-51.
- Ampofo E., Schmitt B.M., Menger M.D., Laschke M.W. Targeting the Microcirculation by indole-3-carbinol and its main derivate 3,3,’-diindolylmethane: effects on angiogenesis, thrombosis and inflammation. Mini Rev. Med. Chem. 2018; 18(11): 962-8. https://dx.doi.org/10.2174/1389557518666180313100144.
- Rahman K.M., Aranha O., Sarkar F.H. Indole-3-carbinol (I3C) induces apoptosis in tumorigenic but not in nontumorigenic breast epithelial cells. Nutr. Cancer. 2003; 45(1): 101-12.
- Feitelson M.A., Arzumanyan A., Kulathinal R.J., Blain S.W., Holcombe R.F., Mahajna J. et al. Sustained proliferation in cancer: mechanisms and novel therapeutic targets. Semin. Cancer Biol. 2015; 35(Suppl.): S25-54. https://dx.doi.org/10.1016/j.semcancer.2015.02.006.
- Fuentes F., Paredes-Gonzalez X., Kong A.N. Dietary glucosinolates sulforaphane, phenethyl isothiocyanate, indole-3-carbinol/3,3’-diindolylmethane: anti-oxidative stress/inflammation, Nrf2, epigenetics/epigenomics and in vivo cancer chemopreventive efficacy. Curr. Pharmacol. Rep. 2015; 1(3): 179-96. https://dx.doi.org/10.1007/s40495-015-0017-y.
- Ahmad A., Sakr W.A., Rahman K.W. Mechanisms and therapeutic implications of cell death induction by indole compounds. Cancers (Basel). 2011; 3(3): 2955-74. https://dx.doi.org/10.3390/cancers3032955.
- Maruthanila V.L., Poornima J., Mirunalini S. Attenuation of carcinogenesis and the mechanism underlying by the influence of indole-3-carbinol and its metabolite 3,3’-diindolylmethane: a therapeutic marvel. Adv. Pharmacol. Sci. 2014;2014:832161. https://dx.doi.org/10.1155/2014/832161.
- Wang S.Q., Cheng L.S., Liu Y., Wang J.Y., Jiang W. Indole-3-carbinol (I3C) and its major derivatives: their pharmacokinetics and important roles in hepatic protection. Curr. Drug Metab. 2016; 17(4): 401-9. https://dx.doi.org/10.2174/1389200217666151210125105.
- Riby J.E., Firestone G.L., Bjeldanes L.F. 3,3’-diindolylmethane reduces levels of HIF-1alpha and HIF-1 activity in hypoxic cultured human cancer cells. Biochem. Pharmacol. 2008; 75(9): 1858-67. https://dx.doi.org/10.1016/j.bcp.2008.01.017.
- Augoulea A., Alexandrou A., Creatsa M., Vrachnis N., Lambrinoudaki I. Pathogenesis of endometriosis: the role of genetics, inflammation and oxidative stress. Arch. Gynecol. Obstet. 2012; 286(1): 99-103. https://dx.doi.org/10.1007/s00404-012-2357-8.
- Киселев В.И., Сметник В.П., Сутурина Л.В., Селиванов С.П., Рудакова Е.Б., Рахматуллина И.Р., Андреева Е.Н., Фадеева Н.И., Хасанов Р.Ш., Кулагина Н.В., Рожкова Н.И., Артымук Н.В., Гависова А.А., Муйжнек Е.Л., Кузнецов И.Н., Друх В.М. Индолкарбинол (Индинол Форто) – метод мультитаргетной терапии при циклической мастодинии. Акушерство и гинекология. 2013; 7: 56-62. [Kiselev V.I., Smetnik V.P.,Suturina L.V., Selivanov S.P., Rudakova E.B., Rakhmatullina I.R., Andreeva E.N., Fadeeva N.I., Khasanov R.Sh., Kulagina N.V., Rozhkova N.I.,Artymuk N.V., Gavisova A.A., Muizhnek E.L., Kuznetsov I.N., Drukh V.M. Indole carbinol (Indinol Forto) is a multitargeted therapy option for cyclic mastodynia. Akusherstvo i Ginekologiya (Obstetrics and Gynecology). 2013; 7: 56-62. (in Russian)].
- Anesthesia and analgesia in laboratory animals at UCSF. The Regents of the University of California. Электронный ресурс: Available at: http://www.iacuc.ucsf.edu/Proc/awRbtFrm.asp Accessed 01.2019.
- Keenan J.A., Williams-Boyce P.K., Massey P.J., Chen T.T., Caudle M.R., Bukovsky A. Regression of endometrial explants in a rat model of endometriosis treated with the immune modulators loxoribine and levamisole. Fertil. Steril. 1999; 72(1): 135-41. https://dx.doi.org/10.1016/s0015-0282(99)00157-0.
- Tosti C., Biscione A., Morgante G., Bifulco G., Luisi S., Petraglia F. Hormonal therapy for endometriosis: from molecular research to bedside. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017; 209: 61-6. https://dx.doi.org/10.1016/j.ejogrb.2016.05.032.
- Simmen R.C., Kelley A.S. Reversal of fortune: estrogen receptor-β in endometriosis. J. Mol. Endocrinol. 2016; 57(2): F23-7. https://dx.doi.org/10.1530/JME-16-0080.
- ACOG Committee on Practice Bulletins-Gynecology. ACOG practice bulletin. Medical management of endometriosis. Number 11, December 1999 (replaces Technical Bulletin Number 184, September 1993). Clinical management guidelines for obstetrician gynecologists. Int. J. Gynaecol. Obstet. 2000; 71(2): 183-96. https://dx.doi.org/10.1016/s0020-7292(00)80034-.
- Shertzer H.G., Senft A.P. The micronutrient indole-3-carbinol: implications for disease and chemoprevention. Drug Metabol. Drug Interact. 2000; 17(1-4): 159-88. https://dx.doi.org/ 10.1515/dmdi.2000.17.1-4.159.
- Rogan E.G. The natural chemopreventive compound indole-3-carbinol: state of the science. In Vivo. 2006; 20(2): 221-8.
- Тихончук Е.Ю., Асатурова А.В., Адамян Л.В. Частота выявления и структура патологических изменений эндометрия у женщин репродуктивного возраста с генитальным эндометриозом. Акушерство и гинекология. 2016; 12: 87-95. [Tikhonchuk E.Yu., Asaturova A.V., Adamyan L.V. The detection rate and pattern of endometrial pathological changes in reproductive-aged women with genital endometriosis. Akusherstvo i Ginekologiya (Obstetrics and Gynecology). 2016; 12: 87-95. (in Russian)]. https://dx.doi.org/10.18565/aig.2016.12.87-95.
- Ищенко А.И., Кудрина Е.А. Эндометриоз: диагностика и лечение. М.: ГЭОТАР-МЕД; 2002. 104 с. [Ishchenko A.I., Kudrina E.A. Endometriosis: diagnosis and treatment. Moscow: GEOTAR-MED; 2002.104 p. (in Russian)].
- Ahmad A., Biersack B., Li Y., Kong D., Bao B., Schobert R. et al. Targeted regulation of PI3K/Akt/mTOR/NF-kB signaling by indole compounds and their derivatives: mechanistic details and biological implications for cancer therapy. Anticancer Agents Med. Chem. 2013; 13(7): 1002-13. https://dx.doi.org/10.2174/18715206113139990078.
- Wang X., He H., Lu Y., Ren W., Teng K.Y., Chiang C.L. et al. Indole-3-carbinol inhibits tumorigenicity of hepatocellular carcinoma cells via suppression of microRNA-21 and upregulation of phosphatase and tensin homolog. Biochim. Biophys. Acta. 2015; 1853(1): 244-53. https://dx.doi.org/10.1016/j.bbamcr.2014.10.017.
- Marconett C.N., Singhal A.K., Sundar S.N., Firestone G.L. Indole-3-carbinol disrupts estrogen receptor-alpha dependent expression of insulin-like growth factor-1 receptor and insulin receptor substrate-1 and proliferation of human breast cancer cells. Mol. Cell. Endocrinol. 2012; 363(1-2): 74-84. https://dx.doi.org/10.1016/j.mce.2012.07.008.
Received 21.11.2019
Accepted 29.11.2019
About the Authors
Vsevolod I. Kiselev, Doctor of Biological Sciences, Professor, Corresponding Member of RAS Professor, Deputy Director if the Institute of Oncogynecology and Mammology, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov. E-mail: vkis10@mail.ru.4 Oparina str., Moscow, 117997, Russian Federation.
Levon A. Ashrafyan, DM, Professor, Academician of the Russian Academy of Sciences, Director оf the Institute of Oncogynecology and Mammology, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov. E-mail: levaa2004@yahoo.com.
4 Oparina str., Moscow, 117997, Russian Federation.
Stanislav M. Pronin, MD, PhD, Oncologist, Senior scientific researcher at the Institute of Oncogynecology and Mammology, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov. E-mail: s_pronin@oparina4.ru. 4 Oparina str., Moscow, 117997, Russian Federation.
Evgeniya V. Gerfanova, MD, Oncologist at the Institute of Oncogynecology and Mammology, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov. E-mail: e_gerfanova@oparina4.ru.
4 Oparina str., Moscow, 117997, Russian Federation.
Igor N. Kuznetsov, PhD, Deputy Director if the Institute of Oncogynecology and Mammology, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov. E-mail: i_kuznetsov@oparina4.ru.
4 Oparina str., Moscow, 117997, Russian Federation.
Vadim M. Drukh, DM, General Director of JSC «MiraxBioFarma». E-mail: info@mbpharma.ru.
5 Bryanskaja str., Moscow, 121059, Москва, Russian Federation.
Vladimir V. Udut, DM, Professor, Corresponding Member of RAS, Deputy Director of Scientific and Medical Work, Head of the Laboratory of Physiology, Molecular and Clinical Pharmacology Research Institute of Pharmacology and Regenerative Medicine named after E.D. Goldberg, Tomsk National Research Medical Center of RAS.
E-mail: udutv@mail.ru. 3 Lenina Ave., Tomsk, 634028, Russian Federation.
Alexey A. Churin, DM, Head of the Department of Drug Toxicology,
Research Institute of Pharmacology and Regenerative Medicine named after E.D. Goldberg, Tomsk National Research Medical Center of RAS. E-mail: churin_aa@pharmso.ru.
3 Lenina Ave., Tomsk, 634028, Russian Federation.
Olga I. Pchelintseva, MSc, Clinical trial manager of JsC «MiraxBioFarma». E-mail: pchelintseva87@mail.ru.
5 Bryanskaja str., Moscow, 121059, Москва, Russian Federation.
For reference: Kiselev V.I., Ashrafyan L.A., Pronin S.M., Gerfanova E.V., Kuznetsov I.N.,
Drukh V.M., Udut V.V., Churin A.A., Pchelintseva O.I. Investigation of the efficacy
of indole-3-carbinol on a rat model of endometriosis (experimental study).
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2020; 5: 122-30 (In Russian).
https://dx.doi.org/10.18565/aig.2020.5.122-30



